Synthesis and Chemical Characterisation of New Bis-Thieno [2,3-b]thiophene Derivatives
Abstract
:1. Introduction
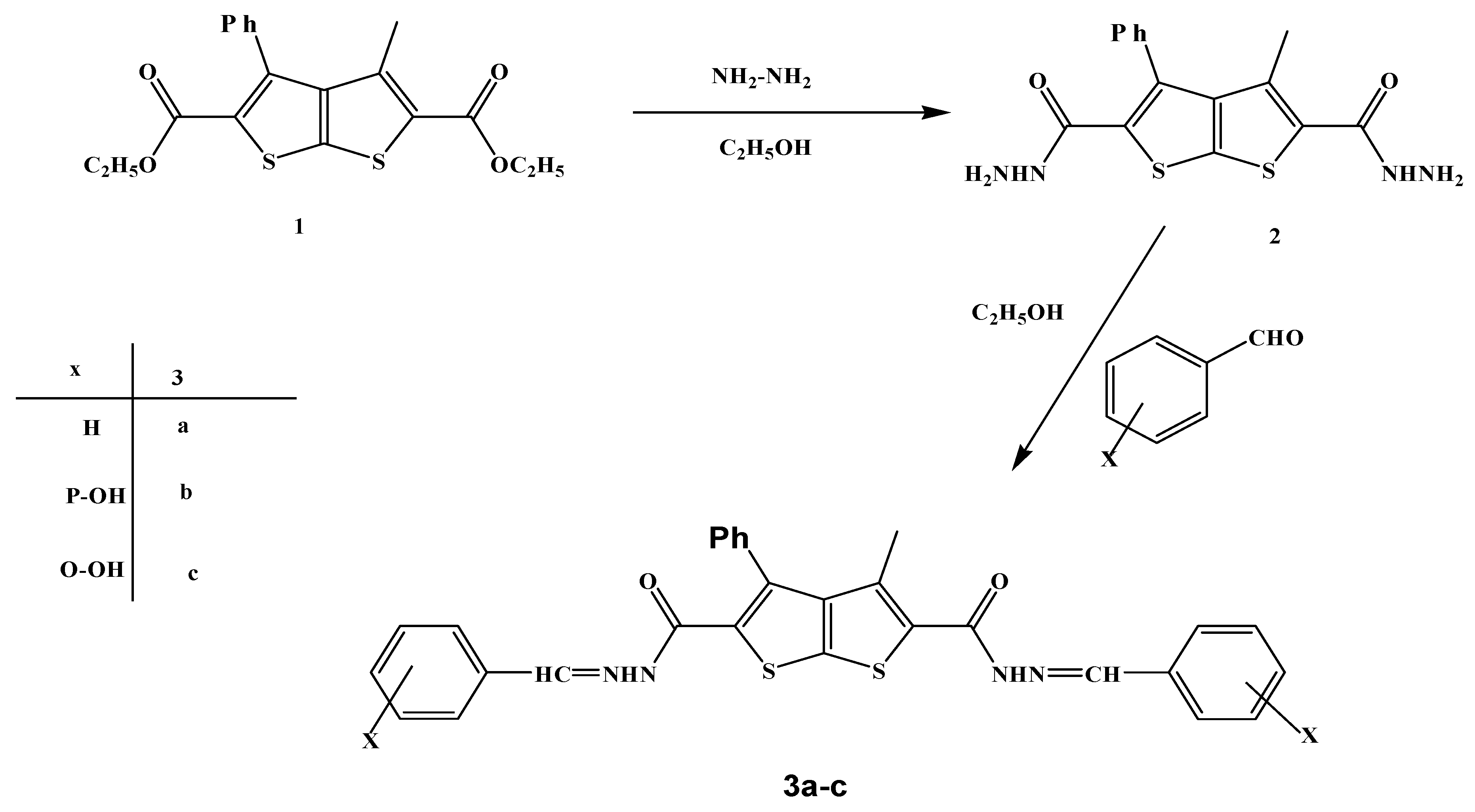
2. Results and Discussion


3. Experimental
3.1. General
3.2. Reaction of 3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarbohydrazide (2) with aldehydes
3.3. Reaction of 3-methyl-4-phenylthieno[2,3-b]thiophene-2,5-dicarbohydrazide (2) with active methylene derivatives
3.4. General procedure for the synthesis of compounds 7a-c
4. Conclusions
Acknowledgements
- Sample Availability: Samples of compounds 1-7a-c are available from the author.
References
- Jarak, I.; Kralj, M.; Piantanida, I.; Suman, L.; Zinic, M.; Pavelic, K.; Karminski-Zamola, G. Novel cyano- and amidino-substituted derivatives of thieno[2,3-b]- and thien- o[3,2-b]thiophene-2-carboxanilides and thieno[30, 20: 4,5]thieno- and thieno [20, 30: 4, 5] thieno[2,3-c]quinolones: Synthesis, photochemical synthesis, DNA binding, and antitumor evaluation. Bioorg. Med. Chem. 2006, 14, 2859–2868. [Google Scholar] [CrossRef]
- Peters, D.; Hornfeldt, A.B.; Gronowitz, S. Synthesis of various 5-substituted uracils. J. Heterocycl. Chem. 1990, 27, 2165–2173. [Google Scholar] [CrossRef]
- Kukolja, S.; Draheim, S.E.; Graves, B.J.; Hunden, D.C.; Pfeil, J.L.; Cooper, R.D.G.; Ott, J.L.; Couter, F.T. Orally absorbable cephalosporin antibiotics. 2. Structure-activity studies of bicyclic glycine derivatives of 7-aminodeacetoxycephalosporanic acid. J. Med. Chem. 1985, 28, 1896–1903. [Google Scholar] [CrossRef]
- Kumar, R.; Nair, R.R.; Dhiman, S.S.; Sharma, J.; Prakash, O. Organoiodine (III)-mediated synthesis of 3-aryl/heteroaryl-5,7-dimethyl-1,2,4-triazolo[4,3-c]pyrimidines as antibacterial agents. Eur. J. Med Chem. 2009, 44, 2260–2264. [Google Scholar] [CrossRef]
- Prugh, J.D.; Hartman, G.D.; Mallorga, P.J.; McKeever, B.M.; Michelson, S.R.; Murcko, M.A.; Schwam, H.; Smith, R.L.; Sondey, J.M.; Springer, J.P.; Surgrue, M.F. New isomeric classes oftopically active ocular hypotensive carbonic anhydrase inhibitors: 5-substituted thieno[2,3-b]thiophene-2-sulfonamides and 5-substituted thieno[3,2-b]thiophene-2-sulfonamides. J. Med. Chem. 1991, 34, 1805–1818. [Google Scholar] [CrossRef]
- Egbertson, M.S.; Cook, J.J.; Bednar, B.; Prugh, J.D.; Bednar, R.A.; Gaul, S.L.; Gould, R.J.; Hartman, G.D.; Homnick, C.F.; Holahan, M.A.; Libby, L.A.; Lynch, J.J., Jr.; Lynch, R.J.; Sitko, G.R.; Stranieri, M.T.; Vassallo, L.M. Non-Peptide GPIIb/IIIa Inhibitors. 20. Centrally Constrained Thienothiophene α-Sulfonamides Are Potent, Long Acting in Vivo Inhibitors of Platelet Aggregation. J. Med. Chem. 1999, 42, 2409–2421. [Google Scholar]
- Eisa, H.M.; Tantawy, A.S.; El-Kerdawy, M.M. Synthesis of certain 2-aminoadamantane derivatives as potential antimicrobial agents. Pharmazie 1991, 46, 182–184. [Google Scholar]
- Sah, P.P.T.; Peoples, S.A. Isonicotinyl hydrazones as antitubercular agents and derivatives for identification of aldehydes and ketones. J. Am. Pharm. Ass. Sci. Ed. 1954, 43, 513–524. [Google Scholar]
- Parmar, S.S.; Gupta, A.K.; Gupta, T.K.; Stenberg, V.I. Synthesis of substituted benzyldinohydrazines and their monoamine oxidase inhibitory and anticonvulsant properties. J. Pharm. Sci. 1975, 64, 154–157. [Google Scholar]
- Kalsi, R.; Pande, K.; Bhalla, T.N.; Bartwall, J.P.; Gupta, G.P.; Parmar, S.S. Anti-inflammatory activity of quinazolinoformazans. J. Pharm. Sci 1990, 79, 317–320. [Google Scholar] [CrossRef]
- Tozkoparan, B.; Gökhan, N.; Aktay, G.; Yesilada, E.; Ertan, M. Benzylidenethiazolo[3,2-b]-1,2,4-triazole-5(6H)-onessubstituted with ibuprofen: synthesis, characterizationand evaluation of anti-inflammatory activity. Eur. J. Med. Chem. 2000, 35, 743–750. [Google Scholar] [CrossRef]
- Demirbas, N.; Ugurluoglu, R.; Demirbas, A. Synthesis of 3-alkyl(Aryl)-4-alkylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-ones and 3-alkyl-4-alkylamino-4,5-dihydro-1H-1,2,4-triazol-5-ones as antitumor agents. Bioorg. Med. Chem. 2002, 10, 3717–3723. [Google Scholar] [CrossRef]
- Holla, B.S.; Akberali, P.M.; Shivananda, M.K. Studies on nitrophenylfuran derivatives part Xii. synthesis, characterization, antibacterial and antiviral activities of some nitrophenylfurfurylidene-1,2,4-triazolo[3,4-b]-1,3,4-thiadiazines. Farmaco 2001, 56, 919–927. [Google Scholar] [CrossRef]
- Holla, B.S.; Kalluraya, B.; Sridhar, K.R.; Drake, E.; Thomas, L.M.; Bhandary, K.K.; Levine, M.J. Synthesis, structural characterization, crystallographic analysis and antibacterial properties of some nitrofuryl triazolo[3,4-b]-1,3,4-thiadiazines. Eur. J. Med.Chem. 1994, 29, 301–308. [Google Scholar] [CrossRef]
- Dawood, K.M.; Farag, A.M.; Abdel-Aziz, H.A. A convenient access to functionalized pyrazole, pyrazolyl-azole, and pyrazolo[3,4-d]pyridazine derivatives. J. Chin. Chem. Soc. 2006, 53, 873–880. [Google Scholar]
- Dawood, K.M.; Farag, A.M.; Abdel-Aziz, H.A. Synthesis and antimicrobial evaluation of some 1,2,4-triazole, 1,3,4-oxa(thia)diazole, and 1,2,4-triazolo[3,4-b]-1,3,4-thiadiazine derivatives. Heteroatom Chem. 2005, 16, 621–627. [Google Scholar] [CrossRef]
- Dawood, K.M.; Farag, A.M.; Abdel-Aziz, H.A. Synthesis of some new benzofuran-based thiophene, 1,3-oxathiole and 1,3,4-oxa(thia)diazole derivatives. Heteroatom Chem. 2007, 18, 294. [Google Scholar] [CrossRef]
- Farag, A.M.; Dawood, K.M.; Abdel-Aziz, H.A. Synthesis of some new pyridazine, 1,2,4-triazine and 1,3,4-thiadiazole derivatives. J. Chem. Res. 2004, 808–810. [Google Scholar]
- Dawood, K.M.; Farag, A.M.; Abdel-Aziz, H.A. Azoles and azolo-azines via 3-(3-methylbenzofuran-2-yl)-3-oxopropanenitrile. J. Chem. Res. 2005, 378–381. [Google Scholar]
- Joshi, S.D.; Joshi, S.D.; Vagdevi, H.M.; Vaidya, V.P.; Gadaginamath, G.S. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial and antitubercular agents. Eur. J. Med. Chem. 2008, 43, 1989–1996. [Google Scholar] [CrossRef]
- Mamolo, M.G. Synthesis and antimycobacterial activity of (3,4-diaryl-3H-thiazol-2-ylidene)-hydrazide derivatives. Farmaco 2003, 78, 631–637. [Google Scholar] [CrossRef]
- Bedia, K.K.; Elçin, O.; Seda, U.; Fatma, K.; Nathaly, S.; Sevim, R.; Dimoglo, A. Synthesis and characterization of novel hydrazide-hydrazones and the study of their structure-antituberculosis activity. Eur. J. Med. Chem. 2006, 41, 1253–1261. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2-yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Convenient Synthesis and Antimicrobial Activity of New 3-Substituted 5-(Benzofuran-2-yl)-pyrazole Derivatives. Monatsh. Chem. 2009, 341, 734–739. [Google Scholar]
- Amr, A.E.; Sabrry, N.M.; Abdalla, M.M.; Abdel-Wahab, B.F. Synthesis, antiarrhythmic and anticoagulant activities of novel thiazolo derivatives from methyl 2-(thiazol-2-ylcarbamoyl)acetate. Eur. J. Med. Chem. 2009, 44, 725–735. [Google Scholar]
- Abdel-Wahab, B.F.; Amr, A.E.; Abdalla, M.M. Synthesis and antimicrobial evaluation of some 1,3-thiazole, 1,3,4-thiadiazole, 1,2,4-triazole, and 1,2,4-triazolo[3,4-b][1,3,4]-thiadiazine derivatives includinga 5-(benzofuran-2-yl)-1-phenylpyrazole moiety. Monatsh. Chem. 2009, 140, 601–605. [Google Scholar] [CrossRef]
- Comel, A.; Kirsch, G. Efficient one pot preparation of variously substituted thieno[2,3-b]thiophene. J. Heterocycl. Chem. 2001, 38, 1167. [Google Scholar] [CrossRef]
© 2010 by the authors;
Share and Cite
Mabkhoot, Y.N. Synthesis and Chemical Characterisation of New Bis-Thieno [2,3-b]thiophene Derivatives. Molecules 2010, 15, 3329-3337. https://doi.org/10.3390/molecules15053329
Mabkhoot YN. Synthesis and Chemical Characterisation of New Bis-Thieno [2,3-b]thiophene Derivatives. Molecules. 2010; 15(5):3329-3337. https://doi.org/10.3390/molecules15053329
Chicago/Turabian StyleMabkhoot, Yahia Nasser. 2010. "Synthesis and Chemical Characterisation of New Bis-Thieno [2,3-b]thiophene Derivatives" Molecules 15, no. 5: 3329-3337. https://doi.org/10.3390/molecules15053329




