Abstract
The organotin monomer di(tri-n-butyltin) citraconate (DTBTC, I) was synthesized. Subsequently this monomer was copolymerized with N-vinylimidazole (VI) using a free radical technique. The overall conversion was kept low (≤14% wt/wt) for all studied samples and the copolymer composition was determined from tin analysis using the Gilman and Rosenberg method. The synthesized monomer and copolymer were further characterized by elemental analysis, 1H- and 13C-NMR, and FTIR spectroscopy.
1. Introduction
A copolymer’s composition is an important factor in the evaluation of its utility [1,2,3,4]. Controlling the polymer property parameters, such as copolymer composition and sequence distribution and molecular weight averages, is of particular importance in copolymerization processes [2]. In order to calculate the rate of polymerization or polymer productivity and copolymer composition, monomer reactivity ratios must be known [5]. Reactivity ratios are among the most important parameters for the composition equation of copolymers, as they can offer information such as the relative reactivity of monomer pairs and help estimate the copolymer composition [2,3]. Determination of the monomer reactivity ratios with small confidence intervals requires sensitive analytical techniques, careful planning of experiments and the use of statistically valid methods of estimation [5,6]. The method which is used most often nowadays for estimating monomer reactivity ratios is to perform low conversion copolymerization at various initial monomer feed compositions. Subsequently, the copolymer composition is determined for each reaction. Traditional methods for estimating monomer reactivity ratios are based on, first, transforming the instantaneous copolymer composition equation into a form that is linear in the parameters r1 and r2 and then estimating the monomer reactivity ratios by graphical plotting or by the linear least-squares method [7,8,9,10]. Linearization of the copolymer composition equation will distort the error distributions associated with the data.
In this paper di(tri-n-butyltin) citraconate (DTBTC, I) was synthesized for use as an organotin monomer. This monomer was then copolymerized with N-vinylimidazole (VI). The structural characterization of the copolymer was performed and the reactivity ratios in the copolymerization determined for the classical copolymerization model using the Finemann–Ross linearization method (FR method) [1,2,11].
2. Results and Discussion
2.1. Monomer Synthesis
Di(tri-n-butyltin) citraconate (DTBTC, I) was prepared using equimolar ratios of bis(tri-n-butyltin) oxide (TBTO) and citraconic anhydride (Scheme 1). The purity of the prepared monomer was checked by Thin Layer Chromatography (TLC) using chloroform as eluant. In addition, the structure was confirmed by elemental analysis, FTIR, 1H- and 13C-NMR spectroscopy.
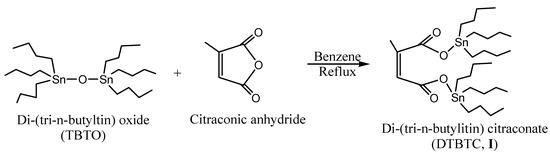
Scheme 1.
Synthesis of di-(tri-n-butyltin)citraconate(DTBTC, I).
Scheme 1.
Synthesis of di-(tri-n-butyltin)citraconate(DTBTC, I).
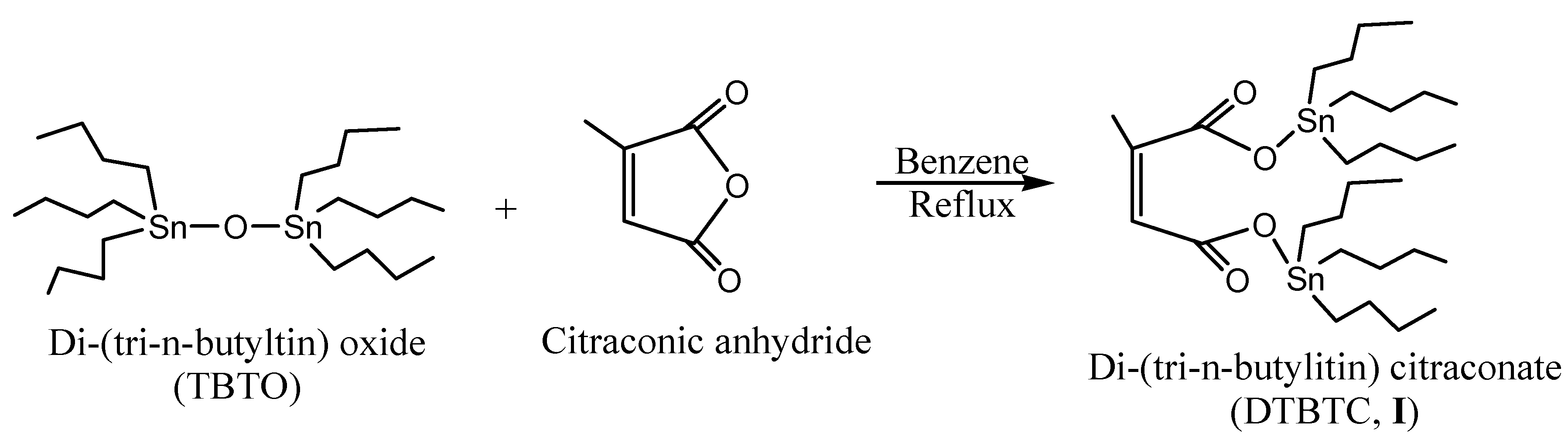
The FTIR spectrum of DTBTC (I) showed characteristic peaks at 2,852, 2,868, 2,922, and 2,954 cm-1 assigned to C-H stretching (-CH2CH2CH2CH3, & -CH=CCH3-), at 1,750 and 1,700 cm-1 assigned to C=O stretching and at 1,642 cm-1, assigned to C=C stretching. Moreover, the disappearance of anhydride group stretching peak at 1,850 cm-1 confirmed the complete conversion of the anhydride group into ester groups.
The 1H-NMR Spectrum (CDCl3) of DTBTC (I) showed peaks at δ 0.86 (triplet, -CH2CH2CH2CH3), 1.26-1.30 (multiplet, -CH2CH2CH2CH3), δ 1.34-1.61 (multiplet, -CH2CH2CH2CH3), δ 1.96 (singlet, ‑CH=CCH3-), 5.73 (singlet, -CH=CCH3-), while the corresponding 13C-NMR spectrum (CDCl3) showed peaks at δ 13.69, 16.55, 27.81, 28.00 (-CH2CH2CH2CH3), 20.00 (-CH=CCH3-), 120.51 and 145.31 (-CH=CH-), 170.50 (-CH=CCH3-C=O) and 174.62 (O=C-CH=CCH3-). The calculated (measured) elemental microanalyses results were in a good agreement [%C: 49.18 (49.73); %H: 8.25 (8.95): %Sn: 33.25 (33.10)]. Tin was estimated using the Gilman and Rosenberg method [12].
2.2. Copolymerization Method
Poly(DTBTC-co-VI) (II) was prepared in DMF solution with a total concentration of 6.67 mol/via a free radical technique using AIBN as initiator at 60 ºC L for different time intervals (Scheme 2). The copolymerization was stopped at overall conversions ≤14% wt/wt and the formed copolymer (II) was precipitated in an excess amount of acetone, and then dried in an oven under vacuum at 60 ºC.
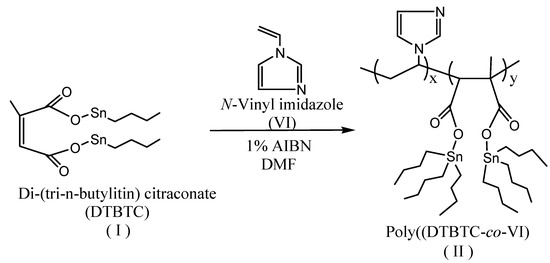
Scheme 2.
Synthesis of Poly(di(tri-n-butyltin) citraconate-co-N-vinylimidazole).
Scheme 2.
Synthesis of Poly(di(tri-n-butyltin) citraconate-co-N-vinylimidazole).
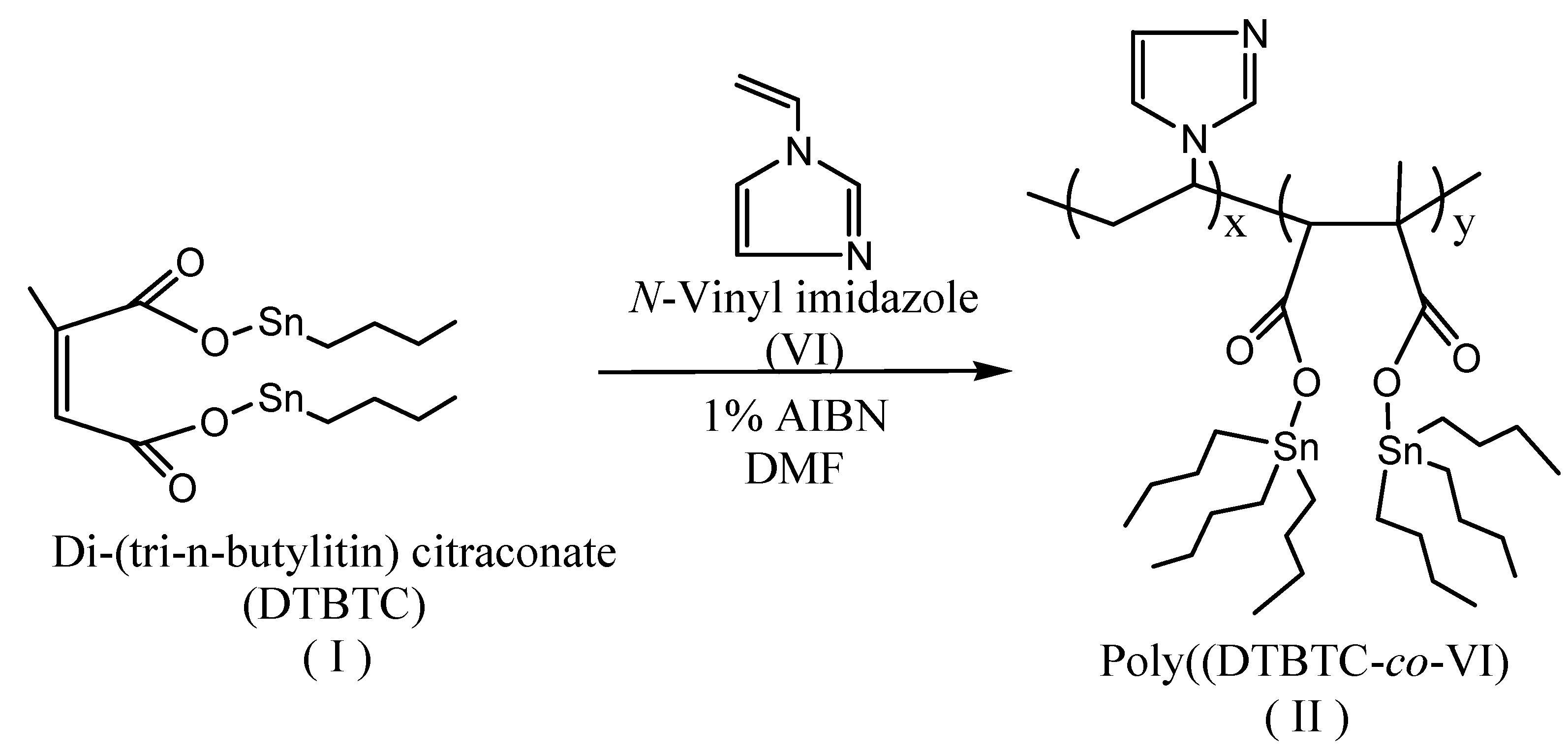
Copolymer (II) was characterized by FTIR and 1H-NMR spectroscopy. After 3 h the FT-IR spectrum of (II) with an overall conversion of 7.81%, was characterized by the disappearance of the C=C stretching bands at 1,650 and 1,670 cm-1 of DTBTC and VI, respectively, which confirm the formation of the copolymer. The FTIR spectrum showed peaks at 1,592, 1,627 and 1,657 cm-1 assigned to C=C stretching of the imidazole ring and characteristic peaks at 1,605 and 1,766 cm-1 assigned to C=O stretching. In addition the spectrum showed peaks at 2,850, 2,924, 3,025, 3,058, and 3,080 cm-1 assigned to aliphatic C-H stretching. The 1H-NMR spectrum (CDCl3) of (II) was characterized by the disappearance of the peaks at δ 5.49–6.04 ppm (-CH=C-, -CH=CCH3- and CH2=CH-) corresponding to DTBTC and VI, respectively, which further confirm the formation of the copolymer. The spectrum of (II) was characterized by the presence of peaks at δ 0.85-1.43 ppm (-CH2CH2CH2CH3), 1.83 ppm (-CH2-CHN-), 2.87 ppm -C-CHCOOSn-), 4.53 ppm (-CH2-CHN-), and at 6.90-7.35 ppm (Harom, imidazole ring).
2.3. Reactivity Ratio Determination
Different copolymers with different ratios were prepared and the percentage of nitrogen was measured, and subsequently the copolymer composition (f) was determined, as shown in Table 1. The monomer reactivity ratios and the content of the reaction mixture and the copolymer were calculated according to the FR method [8,9,10] (Table 2). The FR parameters for DTBTC and VI were calculated by plotting the relation between F(f-1)/f and F2/f, as shown in Figure 1.

Table 1.
The experimental percentage of nitrogen of poly(DTBTC-co-VI) (II) with different ratios and its composition parameters.
| Copolymer Ratio | %N | M1a | Fb | m1c | fd | Conversion (% wt/wt)f |
|---|---|---|---|---|---|---|
| 10/90 | 21.60 | 0.1 | 0.11 | 0.048 | 0.05 | 9.63 |
| 20/80 | 16.34 | 0.2 | 0.25 | 0.099 | 0.11 | 6.91 |
| 30/70 | 13.29 | 0.3 | 0.43 | 0.14 | 0.16 | 9.98 |
| 40/60 | 9.75 | 0.4 | 0.67 | 0.21 | 0.27 | 11.5 |
| 50/50 | 8.18 | 0.5 | 1.0 | 0.25 | 0.35 | 13 |
a Mole fraction of DTBTC in reaction mixture; b Molar ratio of DTBTC to VI in reaction mixture; c Mole fraction of DTBTC in copolymer; d Molar ratio of DTBTC to VI in copolymer; f Overall conversion.

Table 2.
The monomer reactivity ratios and the FR parameters of poly(DTBTC-co-VI) (II).
| Copolymer Ratio | Monomer Ratio F = M1/M2 | M-Unit Ratio in Copolymer | Parameters of the FR Eq. | |
|---|---|---|---|---|
| F2/f | F/f(f-1) | |||
| 10/90 | 0.11 | 0.05 | 0.24 | -2.09 |
| 20/80 | 0.25 | 0.11 | 0.57 | -2.02 |
| 30/70 | 0.43 | 0.16 | 1.16 | -2.26 |
| 40/60 | 0.67 | 0.27 | 1.66 | -181 |
| 50/50 | 1.0 | 0.35 | 2.86 | -1.86 |
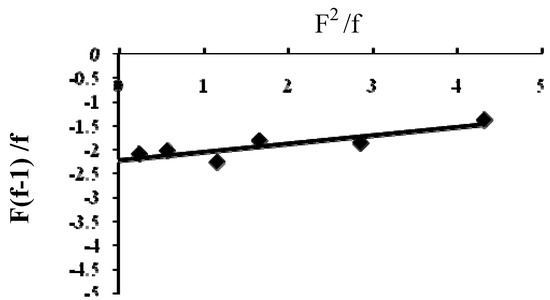
Figure 1.
F(f-1)/f vs F2/f plot for Poly(DTBTC-co-VI) (II).
Figure 1.
F(f-1)/f vs F2/f plot for Poly(DTBTC-co-VI) (II).
As r1r2<1 and r1r2 are close to zero, this implies that monomers tend to form alternating copolymers [13]. From the fact that the values of the experimental reactivity ratio, r1 (k11/k12) are smaller than r2 (k22/k21), it is evident that the DTBTC monomer (r1 = 0.1697) is less reactive towards the addition of its units compared to the addition of VI units, while VI (r2 = 2.2094) is more reactive towards the addition of its units, compared to the addition of DTBTC units.
3. Experimental
3.1. Materials
Citraconic anhydride and bis(tri-n-butyltin) oxide were purchased from Fluka. Benzoyl peroxide was purchased from BDH. 2,2'-Azobisisobutyronitrile (AIBN) was purchased from Riedel-De Haën. All solvents were purchased from BDH and used as received.
3.2. Characterization
1H- and 13C-NMR Spectra were recorded on a JEOL (400 MHz) instrument. FTIR spectra were recorded on a Perkin Elmer 883 spectrophotometer. Elemental analyses were performed on a Perkin Elmer Series II CHN/O Analyzer 2400. Thin-layer chromatography (TLC) was performed using the ascending technique with silica gel 60F 254 precoated aluminum sheets.
3.3. Synthesis of Di(tri-n-butyltin) citraconate (DTBTC, I)
The title monomer di(tri-n-butyltin) citraconate (DTBTC, I) was prepared according to Al-Diab et al. [1]. The method can be summarized as follows: bis(tri-n-butyltin oxide (14.9 g, 25.0 mmol) was added to citraconic anhydride (2.8 g, 25.0 mmol), and benzene (150 mL) in a 500 mL round bottom flask. The reaction mixture was refluxed with stirring for 4 h. The solvent was evaporated on a rotavapor to give pale yellow viscous oil (17.0 g, 95.38% yield).
3.4. Copolymerization Method
In three-necked round bottomed flask, AIBN (1.5 × 10-2 mol/L) was dissolved in DMF (25 mL), and then the calculated molar quantities of the monomers were added to a concentration of 2.5 mol/L. The copolymerization mixture was spurge with nitrogen to expel oxygen and then the copolymerization was done at 60 ºC for the desired period of time. The copolymer formed was precipitated in acetone and was dried in an oven under vacuum at 60 ºC. For reactivity ratio determination, the copolymerization was stopped at overall conversions below 14% wt/wt [14] calculated from the total weight of both monomers by changing the time of polymerization [15].
3.5. Reactivity Ratios Determination
For reactivity ratio determination, copolymerization was performed with different initial feed ratios while maintaining the monomer conversion below 10%. The Fineman–Ross (FR) method was employed. The initiator concentration was kept at 1% relative to the total monomers concentration in benzene or DMF. Monomer reactivity ratios can be calculated from the experimental results depending on the copolymer composition. Copolymer composition can be expressed as follows:
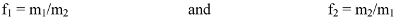 where m1 and m2 are the mole fractions of DTBTC and vinyl imidazole in the copolymer, respectively, and f1 and f2 are its molar ratios in the copolymer.
where m1 and m2 are the mole fractions of DTBTC and vinyl imidazole in the copolymer, respectively, and f1 and f2 are its molar ratios in the copolymer.
Moreover, the feed composition of the reaction mixture is known in advance, so feed composition was used in the calculation of the reactivity ratios and can be expressed as follows:
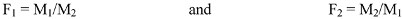 where M1 and M2 are the mole fractions of DTBTC and vinyl imidazole in the reaction mixture, respectively, and F1 and F2 are its molar ratios in the feed composition.
where M1 and M2 are the mole fractions of DTBTC and vinyl imidazole in the reaction mixture, respectively, and F1 and F2 are its molar ratios in the feed composition.
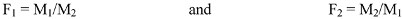
In this research, the calculations were based on the tin content in the copolymer composition [16,17]. The Fineman–Ross (FR) [1,2,11] method is based on the use of copolymer composition and the content of the polymerization mixture. Based on the calculations of the copolymer composition and feed composition and according to the following equation:
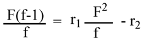 a plot of (F2/f) on X-axis vs {F(f-1)/f} on Y-axis gave a straight line, whose intercept is r2 and the slope is r1.
a plot of (F2/f) on X-axis vs {F(f-1)/f} on Y-axis gave a straight line, whose intercept is r2 and the slope is r1.
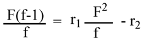
4. Conclusions
The organotin monomer, di(tri-n-butyltin) citraconate (DTBTC, I) was synthesized. This monomer was copolymerized with N-vinylimidazole (VI) via a free radical technique. The overall conversion was kept low (≤14% wt/wt) for all studied samples and the copolymer composition was determined from nitrogen analysis. As r1r2<1, the copolymer tends to form alternating copolymers as r1r2 is close to zero. From the values of the experimental reactivity ratio, r1 is smaller than r2, indicating that the DTBTC monomer is less reactive towards the addition of its units compared to the addition of VI units. On the other hand, compound VI is more reactive towards the addition of its units compared to the addition of DTBTC units.
- Sample Availability: Samples of the compounds are available from the authors.
References
- El-Newehy, M.H.; Al-Deyab, S.S.; Al-Hazmi, A.M. Reactivity Ratios for Organotin Copolymer Systems. Molecules 2010, 15, 2749–2758. [Google Scholar] [CrossRef]
- Al-Deyab, S.S.; Al-Hazmi, A.M.; El-Newehy, M.H. Synthesis and characterization of organotin containing copolymers: reactivity ratio studies. Molecules 2010, 15, 1784–1797. [Google Scholar]
- Erol, I.; Sen, O.; Dedelioglu, A.; Cifci, C. Synthesis and characterization of novel fluorine-containing methacrylate copolymers: Reactivity ratios, thermal properties, and antimicrobial activity. J. Appl. Polym. Sci. 2009, 114, 3351–3359. [Google Scholar] [CrossRef]
- Hou, C.; Liu, J.; Ji, C.; Ying, L.; Sun, H.; Wang, C. Monomer apparent reactivity ratios for acrylonitrile/methyl vinyl ketone copolymerization system. J. Appl. Polym. Sci. 2006, 102, 4045–4048. [Google Scholar] [CrossRef]
- Habibi, A.; Vasheghani-Farahani, E.; Semsarzadeh, M.A.; Sadaghiani, K. Monomer reactivity ratios for lauryl methacrylate–isobutyl methacrylate in bulk free radical copolymerization. Polym. Int. 2003, 52, 1434–1443. [Google Scholar] [CrossRef]
- Wessling, R.A. Kinetics of continuous addition emulsion polymerization. J. Appl. Polym. Sci. 1968, 12, 309–319. [Google Scholar] [CrossRef]
- Dimitratos, J.; Elicabe, G.; Georgakis, C. Control of Emulsion Polymerization Reactors. AIChE J. 1994, 40, 1993–2021. [Google Scholar] [CrossRef]
- Manski, C.F.; Tamer, E.T. Inference on regressions with interval data on a regressor or outcome. Econometrica 2002, 70, 519–547. [Google Scholar] [CrossRef]
- Canegallo, S.; Canu, P.; Morbidelli, M.; Storti, G. Composition control in emulsion copolymerization. II. Application to binary and ternary systems. J. Appl. Polym. Sci. 1994, 54, 1919–1935. [Google Scholar] [CrossRef]
- Al-Deyab, S.S.; El-Newehy, M.H. Synthesis and Characterization of Novel Organotin-Phosphorous Compounds II. Molecules 2010, 15, 1425–1432. [Google Scholar] [CrossRef]
- Finemann, M.; Ross, S.D. Linear method for determining monomer reactivity ratios in copolymerization. J. Polym. Sci. 1950, 5, 259–262. [Google Scholar] [CrossRef]
- Gilman, H.; Rosenberg, D. Reaction of Triphenyltin Hydride with Methyllithium. J. Am. Chem. Soc. 1953, 75, 3592–3593. [Google Scholar] [CrossRef]
- Odian, G. Principles of Polymerization, 3rd ed; John Willy & Sons Inc.: New York, NY, USA, 1995. [Google Scholar]
- Stanely, R.S.; Dannin, J.; Tsou, K.S. Copolymerization of p-triphenyltinstyrene and p-triphenylleadstyrene with styrene or vinyltoluene. J. Polym. Sci. Part A: General Papers 1965, 3, 3199–3207. [Google Scholar]
- Kreisel, M.; Garbatski, U.; David, H.K. Copolymerization of styrene. I. Copolymerization with styrene derivatives containing nitrile groups in the side-chain. J. Polym. Sci. 1964, 2, 105–121. [Google Scholar]
- Shaaban, A.F.; Arief, M.M.; Mahmoud, A.A.; Messiha, N.N. Organotin polymers: 10. Copolymerization parameters for di-(tri-n-butyltin) itaconate with methyl acrylate, ethyl acrylate, N-vinyl pyrrolidone and acrylonitrile. Polymer 1987, 28, 1423–1425. [Google Scholar]
- Alfer, T.; Boher, J.J.; Mark, H. Copolymerization; Interscience: New York, NY, USA, 1952. [Google Scholar]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
