Abstract
The reaction of cyanoacetyl hydrazine (1) with 3-acetylpyridine (2) gave the hydrazide-hydrazone derivative 3. The latter compound undergoes a series of heterocyclization reactions to give new heterocyclic compounds. The antitumor evaluation of the newly synthesized products against three cancer cell lines, namely breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460) and CNS cancer (SF-268) was performed. Most of the synthesized compounds showed high inhibitory effects.
1. Introduction
Hydrazines and their derivatives constitute an important class of compounds that has found wide utility in organic synthesis [1,2]. While hydrazines have traditionally been employed as reagents for the derivatization and characterization of carbonyl compounds, in recent years the N-N linkage has been used as a key structural motif in various bioactive agents. In particular, an increasing number of N-N bond-containing heterocycles and peptidomimetics have made their way into commercial applications as pharmaceutical and agricultural agents [3,4]. Recently, hydrazide-hydrazones have gained great importance due to their diverse biological properties including antibacterial, antifungal, anticonvulsant, anti-inflammatory, antimalarial and antituberculosis activities [5,6,7,8,9,10,11,12,13,14,15,16,17]. With the aim of obtaining novel hydrazide-hydrazones with a wide spectrum of pharmaceutical applications, we report herein the synthesis of a series of hydrazide-hydrazones together with their use in a series of heterocyclic transformations and their evaluation as anti-tumor agents [18,19,20,21].
2. Results and Discussion
Recently, our research group became involved with a comprehensive program involving the synthesis of a series of hydrazide-hydrazone derivatives and their utilization in the synthesis of heterocyclic compounds with potential pharmaceutical and biological activities [22,23]. In continuation to this program, we report herein the reaction of cyanoacetylhydrazine (1) with3-acetylpyridine (2) in 1,4-dioxane to form the hydrazide-hydrazone derivative 3. The structure of compound 3 was confirmed based on its analytical and spectral data. Thus, the 1H-NMR showed a singlet at δ 2.28 for the CH3 group, a singlet at δ 4.26 for the CH2 group, a multiplet at δ 7.43-8.99 for the pyridyl group and a singlet (D2O exchangeable) at δ 10.81 for the NH group. Moreover, the13C- NMR spectrum showed peaks at δ: 14.2 (CH3), 28.9 (CH2), 116.8 (CN), 122.1, 123.4, 133.7, 150.2, 151.0 (pyridine C), 168.1 (C=N), 169.8 (C=O). Further structure elucidation of compound 3 was obtained through the study of its reactivity towards chemical reagents. Thus, the reaction of 3 with either benzaldehyde (4a), 4-chlorobenzaldehyde (4b) or 4-methoxybenzaldehyde (4c) gave the corresponding benzal derivatives 5a-c, respectively (Scheme 1).
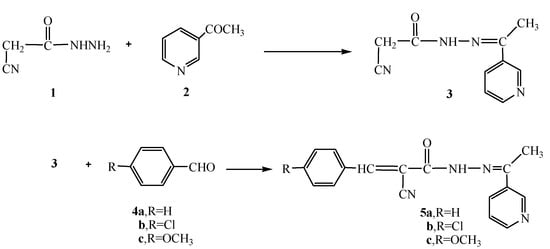
Scheme 1.
Synthesis of the hydrazide-hydrazones 3 and 5a-c.
Scheme 1.
Synthesis of the hydrazide-hydrazones 3 and 5a-c.
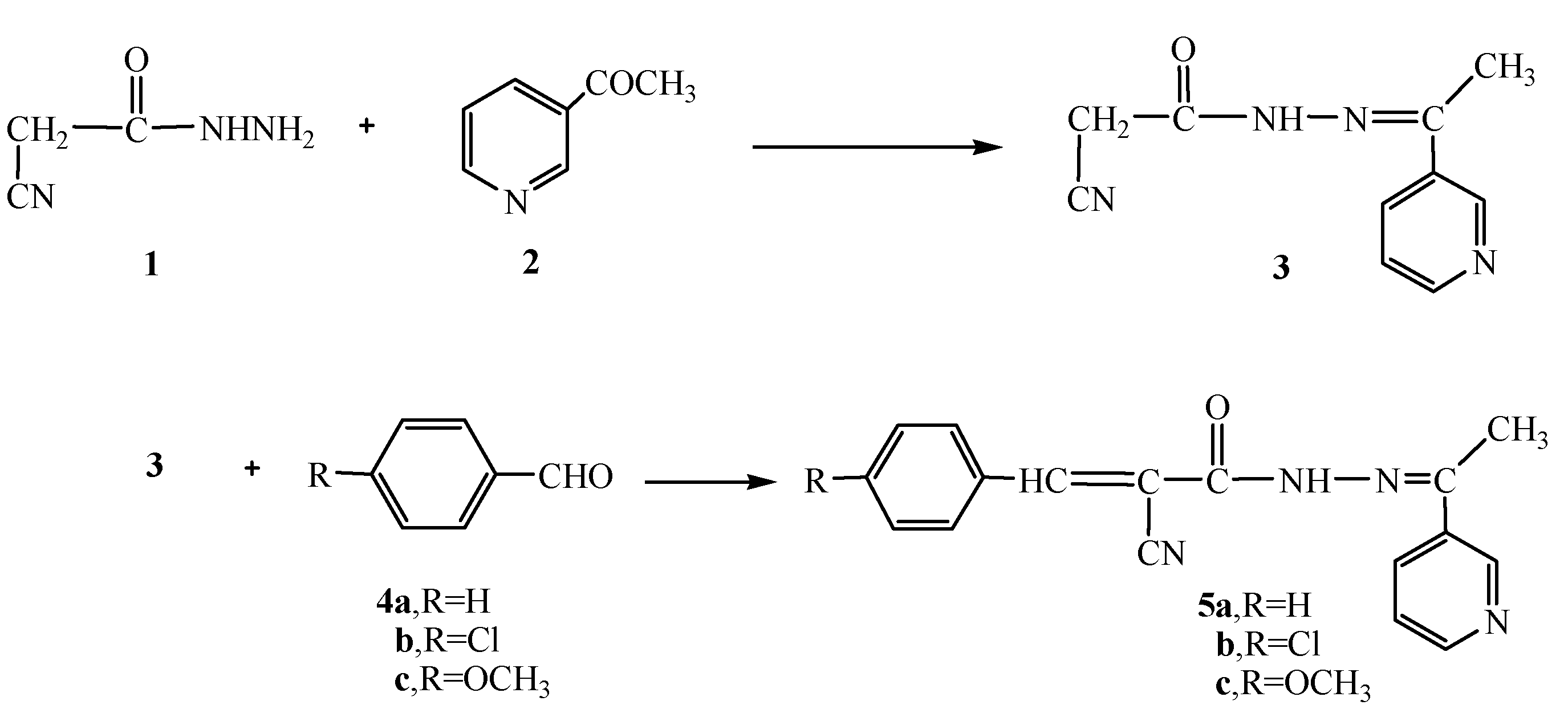
On the other hand, the reaction of compound 3 with salicylaldehyde (6) gave the coumarin derivative 7 (Scheme 2). Analytical and spectral data of the product are in agreement with the proposed structure (see Experimental section).
Scheme 2.
Synthesis of 7.
Scheme 2.
Synthesis of 7.
Next, we studied the reactivity of the active methylene group present in compound 3 towards diazonium salts. Thus, the reaction of 3 with either benzenediazonium chloride (8a), 4-chlorobenzene-diazonium chloride (8b), 4-bromobenzenediazonium chloride (8c) or 4-nitrobenzenediazonium chloride (8d), gave the hydrazone derivatives 9a-d, respectively (Scheme 3). Analytical and spectral data of the latter reaction products are all consistent with the proposed structures.
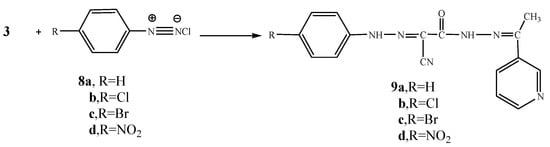
Scheme 3.
Synthesis of phenylhydrazo derivatives 9a-d.
Scheme 3.
Synthesis of phenylhydrazo derivatives 9a-d.
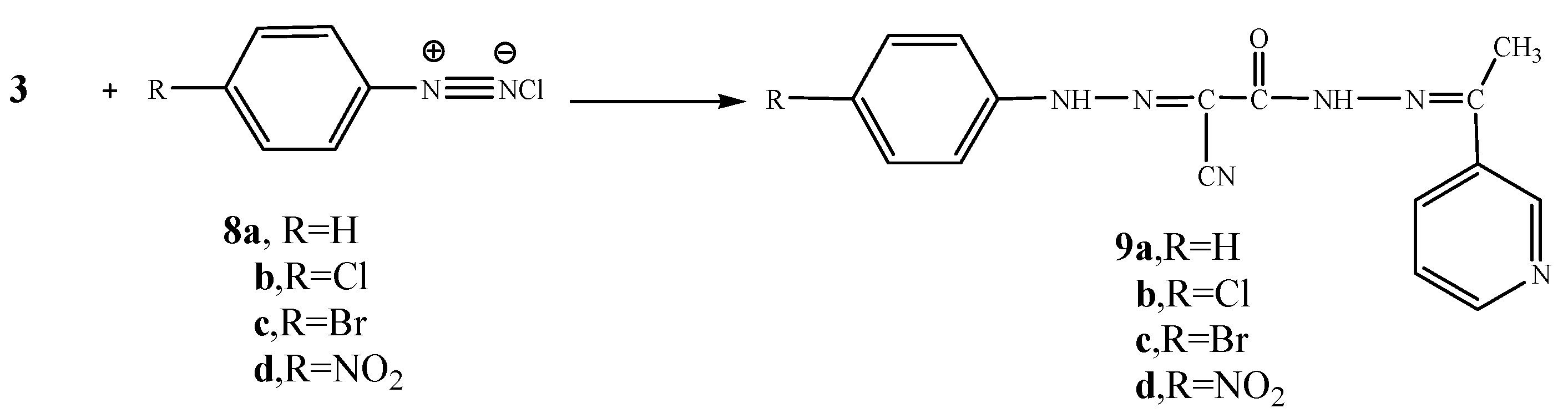
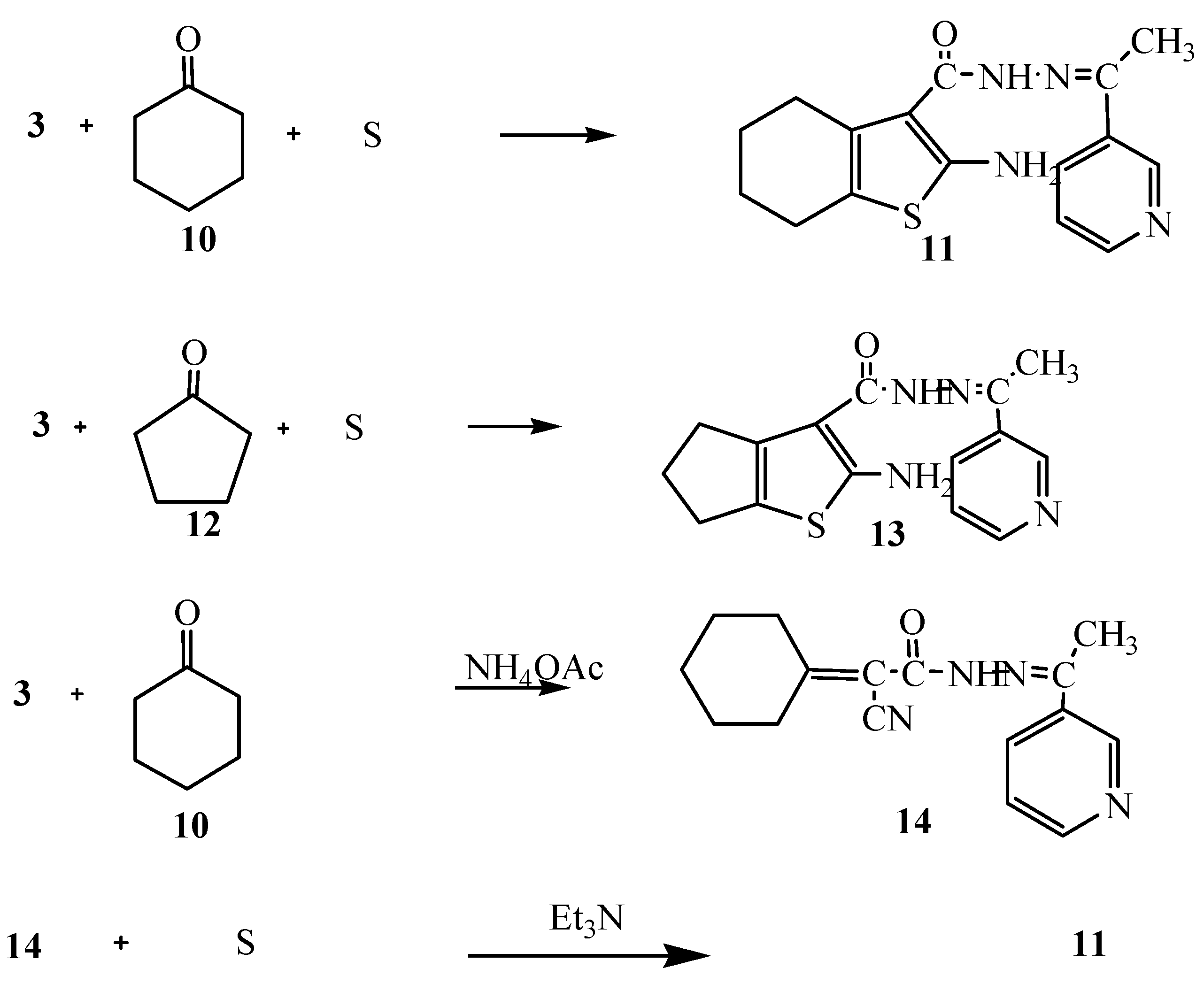
Moreover, the reaction of compound 3 with cyclohexanone (10) and elemental sulfur in the presence of triethylamine was studied as an application of Gewald’s thiophene synthesis. The reaction led to the formation of 4,5,6,7-tetrahydrobenzo[b]thiophene derivative 11. On the other hand, the reaction of compound 3 with cyclopentanone (12) and sulfur gave the cyclopentene[b]thiophene derivative 13. The structures of compounds 11 and 13 were based on analytical and spectral data (see Experimental section). Further confirmation of structure 11 was obtained through its synthesis via another reaction route. Thus, the reaction of compound 3 with cyclohexanone in the presence of ammonium acetate in an oil bath at 140 °C gave the Knoevenagel condensation product 14. The latter reacted with elemental sulfur in the presence of triethylamine to produce the same tetrahydrobenzo[b]thiophene derivative 11. It is convenient to notice that the yield (70%) for formation of compound 11 using the latter procedure was higher than in the synthetic pathway described before (62%) (Scheme 4).
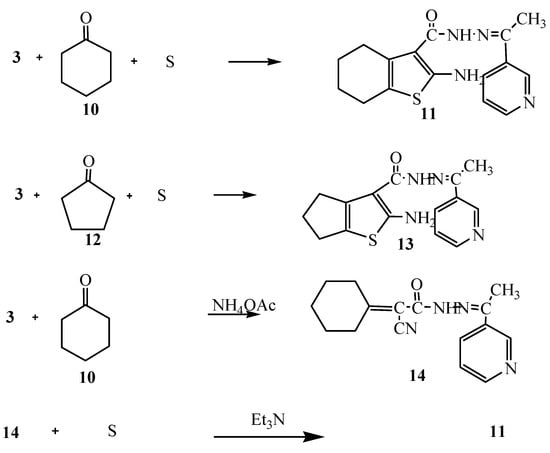
Scheme 4.
Synthesis of 11, 13 and 14.
Scheme 4.
Synthesis of 11, 13 and 14.
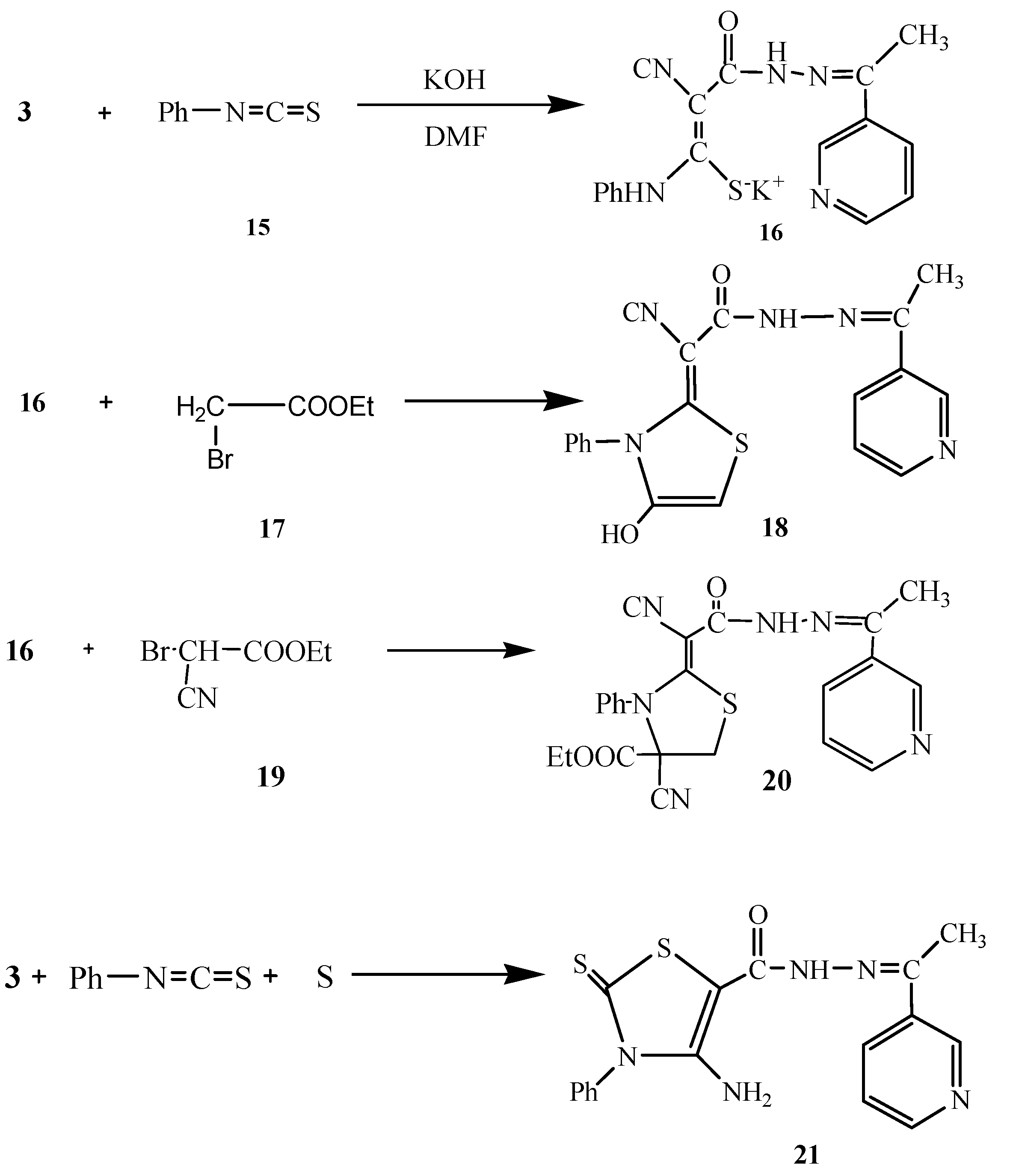
Next, we studied the reactivity of 3 towards phenyl isothiocyanate in basic dimethylformamide solution followed by heterocyclization with active methylene reagents like α-haloketones with the aim of synthesizing thiazole derivatives with potential antitumor activity. Thus, compound 3 reacted with phenylisothiocyanate (15) in DMF/KOH solution at room temperature to give the intermediate potassium sulphide salt 16. Heterocyclization of 16 with α-haloketones like ethyl bromoacetate (17) gave the thiophene derivative 18. The structure of 18 was confirmed based on analytical and spectral data. The 1H-NMR showed a singlet at δ 2.22 for the CH3 group, a singlet at δ 7.46 for the thiazole hydrogen, a multiplet at δ 7.54-8.95 for the pyridine H and C6H5 and two singlets at δ 8.80/10.13 for the NH and OH groups, respectively. Moreover, the 13C-NMR spectrum showed the presence ofpeaks at δ 14.3 (CH3), 93.0, 101.2 (C=C), 115.8 (CN), 119.2, 120.5, 121.8, 122.3, 124.0, 124.8, 127.6, 130.5, 133.2, 137.0, 150.7, 152.2 (C6H5, thiazole, pyridine C), 168.4 (C=N), 170.0 (C=O).
In a similar way, the reaction of 16 with ethyl bromocyanoacetate (19) gave the thiazole derivative 20. Furthermore, compound 3 reacted with phenyl isothiocyanate and elemental sulfur in 1,4-dioxane containing triethylamine to give the thiazole derivative 21 (Scheme 5). The analytical and spectral data of 21 were in agreement with the proposed structure (see Experimental section).
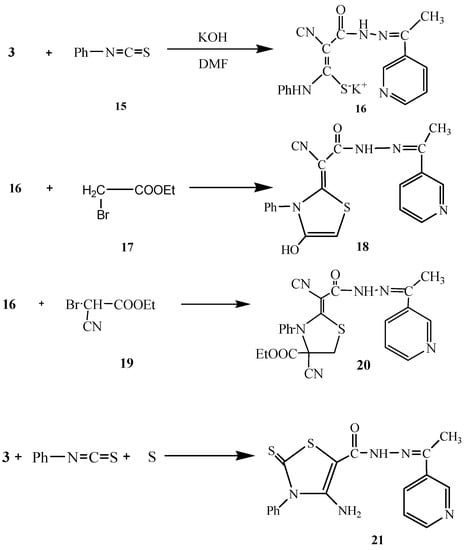
Scheme 5.
Synthesis of 18, 20 and 21
Scheme 5.
Synthesis of 18, 20 and 21
Next, we studied the reactivity of the hydrazide-hydrazone derivative 3 towards cinnamonitrile derivatives. Thus, the reaction of 3 with either 2-benzylidenemalononitrile (22a) or ethyl 2-cyano-3-phenylacrylate (22b) gave the pyridine derivatives 23a and 23b,respectively (Scheme 6).
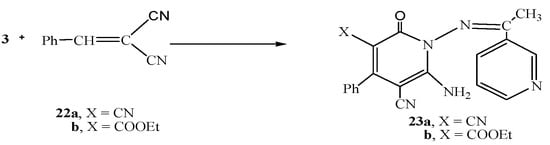
Scheme 6.
Synthesis of 23a,b.
Scheme 6.
Synthesis of 23a,b.
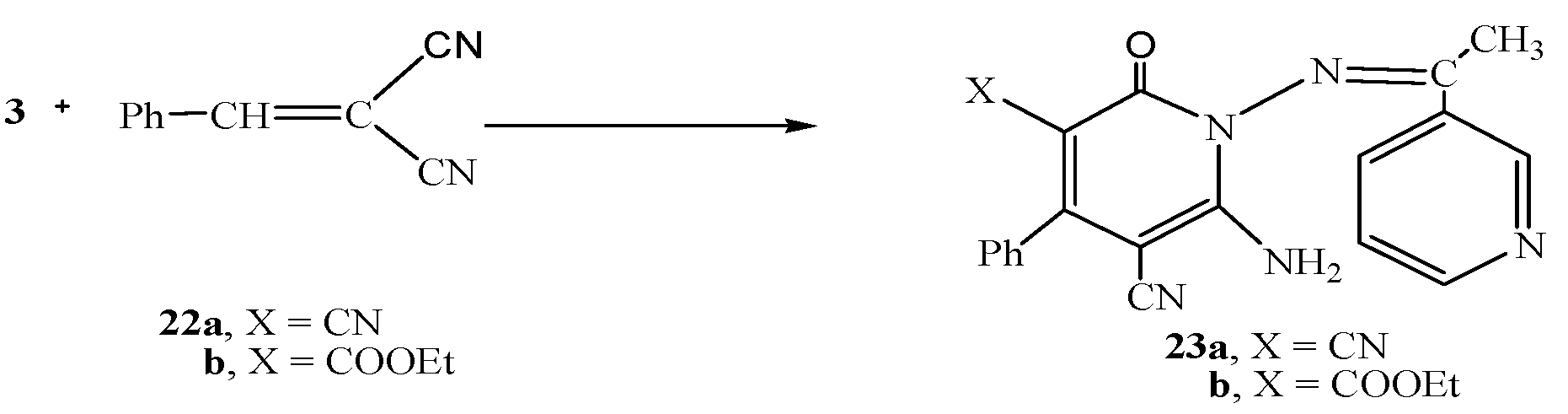
Effect on the Growth of Human Tumor Cell Lines
The effect of compounds 3-25 on the in vitro growth of three human tumor cell lines representing different tumor types, namely, breast adenocarcinoma (MCF-7), non-small cell lung cancer (NCI-H460) and CNS cancer (SF-268) was evaluated after a continuous 48 h exposure. The results are summarized in Table 1. All of the tested compounds were able to inhibit the growth of the tested human tumor cell lines in a dose-dependant manner (data not shown). The results indicated in Table 1 revealed that compound 3 showed the highest inhibitory effect against all the three tumor cell lines. In addition compound 5c showed the best inhibitory effect against CNS cancer (SF-268), while compounds 7 and 23a showed high inhibitory effects against non-small cell lung cancer (NCI-H460) and breast adenocarcinoma (MCF-7), respectively. Compounds 5b, 9a, 23b showed the lowest inhibitory effects. The rest of the compounds showed a moderate growth inhibitory effect. Comparing compound 5b with 5c, it is obvious thatthe presence of the CH3O group in 5c resulted in a higher inhibitory effect than 5b with the Cl group. Comparing the pyridine derivatives 23a (with the cyano group) and 23b (with the carboxyethyl group), the first has a greater inhibitory effect than the second towards the three cell lines.

Table 1.
Effect of the newly synthesized product on the growth of three human tumor cell.
| GI50 (µM) | |||
|---|---|---|---|
| Compound | MCF-7 | NCI-H460 | SF-268 |
| 3 | 0.1 ± 0.009 | 0.2 ± 0.001 | 0.6 ± 0.001 |
| 5a | 20.0 ± 0.2 | 26.6 ± 1.4 | 38.4 ± 0.6 |
| 5b | 60.6 ± 16.9 | 38.9 ± 10.8 | 28.8 ± 8.6 |
| 5c | 2.0 ± 0.2 | 3.0 ± 1.6 | 0.07 ± 0.001 |
| 7 | 66.8 ± 12 | 10 ± 6.2 | 36.8 ± 3.0 |
| 9a | 74.7 ± 17.5 | 48.2 ± 12.8 | 62.0 ± 9.01 |
| 9b | 20 ± 0.4 | 20.3 ± 0.8 | 22.2 ± 0.8 |
| 9c | 28.9 ± 0.9 | 40.6 ± 1.8 | 54.8 ± 0.8 |
| 11 | 30 ± 0.6 | 17.3 ± 1.4 | 22.3 ±1.5 |
| 13 | 36.0 ± 1.8 | 44.0 ± 0.8 | 20.5 ± 1.1 |
| 14 | 50.1 ± 0.7 | 23.2 ± 4.8 | 18.4 ± 1.8 |
| 17 | 22 ± 0.4 | 20.3 ± 0.8 | 30 ± 0.8 |
| 19 | 22.0 ± 0.2 | 24.1 ± 0.8 | 38.4 ± 0.6 |
| 21 | 35.4 ± 10.2 | 24.1 ± 0.8 | 18.9 ± 6.8 |
| 23a | 11.9 ± 0.5 | 14.1 ± 0.6 | 20.3 ± 0.5 |
| 23b | 70.9 ± 0.9 | 40.6 ± 1.8 | 60.8 ± 0.8 |
Results are given in concentrations that were able to cause 50 % of cell growth inhibition (GI50). after a continuous exposure of 48 h and show means ± SEM of three-independent experiments performed in duplicate. Doxorubicin was used as positive control, GI50: MCF-7 = 42.8 ± 8.2 nM; NCI-H460 = 94.0 ± 8.7 nM, and SF-280 = 94.0 ± 7.0 nM.
3. Experimental
3.1. General
Melting points were determined on an Electrothermal melting point apparatus (Electrothermal 9100) and are uncorrected. IR spectra were recorded for KBr discs on a Pye Unicam SP-1000 spectrophotometer. 1H-NMR and 13C-NMR spectra were measured on a Varian EM-390-200 MHz in CD3SOCD3 as solvent using TMS as internal standard, and chemical shifts are expressed as δ. Analytical data were obtained from the Microanalytical Data Unit at Cairo University, Giza, Egypt. Antitumor evaluation for the newly synthesized products were performed by a research group at the National Research Center and the National Cancer Institute at Cairo University.
2-Cyano-N'-(1-(pyridine-3-yl)ethylidene)acetohydrazide (3). To a solution of cyanoacetylhydrazine (2, 0.99 g, 0.01 mol) in 1,4-dioxane (20 mL), 3-acetylpyridine (1.21 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 2 h then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried to give white crystals (from ethanol). Yield: 1.50 g, 74%, m.p. 203-205 °C; IR (KBr) υ/cm−1: 3500-3400 (NH), 3066 (CH-aromatic), 2885 (CH3), 2200 (CN), 1680 (C=O); MS m/z (%) 202 [M+, 8%]; 1H-NMR δ: 2.28 (s, 3H, CH3), 4.26 (s, 2H, CH2), 7.43-8.99 (m, 4H, pyridine-H),10.81(s, 1H, NH); 13C-NMR δ: 14.2 (CH3), 28.9 (CH2), 116.8 (CN), 122.1, 123.4, 133.7, 150.2, 151.0 (pyridine C), 168.1 (C=N), 169.8 (C=O); Anal. Calcd. for C10H10N4O (202.21): C, 59.40; H, 4.98; N, 27.71%. Found: C, 59.72; H, 5.20; N, 28.01%.
3.2. General Procedure for the Synthesis of 5a, 5b or 5c
To a solution of 3 (2.02 g, 0.01 mol) in 1,4-dioxane (20 mL), either benzaldehyde (1.06 g, 0.01 mol), p-chlorobenzaldehyde(1.12 g, 0.01 mol) or p-methoxybenzaldehyde (1.08 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 6 h then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried.
2-Cyano-3-phenyl-N'-(1-(pyridin-3-yl)ethylidene)acrylohydrazide (5a). White crystals (from acetone). Yield: 1.32 g, 45.4%, m.p. 115-118 °C; IR (KBr) υ/cm−1: 3460-3380 (NH), 3050 (CH-aromatic), 2890 (CH3), 2220 (CN), 1683 (C=O), 1635 (C=N); MS m/z (%) 290 [M+, 39.7%];1H-NMR δ: 2.28 (s, 3H, CH3), 3.31 (s, 1H, CH), 7.30-7.91 (m, 9H, C6H5, pyridine-H), 8.71 (s, 1H, NH); 13C-NMR δ: 14.0 (CH3), 98.3 (C=CH), 148.0 (C=CH), 116.8 (CN), 124.3, 125.7, 15.9, 126.2, 128.0, 129.4, 136.5, 150.2, 151.6 (benzene, pyridine C), 165.3 (C=N), 170.0 (C=O); Anal. Calcd. for C17H14N4O (290.32): C, 70.33; H, 4.86; N, 19.30%. Found: C, 70.01; H, 4.92; N, 19.48%.
3-(4-Chlorophenyl)-2-cyano-N'-(1-(pyridin-3-yl)-ethylidene)acrylo-hydrazide (5b). Pale yellow crystals (from acetone). Yield: 1.95 g, 60.1%, m.p. 100 °C; IR (KBr) υ/cm−1: 3460-3380 (NH), 3050 (CH-aromatic), 2890 (CH3), 2200 (CN), 1683 (C=O), 1623 (C=N); MS m/z (%) 324 [M+, 5.17%];1H-NMR δ: 2.27 (s, 3H, CH3), 3.38 (s, 1H, CH), 7.17-7.94 (m, 8H, C6H4, pyridine-H), 10.00 (s, 1H, NH); 13C-NMR δ : 14.1 (CH3), 98.0 (C=CH), 147.8 (C=CH), 116.9 (CN), 122.8, 124.6, 15.9, 126.0, 127.1, 128.9, 136.5, 150.2, 151.6 (benzene, pyridine C), 165.3 (C=N), 170.0 (C=O); Anal. Calcd. for C17H13ClN4O (324.76): C, 62.87; H, 4.03; N, 17.25%. Found: C, 63.01; H, 4.26; N, 17.32%.
2-Cyano-3-(4-methoxyphenyl)-N'-(1-(pyridin-3-yl)ethylidene)acrylo-hydrazide (5c). Yellow crystals (from acetone). Yield: 2.40 g, 75%, m.p. 130-133 °C; IR (KBr) υ/cm−1: 3460-3370 (NH) , 3100 (CH-aromatic), 2890 (CH3), 2200 (CN), 1683 (C=O), 1640 (C=N); MS m/z (%) 320 [M+, 44.28%]; 1H-NMR δ: 2.49 (s, 3H, CH3), 3.31(s, 1H, CH), 3.82 (s, 1H, CH3), 7.03-7.82 (m, 8H, C6H4, pyridine-H), 8.62 (s, 1H, NH); 13C-NMR δ: 14.4, 54.6 (2 CH3), 99.9. (C=CH), 148.3 (C=CH), 116.3 (CN), 122.3, 124.6, 125.3, 125.9, 127.8, 128.4, 135.3, 150.0, 151.9 (benzene, pyridine C), 165.6 (C=N), 168.9 (C=O); Anal. Calcd. for C18H16N4O2 (320.35): C, 67.49; H, 5.03; N, 17.49%. Found: C, 67.55; H, 4.89; N, 17.31%.
2-Oxo-N'-(1-(pyridin-3-yl)ethylidene)-2H-chromene-3-carbohydrazide (7). To a solution of 3 (2.02 g, 0.01 mol) in 1,4-dioxane (20 mL), salicylaldehyde (1.22 g,0.01 mol) was added. The reaction mixture was heated under reflux for 3 hours then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried to give white crystals (from ethanol). Yield: 2.10 g, 68%, m.p. 235-238 °C; IR (KBr) υ/cm−1: 3450-3360 (NH), 3200 (CH-aromatic), 2890 (CH3), 2200 (CN), 1670 (C=O), 1650 (C=N); MS m/z (%) 307 [M+, 2.5%]; 1H-NMR δ2.51(s, 3H, CH3), 7.30 (s, 1H, coumarin H-4), 7.48-9.33 (m, C6H4, pyridine-H), 13.69 (s, 1H, NH); 13C-NMR δ: 13.8 (CH3), 121.8, 122.6, 122.9, 124.5, 127.1, 128.0, 130.9, 131.6, 150.2, 152.3 (C6H4, pyridine C), 159.9 (coumarin C=O), 167.9 (C=N), 169.8 (C=O); Anal. Calcd. for C17H13N3O3 (307.30): C, 66.44; H, 4.26; N, 13.67%. Found: C, 66.38; H, 4.35; N, 13.92%.
3.3. General Procedure for the Synthesis of 9a, 9b, 9c or 9d
To a cold solution of 3 (2.02 g, 0.01 mol) in ethanol (20 mL) containing sodium acetate (3.0 g) was added with continuous stirring either of the appropriate substituted benzenediazonium salt (0.01 mol) [prepared by adding sodium nitrite (1.6 g, 0.02 mol) in water (8 mL) to a cold solution of either of the appropriate substitute aniline 8a-d in the appropriate amount of hydrochloric acid]. The reaction mixture was stirred for 2 h and the formed solid product, in each case, was collected by filtration.
2-Cyano-2-(2-phenylhydrazinylidene)-N'-[1-(pyridin-4-yl)ethylidene]aceto-hydrazide (9a). Orange crystals (from ethanol). Yield: 1.92 g, 63%, m.p. 158-161 °C; IR (KBr) υ/cm−1: 3500-3400 (NH), 3050 (CH-aromatic), 2800 (CH3), 2200 (CN), 1696 (C=O); MS m/z (%) 306 [M+, 93.51%]; 1H-NMR δ2.49 (s, 3H, CH3), 7.11-8.22 (C6H5, pyridine H), 10.25, 12.02 (2s, 2H, 2NH); 13C-NMR δ: 14.1 (CH3), 116.0 (CN), 115.9, 116.8, 118.6, 122.3, 123.9, 129.0, 142.8, 150.2, 152.8 (C6H5, pyridine C), 163.2, 166.7 (2 C=N), 168.8 (C=O); Anal. Calcd. for C16H14N6O (306.32): C, 62.74; H, 4.61; N, 27.44%. Found: C, 62.90; H, 4.51; N, 27.52%.
2-[2-(4-Chlorophenyl)hydrazinylidene]-2-cyano-N'-[1-(pyridin-4-yl)ethylidene] acetohydrazide (9b). Orange crystals (from ethanol). Yield: 2.32 g, 68%, m.p. 174-177 °C; IR (KBr) υ/cm−1: 3600-3500 (NH), 3100 (CH-aromatic), 2890 (CH3), 2200 (CN), 1696 (C=O); MS m/z (%) 340 [M+, 6.17%]; 1H-NMR δ2.49 (s, 3H, CH3), 6.95-7.89 (C6H4-pyridine H), 11.737, 12.75 (2s, 2H, 2NH); 13C-NMR δ: 14.0 (CH3), 115.8 (CN), 116.0, 117.3, 118.9, 121.8, 124.5, 125.4, 143.5, 150.0, 151.8 (C6H5, pyridine C), 163.0, 166.9 (2 C=N), 168.9 (C=O); Anal. Calcd. for C16H13ClN6O (340.77): C, 56.39; H, 3.85; N, 24.66%. Found: C, 56.60; H, 4.01; N, 24.90%.
2-[2-(4-Bromophenyl)hydrazinylidene]-2-cyano-N'-[1-(pyridin-4-yl)ethylidene] acetohydrazide (9c). Orange crystals (from ethanol). Yield: 2.62 g, 68%, m.p. 183-186 °C; IR (KBr) υ/cm−1: 3550-3400 (NH), 3090 (CH-aromatic), 2890 (CH3), 2200 (CN), 1685(C=O); MS m/z (%) 387 [M+, 19 %]; 1H-NMR δ 2.49 (s, 3H, CH3), 6.93-7.84 (C6H4, pyridine H), 9.50, 11.75 (2s, 2H, 2NH); 13C-NMR δ: 14.2 (CH3), 116.6 (CN), 116.3, 118.0, 118.6, 122.8, 124.5, 128.0, 138.9, 150.2, 152.5 (C6H5, pyridine C), 163.0, 166.5 (2 C=N), 168.5 (C=O); Anal. Calcd. for C16H13BrN6O (384): C, 49.89; H, 3.40; N, 21.82%. Found: C, 48.93; H, 3.62; N, 22.02%.
2-Cyano-2-[2-(4-nitrophenyl)hydrazinylidene]-N'-[1-(pyridin-4-yl)ethylidene] acetohydrazide (9d). Orange crystals (from ethanol). Yield: 2.43 g, 69%, m.p. 162-165 °C; IR (KBr) υ/cm−1: 3570-3400 (NH), 3100 (CH-aromatic), 2890 (CH3), 2200 (CN), 1670 (C=O); MS m/z (%) 352 [M+, 20.2 %]; 1H-NMR δ 2.49 (s, 3H, CH3), 6.57-8.39 (C6H4, pyridine H), 10.48, 12.11 (2s, 2H, 2NH); 13C-NMR δ: 14.1 (CH3), 116.0 (CN), 116.8, 116.9, 118.2, 123.8, 126.3, 128.6, 136.1, 151.0, 152.7 (C6H5, pyridine C), 163.1, 166.4 (2 C=N), 168.9 (C=O); Anal. Calcd. for C16H13N7O3 (351.32): C, 54.70; H, 3.73; N, 27.91%. Found: C, 54.85; H, 3.90; N, 29.11%.
2-Amino-4,5,6,7-tetrahydro-N'-(1-(pyridin-3-yl)ethylidene)benzo[b]thiophene-3-carbohydrazide (11). Method A: To a solution of 3 (2.02 g, 0.01 mol) in ethanol (40 mL) containing triethylamine (1 mL) and elemental sulfur (0.32 g, 0.01mol), cyclohexanone 10 (0.98 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 3 hours then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried obtaining pale yellow crystals (from ethanol).
Method B: To a solution of compound 14 (2.82 g, 0.01 mol) in 1,4-dioxane (40 mL) containing triethylamine (0.5 mL), elemental sulfur (0.32 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 2 h then poured onto ice/water containing few drops of hydrochloric acid. The formed solid product was collected by filtration.
Yield: 1.95g, 62% (method A) and 2.20 g, 70% (method B), m.p. 112 °C; IR (KBr) υ/cm−1: 3400-3300 (NH2, NH), 3068 (CH-aromatic), 2886 (CH3), 2250 (CN), 1690 (C=O), 1638 (C=C); MS m/z (%) 314 [M+, 2.19 %]; 1H-NMR δ2.29-2.31 (m, 8H, 4CH2), 2.49 (s, 3H, CH3), 4.25 (s, 2H, NH2), 7.41-8.88 (m, 4H, pyridine-H), 10.76 (s, 1H, NH);13C-NMR δ: 13.8 (CH3), 22.8, 23.6, 24.1, 24.9 (4 CH2), 116.5, 122.6, 124.8, 130.2, 150.6, 151.8 (thiophene, pyridine C), 163.6 (C=O), 170.0 (C=N); Anal. Calcd. for C16H18N4OS (314.41): C, 61.12; H, 5.77; N, 17.82%. Found: C, 60.91; H, 6.01; N, 17.85%.
2-Amino-5,6-dihydro-N'-(1-(pyridin-3-yl)ethylidene)-4H-cyclopenta[b]thiophene-3-carbohydrazide (13). To a solution of 3 (2.02 g, 0.01 mol) in ethanol (40 mL) containing triethylamine (1.0 mL) and elemental sulfur (0.32 g, 0.01 mol), cyclopentanone 12 (0.98 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 3 h then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried obtaining pale yellow crystals (from ethanol).Yield: 1.82 g, 61%, m.p. 140-144 °C; IR (KBr) υ/cm−1: 3450-3300 (NH2, NH), 3080 (CH-aromatic), 2890 (CH3), 2250 (CN), 1690 (C=O); MS m/z (%) 300 [M+, 0.34 %]; 1H-NMR δ2.28-2.35 (m, 6H, 3CH2), 2.48 (s, 3H, CH3), 4.25 (s, 2H, NH2), 7.41-8.99 (m, 4H, pyridine-H),10.78 (s, 1H, NH); 13C-NMR δ: 14.0 (CH3), 20.8, 24.6, 26.9 (3 CH2), 116.9, 123.0, 124.6, 133.1, 150.1, 151.4 (thiophene, pyridine C), 163.4 (C=O), 169.8 (C=N); Anal. Calcd. for C15H16N4OS (300.3): C, 59.98; H, 5.37; N, 18.65%. Found: C, 59.93; H, 5.39; N, 18.81%
2-Cyano-2-cyclohexylidene-N'-(1-(pyridin-3-yl)ethylidene)acetohydrazide (14). Equimolar amounts of compound 3 (2.02 g, 0.01 mol) and cyclohexanone 10 (0.98 g, 0.01 mol) were heated in an oil bath at 140 °C for 1 h in presence of ammonium acetate. After cooling the reaction mixture, it was heated in ethanol, then poured into ice/water mixture and the formed solid product was collected by filtration and dried to give pale yellow crystals (from ethanol). Yield: 1.52 g, 54% , m.p. 145-146 °C; IR (KBr) υ/cm−1: 3355-3370 (NH), 3067 (CH-aromatic), 2930 (CH3), 2200 (CN), 1675 (C=O); MS m/z (%) 282 [M+, 0.40 %]; 1H-NMR δ 2.26-2.34 (m, 10H, 5CH2), 2.31(s, 3H, CH3), 7.41-8.99 (m, 4H, pyridine-H), 10.90 (s, 1H, NH); 13C-NMR δ: 13.9 (CH3), 26.8, 27.4, 26.9, 27.0 (cyclohexane C), 93.0 (C=C), 116.8 (CN), 123.4, 126.8, 126.4, 150.5, 151.2 (pyridine C), 168.2 (C=N), 177.3 (C=O); Anal. Calcd. for C16H18N4O (282.34): C, 68.06; H, 6.43; N, 19.84%. Found: C, 67.85; H, 6.31; N, 20.22%
3.4. General Procedure for the Synthesis of 18 and 20
Compound 3 (2.02 g, 0.01 mol) is dissolved in ethanol and a few sodium hydroxide pellets were added. Phenylisothiocyanate (15, 1.35, 0.01 mol) is then added and the solution is covered and left standing overnight. Equimolar amounts of either ethyl 2-bromoacetate (17) or ethyl 2-bromo-2-cyanoacetate (19) are stirred in the following day, and the solution is covered for another night, after which the reaction mixture is poured onto ice and the precipitated solid is filtered off.
2-(4-Hydroxy-3-phenylthiazol-2(3H)-ylidene)-2-isocyano-N'-(1-(pyridin-3-yl)ethylidene) acetohydrazide (18). Orange crystals (from ethanol). Yield: 2.43 g, 64%, m.p. 125-127 °C; IR (KBr) υ/cm−1: 3500-3370 (NH), 3400 (OH), 3100 (CH-aromatic), 2900 (CH3), 2189 (CN), 1675 (C=O); MS m/z (%) 377 [M+, 7.9 %]; 1H-NMR δ 2.22 (s, 3H, CH3), 7.46 (thiazole H-5),7.54-8.95 (C6H5-pyridine H), 8.80 (NH), 10.13 (OH); 13C-NMR δ: 14.3 (CH3), 93.0, 101.2 (C=C), 115.8 (CN), 119.2, 120.5, 121.8, 122.3, 124.0, 124.8, 127.6, 130.5, 133.2, 137.0, 150.7, 152.2 (C6H5, thiazole, pyridine C), 168.4 (C=N), 170.0 (C=O); Anal. Cald. for C19H15N5O2S (377.42): C, 60.46; H, 4.01; N, 18.56; S, 8.50. Found: C, 60.50; H, 4.01; N, 18.55; S, 8.48%.
(2Z)-Ethyl-2-((1-(pyridin-3-yl)ethylideneaminocarbamoyl)(cyano)methylene)-4-cyano-3-phenylthiazolidine-4-carboxylate (20). Orange crystals (from ethanol).Yield: 3.22 g, 70%, m.p. 125-127 °C; IR (KBr) υ/cm−1: 3577-3370 (NH), 3067 (CH-aromatic), 2930 (CH3), 2200 (CN), 1675 (C=O); MS m/z (%) 461.1 [M+, 25.2 %]; 1H-NMR δ 1.56 (t, 3H, J = 7.02 Hz, CH3), 2.26 (s, 3H, CH3), 4.25 (q, 2H, J = 7.02 Hz, CH2), 6.47 (s, 2H, thiazole, CH2), 7.08-8.12 (m, 9H, C6H5, pyridine-H), 10.72 (s, 1H, NH); 13C-NMR δ : 13.9, 14.5 (2 CH3), 40.2 (thiazole CH2), 58.9 (ester CH2), 93.3, 101.6 (C=C), 116.0, 116.7 (2 CN), 119.2, 120.3, 121.2, 121.8, 122.0, 124.7, 125.3, 133.5, 133.9, 150.4, 152.1 (C6H5, thiazole, pyridine C), 160.2, 164.5 (2 C=O), 168.9 (C=N); Anal. Calcd. for C23H20N6O3S (460.51): C, 59.99; H, 4.38; N, 18.25; S, 6.96%. Found: C, 60.11; H, 4.42; N, 18.13; S, 7.26%.
4-Amino-2,3-dihydro-3-phenyl-N'-(1-(pyridin-3-yl)ethylidene)-2-thioxothiazole-5-carbohydrazide (21). To a solution of 3 (2.02 g, 0.01 mol) in ethanol (40 mL) containing triethylamine (1.0 mL) and elemental sulfur (0.32 g, 0.01 mol), phenylisothiocyanate (15, 1.35 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 3 h then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried obtaining yellow crystals (from ethanol). Yield: 2.46 g, 67% ,m.p. 164-167 °C; IR (KBr) υ/cm−1 : 3465-3300 (NH2, NH), 3166 (CH-aromatic), 2980 (CH3), 1680 (C=O), 1658 (C=N), 1466 (C=C), 1241(C=S); MS m/z (%) 369.9 [M+, 13.27%]; 1H-NMR δ 2.33 (s, 3H, CH3), 3.31(s, 2H, NH2),7.28-7.38 (m, C6H4-pyridine H), 10.67 (s, 1H, NH); 13C-NMR δ: 14.2 (CH3), 120.3, 122.5, 124.1, 127.9, 128.3, 130.1, 133.4, 138.9, 150.0, 152.3 (C6H5, thiazole, pyridine C), 168.2 (C=N), 170.2 (C=O), 180.3 (C=S); Anal. Calcd. for C17H15N5OS2 (369.46): C, 55.26; H, 4.09; N, 18.96; S, 17.36 %. Found: C, 55.40; H, 4.31; N, 19.15; S, 17.60%.
3.5. General Procedure for the Synthesis of 23a or 23b
To a solution of 3 (2.02 g, 0.01 mol) in 1,4-dioxane (20 mL) either 2-benzylidenemalononitrile (22a, 1.54 g, 0.01 mol) or ethyl 2-cyano-3-phenylacrylate (22b, 2.01 g, 0.01 mol) was added. The reaction mixture was heated under reflux for 3 h then poured onto a beaker containing an ice/water mixture. The formed solid product was collected by filtration and dried.
1-(1-Phenylethylideneamino)-6-amino-1,2-dihydro-2-hydroxy-4-phenylpyridine-3,5-dicarbonitrile (23a). White crystals (from ethanol). Yield: 2.40 g, 68%, m.p. >300 °C; IR (KBr) υ/cm−1: 3458-3328 (NH2, NH), 3215 (CH-aromatic), 2890 (CH3), 2192, 2225 (2 CN), 1688 (C=O), 1640 (C=N); MS m/z (%) 355 [M+, 4.9 %]; 1H-NMR δ 2.301 (s, 3H, CH3), 3.56 (s, 2H, NH2), 7.46-9.22 (m, C6H5, pyridine H); 13C-NMR δ: 18.9 (CH3), 116.9, 118.0 (2 CN), 110.2, 118.9, 120.6, 128.4, 137.2, 150.2, 152.4 (two pyridine C), 168.9 (C=N), 172.3 (C=O); Anal. Calcd. for C20H14N6O (354.36): C, 67.79; H, 3.98; N, 23.72%. Found: C, 67.80; H, 4.00; N, 23.71%.
Ethyl-1-(1-phenylethylideneamino)-6-amino-5-cyano-1,2-dihydro-2-hydroxy-4-phenylpyridine-3-carboxylate (23b). White crystals (from ethanol).Yield: 1.96 g, 49%, m.p. 255-259 °C; IR (KBr) υ/cm−1: 3464-3339 (NH2), 3200 (CH-aromatic), 2890 (CH3), 2180 (CN), 1680 (C=O), 1640 (C=N); MS m/z (%) 401 [M+, 2.8 %]; 1H-NMR δ: 1.65 (t, 3H, J = 6.83 Hz, CH3), 2.31 (s, 3H, CH3), 3.56 (s, 2H, NH2), 4.18 (q, 2H, J = 6.83 Hz, CH2), 7.27-8.79 (m, C6H5, pyridine H); 13C-NMR δ: 13.7, 19.0 (2 CH3), 59.8 (CH2), 116.9 (CN), 108.0, 117.4, 120.3, 124.6, 125.1, 127.0, 132.0, 138.3, 150.2, 152.4 (C6H5, two pyridine C), 167.3 (C=N), 170.1, 172.6 (2C=O); Anal. Calcd. for C22H19N5O3 (401.42): C, 65.83; H, 4.77; N, 17.45%. Found: C, 66.04; H, 4.83; N, 17.61%.
4. Conclusions
In this work, cyanoacetylhydrazine (1) reacted with 3-acetylpyridine (2) to afford the hydrazide-hydrazone derivative 3. The latter was reacted with different reagents to give coumarin, pyridine, thiazole and thiophene derivatives. The antitumor evaluations of the newly synthesized products were carried out, showing that both the hydrazide-hydrazone derivative 3 and the benzylidene derivative 5c have the highest inhibitory effects.
Acknowledgements
R. M. Mohareb would like to express his deepest thanks to the Alexander Von Humboldt Foundation in Bonn for affording him a fellowship during Summer 2009. Financial support from the American University in Cairo is greatly acknowledged.
References and Notes
- Rallas, S.; Gulerman, N.; Erdeniz, H. Synthesis and antimicrobial activity of some new hydrazones of 4-fluorobenzoic acid hydrazide and 3-acetyl-2,5- disubstituted-1,3,4-oxadiazolines. Farmaco 2002, 57, 171–174. [Google Scholar] [CrossRef]
- Gursoy, A.; Terzioglu, N.; Otuk, G. Synthesis of some new hydrazide-hydrazones, thiosemicarbazides and thiazolidinones as possible antimicrobials. Eur. J. Med. Chem. 1997, 32, 753–757. [Google Scholar] [CrossRef]
- Vicini, P.; Zani, F.; Cozzini, P.; Doytchinova, I. Hydrazones of 1,2-benzisothiazole hydrazides: synthesis, antimicrobial activity and QSAR investigations. Eur. J. Med. Chem. 2002, 37, 553–564. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Falagiani, V.; Zampieri, D.; Vio, L.; Banfo, E. Synthesis and antimycobacterial activity of [5-(pyridin-2-yl)-1,3,4-thiadiazol-2-ylthio]acetic acid arylidene-hydrazide derivatives. IIFARMACO 2001, 56, 587–592. [Google Scholar]
- Rahman, V.M.; Mukhtar, S.; Ansari, W.H.; Lemiere, G. Synthesis, stereochemistry and biological activity of some novel long alkyl chain substituted thiazolidin-4-ones and thiazan-4-one from 10-undecenoic acid hydrazide. Eur. J. Med. Chem. 2005, 40, 173–184. [Google Scholar] [CrossRef]
- Dimmock, J.R.; Vashishtha, S.C.; Stables, J.P. Anticonvulsant properties of various acetylhydrazones, oxamoylhydrazones and semicarbazones derived from aromatic and unsaturated carbonyl compounds. Eur. J. Med. Chem. 2000, 35, 241–248. [Google Scholar] [CrossRef]
- Yapia, R.; La Mara, M.P.; Massieu, G.H. Modifications of brain glutamate decarboxylase activity by pyridoxal phosphate-β-glutamyl hydrazone. Biochem. Pharmacol. 1967, 16, 1211–1218. [Google Scholar]
- Sava, G.; Perissin, L.; Lassiani, L.; Zabucchi, G. Antiinflammatory action of hydrosoluble dimethyl-triazenes on the carrageen induced edema in guinea pigs. Chem. Biol. Interact. 1985, 53, 37–43. [Google Scholar] [CrossRef]
- Xia, Y.L; Chuan-Dong, F.; Zhao, B.X.; Zhao, J.; Shin, D.S.; Miaom, J.Y. Synthesis and structure-activity relationships of novel 1-arylmethyl-3-aryl-1H- pyrazole-5-carbohydrazide hydrazone derivatives as potential agents A549 lung cancer cells. Eur. J. Med. Chem. 2008, 43, 2347–2353. [Google Scholar]
- Melnyk, P.; Leroux, V.; Serghergert, C.; Grellier, P. Design, synthesis and in vitro antimalarial activity of an acylhydrazone library. Bioorg. Med. Chem. Lett. 2006, 16, 31–35. [Google Scholar] [CrossRef]
- Ajani, O.O.; Obafemi, C.A.; Nwinyi, O.C.; Akinpelu, D.A. Microwave assisted synthesis and antimicrobial activity of 2- quinoxalinone-3-hydrazone derivatives. Bioorg. Med. Chem. 2010, 18, 214–221. [Google Scholar] [CrossRef]
- Zheng, L.W.; Wu, L.L.; Zhao, B.X.; Dong, W.L.; Miao, Y.J. Synthesis of novel substituted pyrazole-5-carbohydrazide hydrazone derivatives and discovery of a potent apoptosis inducer in A549 lung cancer cells. Bioorg. Med. Chem. 2009, 17, 1957–1962. [Google Scholar] [CrossRef]
- Bhagavan, N.V. Medical Biochemistry; Elsevier Science B.V.: Amsterdam, The Netherlands, 2002; Volume 17, pp. 331–363. [Google Scholar]
- Saulnier, M.G.; Velaprthi, U.; Zimmermann, K. Progress In Heterocyclic Synthesis; Gribble, G., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2005; Volume 16, pp. 228–271. [Google Scholar]
- Short, E.I. Studies on the inactivation of isonicotinyl acid hydrazide in normal subjects and tuberculous patients. Tubercle 1962, 43, 33–42. [Google Scholar] [CrossRef]
- Holdiness, M.R. A review of blood dyecrasias induced by the antituberculosis drugs. Tubercle 1987, 68, 301–309. [Google Scholar] [CrossRef]
- Faroumadi, A.; Kiano, Z.; Soltani, F. Antituberculosis agents VIII: Synthesis and in vitro antimycobacterial activity of alkyl a-[5-(5-nitro-2-thienyl)-1,3,4- thiadiazole-2-ylthio]acetates. Farmaco 2003, 58, 1073–1076. [Google Scholar] [CrossRef]
- Bokharev, V.V.; Ghidaspov, A.A.; Peresedova, E.V. Reaction of potassium salts of 2- amino-4-methoxy-6-dinitro-methyl-1,3,5-triazines with N2O4. Chem. Heterocycl. Comp. 2006, 42, 1096–1106. [Google Scholar]
- Fernando, R.P.; Maia, P.I.; Leite, S.R.; Deflon, V.M.; Batista, A.A.; Sato, D.N.; Franzblau, S.G.; Leite, C.Q. Thiosemicarbazones, semicarbazones, dithiocarbazates and hydrazide/hydrazone: Anti – Mycobacterium tuberculosis activity and cytotoxicity. Eur. J. Med. Chem. 2010, 45, 1898–1905. [Google Scholar]
- Brzozowski, Z; Czewski, F.S. Synthesis and antitumor activity of novel 2- amino-4-(3,5,5-trimethyl-2-pyrazolino)-1,3,5-triazine derivatives. Eur. J. Med. Chem. 2002, 37, 709–720. [Google Scholar] [CrossRef]
- Sherif, A., Rostom. Polysubstituted pyrazoles, part 6. Synthesis of some 1-(4-chlorophenyl)-4-hydroxy-1H-pyrazol-3-carbonyl derivatives linked to nitrogenous heterocyclic ring systems as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 2767–2776. [Google Scholar] [CrossRef]
- Mohareb, R.M.; Mohamed, A.A. The reaction of cyanoacetylhydrazine with w-bromo (4-methyl)acetophenone: Synthesis of heterocyclic derivatives with antitumor activity. Molecules 2010, 15, 3602–3617. [Google Scholar] [CrossRef]
- Mohareb, R.M.; El-Arab, E.E.; El-Sharkaway, K.A. The reaction of cyanoacetic acid Hydrazide with 2-acetylfuran: synthesis of coumarin, pyridine, thiophene and thiazole derivative. Sci. Pharm. 2009, 77, 355–365. [Google Scholar] [CrossRef]
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
