Helichrysum gymnocephalum Essential Oil: Chemical Composition and Cytotoxic, Antimalarial and Antioxidant Activities, Attribution of the Activity Origin by Correlations
Abstract
:1. Introduction
2. Results and discussion
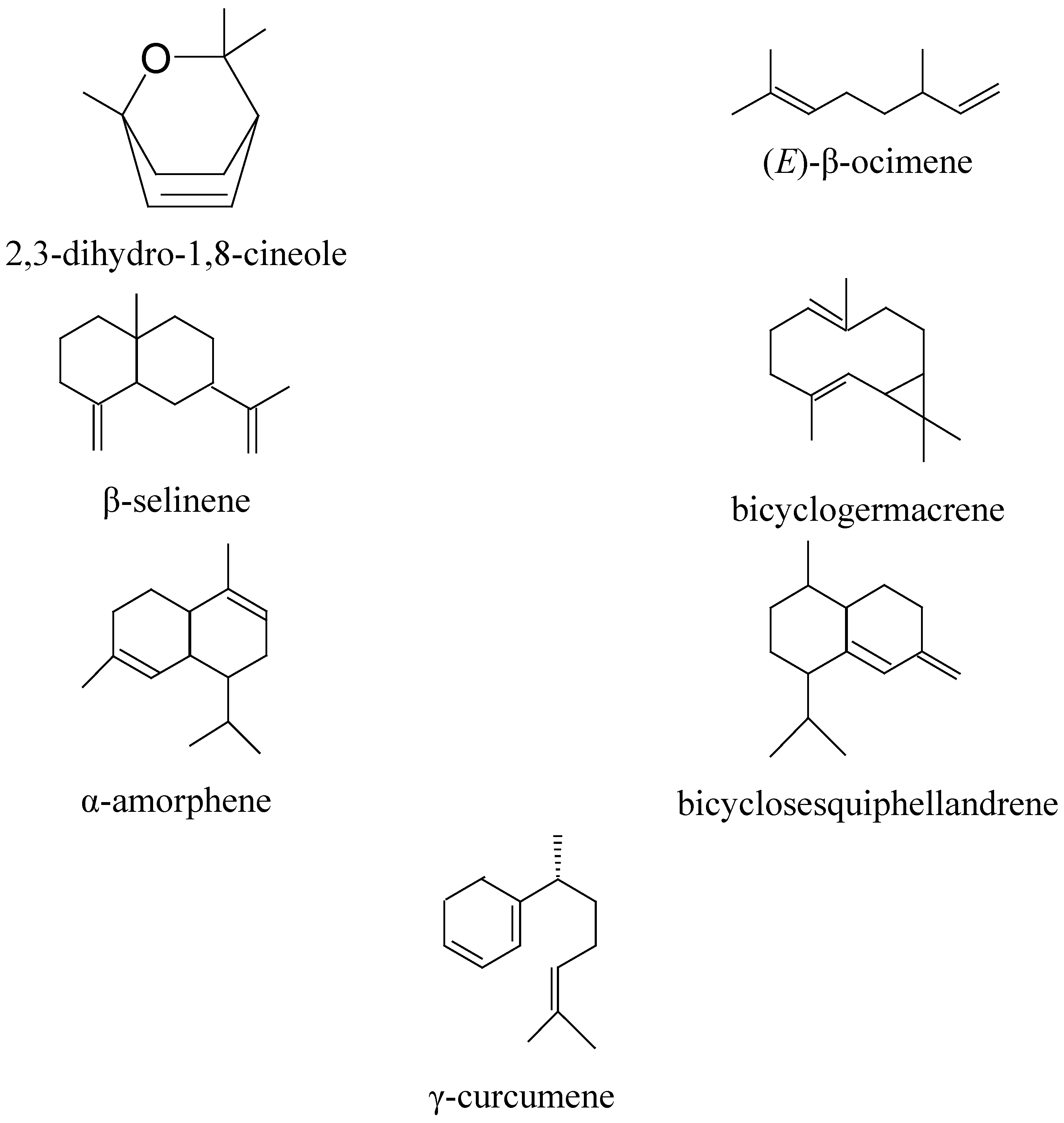
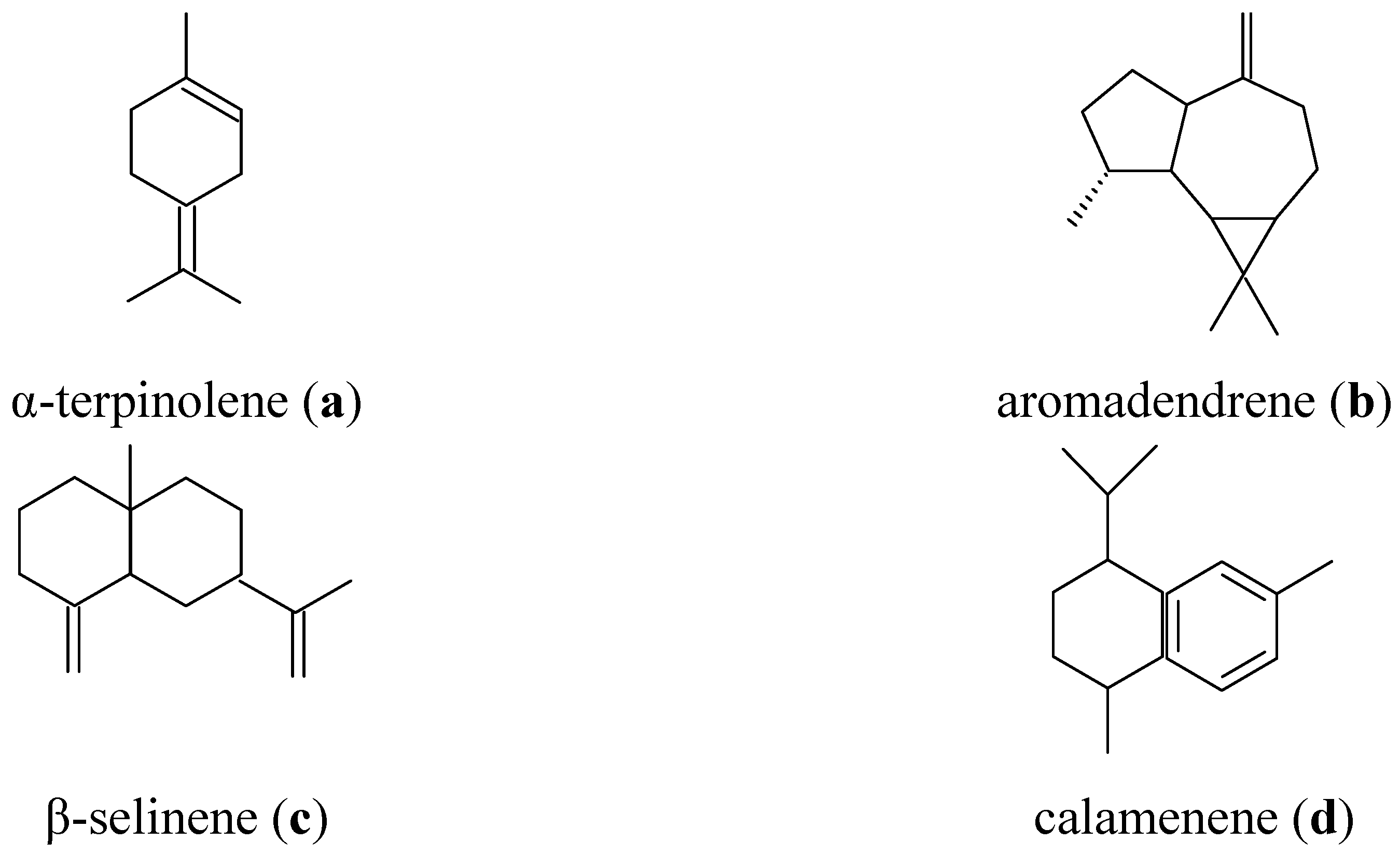
2.1. Chemical Composition

| N | RI | Compounds | (%) |
|---|---|---|---|
| 1 | 928 | α-Thujene | 1.0 |
| 2 | 967 | Sabinene | 0.3 |
| 3 | 971 | β-Pinene | 1.1 |
| 4 | 984 | 2,3-Dihydro-1,8-cineole | 2.1 |
| 5 | 1010 | α-Terpinene | 1.3 |
| 6 | 1018 | p-Cymene | 4.3 |
| 7 | 1022 | Limonene | 0.5 |
| 8 | 1026 | 1,8-Cineole | 47.4 |
| 9 | 1052 | (E)-β-Ocimene | 2.4 |
| 10 | 1084 | α-Terpinolene | 1.3 |
| 11 | 1164 | α-Phellandrene | 0.2 |
| 12 | 1175 | Terpinen-4-ol | 2.7 |
| 13 | 1187 | α-Terpineol | 1.8 |
| 14 | 1373 | α-Copaene | 0.4 |
| 15 | 1438 | Aromadendrene | 2.0 |
| 16 | 1470 | Bicyclosesquiphellandrene | 5.6 |
| 17 | 1473 | γ-Curcumene | 5.6 |
| 18 | 1485 | β-Selinene | 3.3 |
| 19 | 1494 | Bicyclogermacrene | 5.0 |
| 20 | 1497 | α-Amorphene | 5.1 |
| 21 | 1502 | 2,3-di-tert-butylphenol | 0.5 |
| 22 | 1512 | Calamenene | 1.8 |
| 23 | 1521 | δ-Cadinene | 3.6 |
| Identified components | 99.3 | ||
| Monoterpene hydrocarbons | 8.1 | ||
| Monoterpenes oxygenated | 54.0 | ||
| Sesquiterpenes hydrocarbons | 32.4 | ||
| Others | 4.8 | ||
2.2. Antioxidant Activity
| Sample | Anticanceractivity | Antiplasmodial activity | Antioxidant activity (DPPH assay) | Antioxidant activity (ABTS assay) |
|---|---|---|---|---|
| Essential oil | 16 ± 2 | 25 ± 1 | >10000 | 1487.67±47.70 |
| Control | 0.218 a ± 0.04 | 0.10 b ± 0.09 | 3.75 c ± 0.01 | 1.84 c ± 0.03 |
2.3. Cytotoxic Activity
2.4. Antimalarial Activity
| Essential oil | I | II | III | IV | V | VI | VII | VIII | IX |
| Anticancer activity (IC50 mg/L) | 16 ± 2 | 7.3 | 101.7 ± 7.9 | 130 ± 52.2 | 30.1 ± 1.14 | 174.3 ± 73.04 | 150 * | 54 ± 10 | 47 ± 9 |
| Component | |||||||||
| α-Thujene | 1 | 0.62 | 0.47 | 0.54 | 0.38 | ||||
| Sabinene | 0.3 | 6.92 | 0.35 | 5.56 | 0.24 | 0.02 | 0.02 | ||
| β-Pinene | 1 | 22.24 | 4.55 | 0.6 | 1.3 | 8.89 | 4.96 | 3.09 | |
| 2,3-Dihydro-1,8-cineole | 2 | ||||||||
| α-Terpinene | 1.2 | 0.7 | 0.37 | 4.46 | 0.44 | ||||
| p-Cymene | 4.2 | 0.74 | 30.22 | 3.83 | 0.34 | 1.5 | 2.49 | 7.34 | |
| Limonene | 0.5 | 3.61 | 2.1 | 1.68 | 0.9 | ||||
| 1,8-Cineole | 47.4 | 40.91 | 0.27 | 45.16 | 0.4 | ||||
| (E)-β-Ocimene | 2.4 | ||||||||
| α-Terpinolene | 1.3 | 0.35 | 0.43 | ||||||
| α-Phellandrene | 0.2 | 46.52 | 34.38 | ||||||
| Terpinen-4-ol | 2.7 | 1.55 | 1.09 | 0.07 | 0.03 | ||||
| α-Terpineol | 1.8 | 0.62 | 3.6 | 8.38 | 5.6 | ||||
| α-Copaene | 0.4 | 0.6 | 0.11 | 0.19 | |||||
| Aromadendrene | 2 | 0.49 | 0.19 | ||||||
| Bicyclosesquiphellandrene | 5.6 | ||||||||
| γ-Curcumene | 5.6 | ||||||||
| β-Selinene | 3.3 | 0.3 | 1.1 | ||||||
| Bicyclogermacrene | 5 | 1.05 | |||||||
| α-Amorphene | 5.1 | ||||||||
| 2,3-Di-tert-butylphenol | 0.5 | ||||||||
| Calamenene | 1.8 | ||||||||
| δ-Cadinene | 3.6 | 2.8 | 0.27 | 0.69 | |||||
| Essential oil | X | XI | XII | XIII | XIV | XV | |||
| [37] | |||||||||
| Anticancer activity (IC50 mg/L) | 554.4 ± 1.5 | 310 * | 10 | 1 | 0.5 | 14.1 | |||
| Component | |||||||||
| α-Thujene | 0.31 | ||||||||
| Sabinene | 0.41 | 0.1 | |||||||
| β-Pinene | 2.57 | 0.91 | 16.3 | 3.7 | |||||
| 2,3-Dihydro-1,8-cineole | |||||||||
| α-Terpinene | 0.2 | 5.76 | 0.1 | ||||||
| p-Cymene | |||||||||
| Limonene | 1.7 | 98.4 | 56.6 | 98.4 | 0.8 | ||||
| 1,8-Cineole | 17.52 | 19.29 | |||||||
| (E)-β-Ocimene | 0.1 | ||||||||
| α-Terpinolene | |||||||||
| α-Phellandrene | |||||||||
| Terpinen-4-ol | 1.01 | 42.62 | |||||||
| α-Terpineol | 0.27 | 11.3 | |||||||
| α-Copaene | 0.1 | ||||||||
| Aromadendrene | |||||||||
| Bicyclosesquiphellandrene | |||||||||
| γ-Curcumene | |||||||||
| β-Selinene | |||||||||
| Bicyclogermacrene | 2.1 | ||||||||
| α-Amorphene | |||||||||
| 2,3-Di-tert-butylphenol | |||||||||
| Calamenene | |||||||||
| δ-Cadinene | 3.3 | ||||||||
| Essential oil | I | II | III | IV | V | VI | VII | VIII | IX | X | XI |
| Antipalaudic activity (IC50 mg/L) | 25 ± 1 | 17.9 | 16.6 | 29.4 | 17.8 | 2.0 | 4.38 ± 1.07 | 1.23 ± 0.31 | 1.68 ± 0.26 | 6.4 ± 2.0 | 4.8 ± 0.7 |
| Component | |||||||||||
| α-Thujene | 1.0 | 0.59 | 0.61 | ||||||||
| Sabinene | 0.3 | 0.59 | 0.46 | 0.1 | 0.2 | 0.1 | |||||
| β-Pinene | 1.0 | 0.68 | 10.07 | 0.12 | 0.7 | 0.8 | 3.0 | ||||
| 2,3-Dihydro-1,8-cineole | 2.0 | ||||||||||
| α-Terpinene | 1.2 | 0.48 | 0.43 | 0.3 | 0.2 | ||||||
| p-Cymene | 4.2 | 0.35 | 0.32 | 1.72 | 0.2 | 0.7 | 2.5 | 0.2 | |||
| Limonene | 0.5 | 5.3 | 0.6 | 9.8 | 9.4 | 0.6 | |||||
| 1,8-Cineole | 47.4 | 2.0 | 9.4 | ||||||||
| (E)-β-Ocimene | 2.4 | 1.93 | 1.13 | 0.8 | 1.5 | ||||||
| α-Terpinolene | 1.3 | ||||||||||
| α-Phellandrene | 0.2 | ||||||||||
| Terpinen-4-ol | 2.7 | 0.16 | 0.49 | 0.16 | 0.8 | ||||||
| α-Terpineol | 1.8 | 4.99 | 2.7 | 6.2 | |||||||
| α-Copaene | 0.4 | 0.53 | 7.06 | 2.2 | 4.07 | 13.27 | 0.1 | ||||
| Aromadendrene | 2.0 | 1.08 | 2 | ||||||||
| Bicyclosesquiphellandrene | 5.6 | ||||||||||
| γ-Curcumene | 5.6 | ||||||||||
| β-Selinene | 3.3 | 0.28 | 2.2 | ||||||||
| Bicyclogermacrene | 5.0 | ||||||||||
| α-Amorphene | 5.1 | ||||||||||
| 2,3-Di-tert-butylphenol | 0.5 | ||||||||||
| Calamenene | 1.8 | 2.32 | 0.93 | 1.09 | |||||||
| δ-Cadinene | 3.6 | 15.11 | 8.06 | 1.3 | 4.3 | 10.07 | 0.5 | 0.3 | |||
| Essential oil | XII | XIII | XIV | XV | XVI | XVII | XVIII | ||||
| Antipalaudic activity (IC50 mg/L) | 2–4 | 1.25 ± 0.77 | 5.2 ± 0.77 | na | na | na | 40.15 * | 205.19 * | 10.82 * | 72.68 * | |
| Component | |||||||||||
| α-Thujene | 0.8 | 0.8 | |||||||||
| Sabinene | 0.3 | 0.2 | |||||||||
| β-Pinene | 3.7 | 96.32 | |||||||||
| 2,3-Dihydro-1,8-cineole | |||||||||||
| α-Terpinene | 1.2 | 1.0 | |||||||||
| p-Cymene | 0.2 | 2.4 | 0.1 | 0.1 | 0.6 | 93.19 | |||||
| Limonene | 0.2 | 7.2 | 4.7 | 92.98 | |||||||
| 1,8-Cineole | 3.1 | 20.4 | 0.1 | 0.1 | 93.13 | ||||||
| (E)-β-Ocimene | 3.6 | 0.3 | |||||||||
| α-Terpinolene | |||||||||||
| α-Phellandrene | 1.8 | ||||||||||
| Terpinen-4-ol | 0.2 | ||||||||||
| α-Terpineol | 0.3 | 2.6 | 0.2 | 0.2 | 0.1 | 0.3 | |||||
| α-Copaene | 0.3 | 1.2 | 0.2 | ||||||||
| Aromadendrene | 1.5 | ||||||||||
| Bicyclosesquiphellandrene | |||||||||||
| γ-Curcumene | 0.5 | ||||||||||
| β-Selinene | 0.3 | ||||||||||
| Bicyclogermacrene | |||||||||||
| α-Amorphene | |||||||||||
| 2,3-Di-tert-butylphenol | |||||||||||
| Calamenene | 0.1 | 0.2 | |||||||||
| δ-Cadinene | 0.2 | 0.4 | 0.8 | ||||||||
2.5. Cytotoxic Activity Correlations
2.6. Correlations for Antimalarial Activity
3. Experimental
3.1. Extraction of the Essential Oil
3.2. Chemicals
3.3. Gas Chromatography and Gas Chromatography-Mass Spectrometry
3.4. Antioxidant Activity
Free Radical Scavenging Activity: DPPH Test
3.5. ABTS Radical-Scavenging Assay
3.6. Antiplasmodial Activity
3.7. Cytotoxicity Evaluation
3.8. Statistical Analysis
4. Conclusions
References
- Humbert, H. Flore de Madagascar et des Comores. 189e Famille, Composées (Compositae); Firmin-Didot et Cie: Paris, France, 1962; pp. 339–622. [Google Scholar]
- Zechmeister, L.; Herz, W.; Grisebach, H.; Kirby, G.W.; Chang, C.W.J.; Flament, I. The Chemistry of Organic Natural Products; Springer-Verlag: New York, NY, USA, 1979; p. 302. [Google Scholar]
- Ramanoelina, A.R.P.; Terrom, G.P.; Bianchini, J.P.; Coulanges, P. Contribution à l’étude de l’action antibactérienne de quelques huiles essentielles de plantes malgaches. Arch. Inst. Past. Madag. 1987, 53, 217–226. [Google Scholar]
- De Medici, D.; Pieretti, S.; Salvatore, G.; Nicoletti, M.; Rasoanaivo, P. Chemical analysis of essential oils of malagasy medicinal plants by gas chromatography and NMR spectroscopy. Flav. Fragr. J. 1992, 7, 275–281. [Google Scholar] [CrossRef]
- Afolayan, A.J.; Meyer, J.J.M. The antimicrobial activity of 3,5,7-trihydroxyflavone isolated from the shoots of Helichrysum aureonitens. J. Ethnopharmacol. 1997, 57, 177–181. [Google Scholar] [CrossRef]
- Boiteau, P.; Allorge-Boiteau, L. Plantes médicinales de Madagascar; ACCT: Paris, France, 1993; pp. 135–136. [Google Scholar]
- Debray, M.; Jacquemin, H.; Razafindrambao, R. Contribution à l’inventaire des plantes médicinales de Madagascar; ORSTOM: Paris, France, 1971; p. 150. [Google Scholar]
- Cabanis, Y.; Chabouis, L.; Chabouis, F. Végétaux et groupements végétaux de Madagascar et des Mascareignes; BDPA: Tananarive, Madagascar, 1969; p. 1342, Tome I–IV. [Google Scholar]
- Boiteau, P. Médecine traditionnelle et pharmacopée, précis de matière médicale malgache; ACCT: Paris, France, 1986; p. 141. [Google Scholar]
- Cavalli, J.F. Caractérisation par CPG/IK, CPG/SM et RMN du Carbone-13 d’huiles essentielles de Madagascar. Ph.D. Thesis, Faculty of Science and Technology, Pascal Paoli University of Corsica, Corse, France, 2002. [Google Scholar]
- Guerin, J.P.; Olliaro, P.; Nosten, F.; Druilhe, P.; Laxminarayan, R.; Binka, F.; Kilama, W.L.; Ford, N.; White, N.J. Malaria, current status of control, diagnosis, treatment, and a proposed agenda for research and development. Lancet Infect. Dis. 2002, 2, 564–573. [Google Scholar] [CrossRef]
- Greenlee, R.T.; Murray, T.; Bolden, S.; Wingo, P.A. Cancer statistics. Cancer J. Clin. 2000, 50, 7–33. [Google Scholar] [CrossRef]
- Cordell, G.A.; Beecher, C.W.; Pezzut, J.M. Can ethnopharmacology contribute to development of new anti-cancer? J. Ethnopharmacol. 1991, 32, 117–133. [Google Scholar] [CrossRef]
- Popoca, J.; Aguilar, A.; Alonso, D.; Villarreal, M.L. Cytotoxic activity of selected plant used as antitumorals in Mexican traditional medicine. J. Ethnopharmacol. 1998, 59, 173–177. [Google Scholar]
- European Pharmacopoeia; Maissoneuve SA: Sainte Ruffine, France, 1983.
- Blois, M.S. Antioxidant determination by use of free radical stable. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Trager, W.; Jensen, J.B. Human malarial parasites in continuous culture. Science 1976, 193, 673–675. [Google Scholar]
- Desoubzdanne, D.; Marcourt, L.; Raux, R.; Chevalley, S.; Dorin, D.; Doerig, C.; Valentin, A.; Ausseil, F.; Debitus, C. Alisiaquinones and alisiaquinol, dual inhibitors of Plasmodium falciparum enzyme targets from a New Caledonian deep water sponge. J. Nat. Prod. 2008, 71, 1189–1192. [Google Scholar] [CrossRef]
- Ribaut, C.; Berry, A.; Chevalley, S.; Reybier, K.; Morlais, I.; Parzy, D.; Nepveu, F.; Benoit-Vical, F.; Valentin, A. Concentration and purification by magnetic separation of the erythrocytic stages of all human Plasmodium species. Malar. J. 2008, 7, 45. [Google Scholar] [CrossRef]
- Lambros, C.; Vanderberg, J.P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J. Parasitol. 1979, 65, 418–420. [Google Scholar] [CrossRef]
- Benoit-Vical, F.; Valentin, A.; Mallié, M.; Bastide, J.M.; Bessière, J.M. In vitro antimalarial activity and cytotoxicity of Cochlospermum tinctorium and C. planchonii leaf extracts and essential oils. Planta Med. 1999, 65, 378–381. [Google Scholar]
- Muñoz, V.; Sauvain, M.; Mollinedo, P.; Callapa, J.; Rojas, I.; Gimenez, A.; Valentin, A.; Mallié, M. Antimalarial activity and cytotoxicity of (−) roemrefidine isolated from the stem bark of Sparattanthelium amazonum. Planta Med. 1999, 65, 448–449. [Google Scholar] [CrossRef]
- Ouattara, Y.; Sanon, S.; Traoré, Y.; Mahiou, V.; Azas, N.; Sawadogo, L. Antimalarial activity of swartzia madagascarensis desv. (leguminosae), combretum glutinosum Guill. & Perr. (combretaceae) and tinospora bakis miers. (menispermaceae) Burkina Fasso medicinal plants. Afr. J. Trad. Cam. 2006, 3, 75–81. [Google Scholar]
- Cachet, N.; Hoakwie, F.; Bertani, S.; Bourdy, G.; Deharo, E.; Stien, D.; Houel, E.; Gornitzka, H.; Fillaux, J.; Chevalley, S.; Valentin, A.; Jullian, V. Antimalarial activity of simalikalactone E, a new quassinoid from Quassia amara L. (Simaroubaceae). Antimicrob. Agents Chemother. 2009, 53, 4393–4398. [Google Scholar] [CrossRef]
- Mollenbeck, S.; Konig, T.; Schreier, P.; Schwab, W.; Rajaonarivony, J.; Ranarivelo, L. Chemical composition and analyses of enantiomers of essential oils from Madagascar. Flav. Frag. J. 1997, 12, 63–69. [Google Scholar] [CrossRef]
- Cavalli, J.F.; Ranarivelo, L.; Ratsimbason, M.; Bernardini, A.F.; Casanova, J. Constituents of the essential oil of six Helichrysum species from Madagascar. Flav. Fragr. J. 2001, 116, 253–256. [Google Scholar]
- Lourens, A.C.U.; Reddya, D.; Baser, K.H.C.; Viljoena, A.M.; Van Vuuren, S.F. In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 2004, 95, 253–258. [Google Scholar] [CrossRef]
- Sharma, P.R.; Dilip, M.; Mondhe, D.M.; Muthiah, S.; Pal, H.C.; Ashok, K.; Shahi, A.K.; Ajit, K.; Saxena, A.K.; Qazi, G.N. Anticancer activity of an essential oil from Cymbopogon flexuosus. Chem.-Biol. Inter. 2009, 179, 160–168. [Google Scholar] [CrossRef]
- Li, Y.L.; Yeung, C.M.; Chiu, L.C.M.; Cen, Y.Z.; Ooi, V.E.C. Chemical composition and antiproliferative activity of essential oil from the leaves of a medicinal herb, Schefflera heptaphylla. Phytother. Res. 2009, 23, 140–142. [Google Scholar] [CrossRef]
- Al-Kalaldeh, J.Z.; Abu-Dahab, R.; Afifi, F.U. Volatile oil composition and antiproliferative activity of Laurus nobilis, Origanum syriacum, Origanum vulgare, and Salvia triloba against human breast adenocarcinoma cells. Nutr. Res. 2010, 30, 271–278. [Google Scholar] [CrossRef]
- Sibanda, S.; Chigwada, G.; Poole, M.; Gwebu, E.T.; Noletto, J.A.; Schmidt, J.M.; Rea, A.I; Setzer, W.N. Composition and bioactivity of the leaf essential oil of Heteropyxis dehniae from Zimbabwe. J. Ethnopharmacol. 2004, 92, 107–111. [Google Scholar] [CrossRef]
- Bendaoud, H.; Romdhane, M.; Souchard, J.P.; Cazaux, S.; Bouajila, J. Chemical composition and anticancer and antioxidant activities of Schinus Molle L. and Schinus Terebinthifolius Raddi Berries essential oils. J. Food Sci. 2010, 75, 466–472. [Google Scholar] [CrossRef]
- El Hadri, A.; Gómez del Río, M.A.; Sanz, J.; González Coloma, A.; Idaomar, M.; Ozonas, B.R.; González, J.B.; Sánchez Reus, M.I. Cytotoxic activity of α-humulene and trans-caryophyllene from Salvia officinalis in animal and human tumor cells. An. R. Acad. Nac. Farm. 2010, 76, 343–356. [Google Scholar]
- Liu, X.; Zu, Y.; Fu, Y.; Yao, L.; Gu, C.; Wang, W.; Efferth, T. Antimicrobial activity and cytotoxicity towards cancer cells of Melaleuca alternifolia (tea tree) oil. Eur. Food Res. Technol. 2009, 229, 247–253. [Google Scholar] [CrossRef]
- Monajemi, R.; Oryan, S.; Haeri-Roohani, A.; Ghannadi, A.; Jafarian, A. Cytotoxic effects of essential oils of some Iranian citrus peels. Iranian J. Pharm. Res. 2005, 3, 183–187. [Google Scholar]
- Haber, W.A.; Agius, B.R.; Stokes, S.L.; Setzer, W.N. Bioactivity and chemical composition of the leaf essential oil of Talauma gloriensis Pittier (Magnoliaceae) from Monteverde, Costa Rica. Rec. Nat. Prod. 2008, 2, 1–5. [Google Scholar]
- Lopes, N.P.; Kato, M.J.; De A Andrade, E.H.; Maiaa, J.G.S.; Yoshida, M.; Planchart, A.R.; Katzin, A.M. Antimalarial use of volatile oil from leaves of Virola surinamensis (Rol.) Warb. by Waiǎpi Amazon Indians. J. Ethnopharmacol. 1999, 67, 313–319. [Google Scholar] [CrossRef]
- Van Zyl, R.L.; Seatlholo, S.T.; Van Vuuren, S.F.; Viljoen, A.M. The biological activity of 20 nature identical essential oil constituents. J. Essential Oil Res. 2006, 18, 129–133. [Google Scholar] [CrossRef]
- Boyom, F.F.; Ngouana, V.; Amvam Zollo, P.H.; Menut, C.; Bessiere, J.M.; Gut, J.; Rosenthal, P.J. Composition and anti-plasmodial activities of essential oils from some Cameroonian medicinal plants. Photochemistry 2003, 64, 1269–1275. [Google Scholar]
- Kamatou, G.P.P.; Viljoen, A.M.; Gono-Bwalya, A.B.; Van Zyl, R.L.; Van Vuuren, S.F.; Lourens, A.C.U.; Baser, K.H.C.; Demirci, B.; Lindsey, K.L.; Van Staden, J.; Steenkamp, P. The in vitro pharmacological activities and a chemical investigation of three South African Salvia species. J. Ethnopharmacol. 2005, 102, 382–390. [Google Scholar] [CrossRef]
- Kamatou, G.P.P.; Viljoen, A.M.; Figueiredo, A.C.; Tilney, P.M.; Van Zyl, R.L.; Barroso, J.G.; Pedro, L.G.; Van Vuuren, S.F. Trichomes, essential oil composition and biological activities of Salvia albicaulis Benth. and S. dolomitica Codd, two species from the Cape region of South Africa. S. Afr. J. Bot. 2007, 73, 102–108. [Google Scholar] [CrossRef]
- Valentin, A.; Pélissier, Y.; Benoit, F.; Marion, C.; Kone, D.; Mallie, M.; Bastide, J.M.; Bessiere, J.M. Composition and antimalarial activity in vitro of volatile components of lippia multiflora. Photochemistry 1995, 40, 1439–1442. [Google Scholar]
- Van Vuuren, S.F.; Viljoen, A.M.; Van Zyl, R.L.; Van Heerden, F.R.; Baser, K.H.C. The antimicrobial, antimalarial and toxicity profiles of helihumulone, leaf essential oil and extracts of Helichrysum cymosum (L.) D. Don subsp. S. Afr. J. Bot. 2006, 72, 287–290. [Google Scholar] [CrossRef]
- Ortet, R.; Thomas, O.P.; Regalado, E.L.; Pino, J.A.; Filippi, J.J.; Fernandez, M.D. Composition and biological properties of the volatile oil of Artemisia gorgonum Webb. Chem. Biodivers. 2010, 7, 1325–1333. [Google Scholar] [CrossRef]
- Tabanca, N.; Demirci, B.; Crockett, S.L.; Baser, K.H.C.; Wedge, D.E. Chemical composition and antifungal activity of Arnica longifolia, Aster hesperius, and Chrysothamnus nauseosus essential oils. J. Agric. Food Chem. 2007, 55, 8430–8435. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Helichrysum gymnocephalum Essential Oil: Chemical Composition and Cytotoxic, Antimalarial and Antioxidant Activities, Attribution of the Activity Origin by Correlations. Molecules 2011, 16, 8273-8291. https://doi.org/10.3390/molecules16108273
Afoulous S, Ferhout H, Raoelison EG, Valentin A, Moukarzel B, Couderc F, Bouajila J. Helichrysum gymnocephalum Essential Oil: Chemical Composition and Cytotoxic, Antimalarial and Antioxidant Activities, Attribution of the Activity Origin by Correlations. Molecules. 2011; 16(10):8273-8291. https://doi.org/10.3390/molecules16108273
Chicago/Turabian StyleAfoulous, Samia, Hicham Ferhout, Emmanuel Guy Raoelison, Alexis Valentin, Béatrice Moukarzel, François Couderc, and Jalloul Bouajila. 2011. "Helichrysum gymnocephalum Essential Oil: Chemical Composition and Cytotoxic, Antimalarial and Antioxidant Activities, Attribution of the Activity Origin by Correlations" Molecules 16, no. 10: 8273-8291. https://doi.org/10.3390/molecules16108273
APA StyleAfoulous, S., Ferhout, H., Raoelison, E. G., Valentin, A., Moukarzel, B., Couderc, F., & Bouajila, J. (2011). Helichrysum gymnocephalum Essential Oil: Chemical Composition and Cytotoxic, Antimalarial and Antioxidant Activities, Attribution of the Activity Origin by Correlations. Molecules, 16(10), 8273-8291. https://doi.org/10.3390/molecules16108273




