Abstract
The synthesis of some thio-oxocrown ether ligands, B1 (1,4-dithio-12-crown-4), B2 (1,7-dithio-12-crown-4), B3 (1,7-dithio-15-Crown-5), B4 (1,7-dithio-18-crown-6), B5 (1,10-dithio-18-crown-6), B6 (1,10-dithio-21-crown-7), under mild conditions, were reported. The ligands were characterized by FT-IR, 1H NMR and GC-MS spectroscopy. The formation of 1:1 ligand complexes with a variety of metal salts (Ag+, Ca+2, K+, Na+, Mg+2, Zn+2 and Fe+2) were investigated by a conductometric method in a 1:1 dioxane–water system at 25 °C, and the complexation constants (Ke = (ΛMAm -Λ) / ((Λ-ΛMaLbAm) [L]) and free energy (∆Go= - RT lnKe) values are calculated. Details of the specific molecular interactions between the ligands and metals were proposed. We also performed DFT calculations to explain their geometrical properties, charges and frontier molecular orbitals.
1. Introduction
Synthetic macrocycles, in particular crown ethers, have been known for over three quarters of a century, although a real spate of publications in this area was observed in the late 1960s [1,2]. In that period, thousands of macrocyclic compounds were reported, and since then their number has increased markedly from year to year. Crown ethers contains “hard” ether–oxygen-bridges and show a binding preference toward “hard” metals (such as alkali and alkaline earth metal cation). The replacement of oxygen-bridge with “soft” sulfide or amine linkages can shift their preference towards “soft” heavy metal cations [3]. Thus, selectivity can be tuned by combining different hard/soft donor atoms in one ring system. In this regard oxo-thiocrown ethers are prepared and used as potential heavy-metal receptors. In this work, oxo-thiocrown ethers having different ring size or arrangement have been prepared and their complexation properties have been investigated with various metal cations by conductometry [4,5,6,7].
More recently, interest in differences in the properties of metal complexes caused by replacement of one or more thioether sulfurs with amine nitrogen atoms has been apparent in the literature [8,9]. In spite of this and the obvious connection between crown ethers and thiocrown ethers, until quite recently [10,11], less effort has been devoted to a comparison of O,S donor complexes with complexes of these other ligand systems. As part of our continuing interest in the properties of both acyclic and cyclic ligands that have thioether and other donors [12,13], we have examined the properties of some metal complexes of ligand L and the results are reported herein for the purpose of comparison with those from homothioether and mixed S,N donor macrocyclic ligand complexes [14].
Binary mixed aqueous solvents are frequently employed in broad areas of chemistry. Their applicability ranges from synthetic and mechanistic studies in organic chemistry to biophysical chemistry, with emphasis on molecular interactions in biologically significant structures. Stability constants of crown compound-action complexes are determined by various methods, such as potentiometry (with ion selective electrodes), polarography, voltammetry, spectrophotometry, NMR, calorimetry and solubility. The ability of macrocyclic polyethers (crown ethers) to form stable complexes with cations, mainly with alkali and alkaline earth cations, has spurred interest in these compounds [15,16,17]. Recently published studies include conductometric measurement of some electrolytes in non-aqueous solvents (tetrahydrofuran [18], tetrahydropyran [18], acetonitrile [19,20] and methanol [20]) in the presence of crown-ethers. Studies carried out in solutions of alkali metal salts and crown-ethers in acetonitrile and methanol showed decreases in conductivities of the solutions.
The object of the present work was to study the conductance behavior of AgNO3, CaCl2, KCl, NaCl, MgCI2, ZnCl2 and FeSO4 and with B1 (1,4-dithio-12-crown-4), B2 (1,7-dithio-12-crown-4), B3 (1,7-dithio-15-Crown-5), B4 (1,7-dithio-18-crown-6), B5 (1,10-dithio-18-crown-6), B6 (1,10-dithio-21-crown-7) in the dioxane/water mixtures (50%) at 25 °C. The study indicates 1:1 complex formation between the metal-ions and electrically neutral crown ethers.
The experimental findings were also supported by theoretical computations. The gas phase molecular mechanical and quantum chemical calculations of B1, B2, B3, B4, B5, and B6 were performed at B3LYP/6-31G(d) level with the help of the Gaussian03 program.
2. Results and Discussion
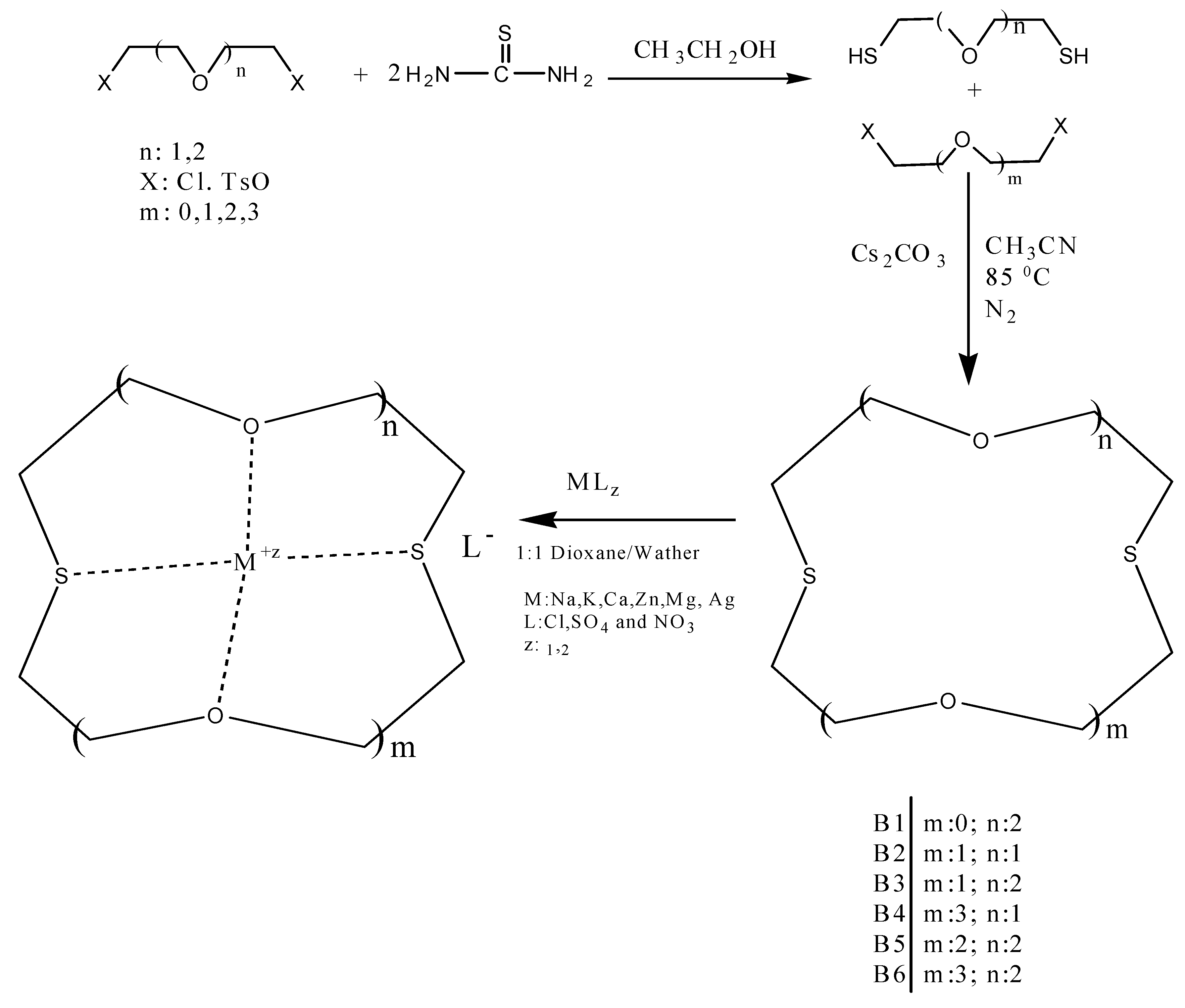
In this work, several oxo-thiocrown ethers B1-B6 with different ring sizes and structural isomers, were synthesized in acetonitrile under high dilution conditions by the SN2 reaction of di/tri-ethyleneglycol dithiols with mono/di/tri-ethyleneglycol dichlorides/ditosylates in the presence of CsCO3. From this the folowing oxo-thiocrown ether ligands were obtained in low (8%) to moderate (48%) to high yield (73%): B1 (1,4-dithio-12-crown-4), B2 (1,7-dithio-12-crown-4), B3 (1,7-dithio-15-Crown-5), B4 (1,7-dithio-18-crown-6), B5 (1,10-dithio-18-crown-6), B6 (1,10-dithio-21-crown-7). The oxo-thiocrown ethers (B1-B5) were previously reported by others; however B6 is new to the best of our knowledge (Scheme 1). The reported procedure was modified slightly by replacing DMF with acetonitrile, which is easy to evaporate.
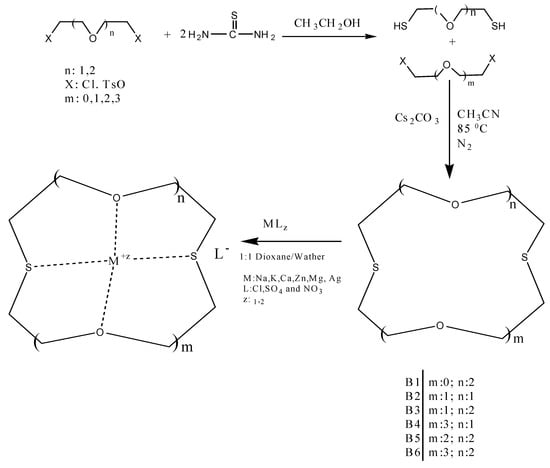
Scheme 1.
Syntheses of several oxo-thiocrown ethers (B1-B6) and complexation with metals.
Scheme 1.
Syntheses of several oxo-thiocrown ethers (B1-B6) and complexation with metals.
The oxo-thiocrown ethers (B1-B6) are hygroscopic materials, supported by the presence of O-H stretching vibrations at 3,500 cm−1. The dithiols used in the synthesis of oxo-thiocrown ethers were obtained by two different methods. Generally thio derivatives, such as dithiols or thio-crown ethers are expensive materials; hence ethyleneglycol derivatives are frequently used as cheap starting materials. In this regard, initially Method A was explored. This involves the reaction of di/tri-ethyleneglycol with thiourea in the presence of HCl, where dichlorides were generated in situ, followed by the addition of KOH. On the other hand Method B involves direct reaction of di/tri-ethyleneglycol dichlorides with thiourea in the presence of KOH. The both method gave moderate yields (30–36%). Dithiols were purified by using vacuum distillation and then characterized with FT-IR, 1H NMR and GC-MS. The S-H streching vibration for dithiols was characterized by the presence of band at 2,500 cm−1.
For example, if we have prepared dithio-18-crown-6 using both ways, the product obtained from ditosylate is less than the product made from the dichloride because, the tosylate is a group that can be separated in SN2 mechanism under normal conditions but it is too hard to remove it from complex donor crown ethers. Especially, that’s hard to purify the molecules which have –S center oxygen, so their yields are lower.
Various methods to calculate stability constants from measurements of properties involving intrinsic factors, such as molar conductivity etc. have been described in the literature [21-23]. When an oxo-thiocrown ether ligand (L) forms a complex (MaLbm+) with a cation (Mm+), the equilibrium equation can be written as:
where Mm+, L, and α are respectively the cation, ligand and fraction of free cations. Thus the equilibrium constants Ke of different ratios of complex formation were calculated using the following equations (2)–(13):
a Mm+ + bL ⇌ MaLbm+
αCM CL -(1-α)CM (1- α)CM
Ke = [MaLbm+] / [Mm+]a [L]b
CM / CL = 1
CM = [Mam+] +[MaLbm+]
CL = [Lb] +[MaLbm+]
α = [Mam+] / CM
P = [MaLbm+] / CM =Ke[Lb] / (1+ Ke[Lb] )
The observed conductivity, κ, is given by:
κ= κMam+ +κMaLbm+
The molar conductivities are:
ΛMAm = κMAam+ / [Mam+]
ΛMaLbm+ = κ MaLbm+ / [MaLbm+]
Λ= κ / CM
Λ= αΛMam+ + (1- α)ΛMaLbm+
As a result of Eq. (12), Eq. (2) can be transformed into:
where [Lb] = CL - CM.P and [Lb] = CL - CM.(ΛMam+ - Λ)/(ΛMam+ - Λ MaLbm+) and CM, CL are the total concentrations of metal ion and crown ether, respectively; [Mam+], [Lb] and [MaLbm+], are the concentrations of uncomplexed action, uncomplexed crown ether and complexed cation, respectively; P, is the experimental mole fraction of the complexed cation or the ligand, and a and b are the complexing degrees of both sides in the case of several degrees of complexing [eqns. (1)-(13)] κMam+, κMaLbm+, are the observed conductivities of the electrolyte and the crown compound-electrolyte complex, respectively; ΛMam+ and ΛMaLbm+ are the designated molar conductivities of the electrolyte and the crown compound-electrolyte complex, respectively [21].
Ke = (ΛMam+ - Λ) / ((Λ-ΛMaLbm+) [Lb] )
In the present work, considerable solvent effects of water were displayed for B1 (1,4-dithio-12-crown-4), B2 (1,7-dithio-12-crown-4), B3 (1,7-dithio-15-crown-5), B4 (1,7-dithio-18-crown-6), B5 (1,10-dithio-18-crown-6), B6 (1,10-dithio-21-crown-7), owing to differences in Ke depending on the solvent composition. The selectivity for a given salt is a factor not only of the crown ether, but of the solvent as well [21,24]. These various selectivities depend on the crown ether interactions with the cations governed by charge density.
The complexation of the synthesized products with Na+ , K+ , Ca2+, Zn2+, Mg2+, Ag+ and Fe2+ was studied by conductometry in 50% dioxane-water at 25 °C and original results were obtained. The complexation selectivity of B1 with Na+, K+, Ca2+, Zn2+, Mg2+, Ag+ and Fe2+ ions, were observed to be as follows: Na+ > Fe2+ > Mg2+ > Ca2+ > Ag+ > K+ > Zn2+. The best complexation of with B1 with Na+ can be explained by the possibility of having sandwich molecules with more than one ion because its ring cavity is bigger.
The complexation sequence for B2 was obtained as follows: Fe2+ > Ca2+ > K+ > Mg2+ > Na+ > Zn2+ > Ag+. The best complexation with B2 is shown by Fe2+ ion and the least is Ag+ ion .
B3 gave the sequence K+ > Ca2+ > Na+ > Zn2+ >Fe2+ > Ag+ > Mg2+. Thus, the highest association constant for complexation of B3 was found for K+ ion.
However, for B4, the order is Na+ > Zn2+ > Ca2+ > Fe2+ > Ag+ > K+ > Mg2+. Hence, the best complexing with B4 is Na+.
Moreover, B5, gives the order K+ > Ag+ > Ca2+ > Zn2+ > Mg2+ > Na+ > Fe2+. The best complexing with B5 is K+.
B6, gives the order Mg2+ > Na+ > Ca2+ > K+ > Zn2+ >Fe2+ > Ag+. The best complexing with B6 is Mg2+.
All oxo-thio crown ethers B1-B6 have systematic variations in the ring size of the crown ether ring from [12]crown-4 to [15]crown-5 to [18]crown-6 to [21]crown-7, respectively. Results of our alkali metal complexation study using oxo-thio crown ether derivatives are presented in Table 1. One of the most impressive results of this study is the dithio-12-crown-4 and dithio-18-crown-6 derivatives’ complexing ability in dioxane-water system. Some specific results were obtained by exchanging of oxygen and sulphur in the crown-ring. For example, for Ag+ the best are B1, B3, B2 and then, B6 is lesser. Mg++ has the highest complexing ability with B6, and it complexes with B3 and B5. This is caused by the conformational difference between the oxygens and sulphurs in the structure. The ligands which best complex with the metals were determined in this sequence: for NaCI and KCl B1 is the best, for ZnCl2 B3, for MgCl2 B6, for FeSO4 and CaCl2 B2.
2.1. Computational Results
The difficulty of the theoretical calculations can be attributed to the complication of supramolecular structures. Various quantum-chemical and force-field computations of crown ethers and of their metal complexes have been reported recently [24,26,27,28], even on the ab initio level [29,30] and the density functional level of theory (DFT) [31,32,33]. The structures of molecules play an especially significant role in determining their chemical properties. Therefore, we study the most stable geometries of the six crown ethers. In order to obtain the preferred conformers, the simulated anneling technique was employed initially [34]. The simulation protocol involving a heating time of 0.1 ps, followed by a 1 ps simulation at 3,000 K and cooling to 298 K within 50 ps was applied at AM1 self-consistent field molecular orbital level [35]. Quantum-mechanical calculations were carried out on initially geometry optimized structures at the DFT level using the Gaussian03 suite of programs [36]. The DFT methods are effective for the theoretical studies of supramolecular structures [37,38,39]. The B3LYP, a version of the DFT method, which uses Becke’s three-parameter functional (B3) [40] and includes a mixture of HF and DFT exchange terms associated with the gradient corrected correlation functional of Lee, Yang, and Parr (LYP) [41]. Hence, B3LYP method is used in this article to perform quantum chemical calculations.

Table 1.
The Association Constants (Ke) and Free Enthalpies (ΔGθ) of NaCI, KCI, CaCl2, ZnCl2, MgCl2, AgNO3 and FeSO4 with B1 (1,4-dithio-12-crown-4), B2 (1,7-dithio-12-crown-4), B3 (1,7-dithio-15-Crown-5), B4 (1,7-dithio-18-crown-6),B5 (1,10-dithio-18-crown-6) and B6 (1,10-dithio-21-crown-7), with (1:1) in %50 Dioxane/Water Mixtures at 25 °C.
| Thiocrown ethers | Cation | Ke (1:1) | LogKe(1:1) | -ΔGθ1:1 |
|---|---|---|---|---|
 | Na+ | 159005,00 | 5,201411 | 7091,71 |
| K+ | 4659,09 | 3,668301 | 5001,44 | |
| Ca2+ | 21002,62 | 4,322273 | 5893,08 | |
| Zn2+ | 4134,76 | 3,61645 | 4930,72 | |
| Mg2+ | 22862,18 | 4,359118 | 5943,31 | |
| Ag+ | 15602,02 | 4,193181 | 5717,07 | |
| Fe2+ | 63856,70 | 4,805206 | 6551,52 | |
 | Na+ | 24556,00 | 4,390158 | 5985,63 |
| K+ | 1177370,00 | 6,070913 | 8277,21 | |
| Ca2+ | 1334785,00 | 6,125411 | 8351,51 | |
| Zn2+ | 9608,34 | 3,982648 | 5430,02 | |
| Mg2+ | 113650,30 | 5,055571 | 6892,87 | |
| Ag+ | 6486,80 | 3,812031 | 5197,40 | |
| Fe2+ | 2542753,00 | 6,405304 | 8733,12 | |
 | Na+ | 128719,00 | 5,109643 | 6966,59 |
| K+ | 1073250,00 | 6,030701 | 8222,38 | |
| Ca2+ | 516059,00 | 5,712699 | 7788,81 | |
| Zn2+ | 66872,94 | 4,82525 | 6578,85 | |
| Mg2+ | - | - | - | |
| Ag+ | 15602,07 | 4,193182 | 5717,07 | |
| Fe2+ | 30157,18 | 4,479391 | 6107,29 | |
 | Na+ | 136555,00 | 5,135308 | 7001,58 |
| K+ | 217,76 | 2,337978 | 3187,65 | |
| Ca2+ | 24444,83 | 4,388187 | 5982,94 | |
| Zn2+ | 58836,12 | 4,769644 | 6503,03 | |
| Mg2+ | - | - | - | |
| Ag+ | 246,23 | 2,391341 | 3260,40 | |
| Fe2+ | 3701,47 | 3,568374 | 4865,20 | |
 | Na+ | 4144,79 | 3,617503 | 4932,18 |
| K+ | 57052,90 | 4,756278 | 6484,81 | |
| Ca2+ | 37945,99 | 4,579166 | 6243,33 | |
| Zn2+ | 31015,46 | 4,491578 | 6123,91 | |
| Mg2+ | 22825,90 | 4,358428 | 5942,37 | |
| Ag+ | 54208,9 | 4,734071 | 6454,529 | |
| Fe2+ | 390.0 | 2,591065 | 3532,71 | |
 | Na+ | 127313,00 | 5,104873 | 6960,09 |
| K+ | 22056,90 | 4,343544 | 5922,08 | |
| Ca2+ | 37945,99 | 4,579166 | 6243,33 | |
| Zn2+ | 11118,95 | 4,046064 | 5516,49 | |
| Mg2+ | 517167,30 | 5,713631 | 7790,08 | |
| Ag+ | 298,72 | 2,475264 | 3374,83 | |
| Fe2+ | 6359,96 | 3,803454 | 5185,71 |
Corr.Coeff: Na+ :0,9993, K+: 0,9995, Ca2+: 0,9997, Zn2+, : 0,9995, Mg2+:0,9994, Ag+: 0,9996 and Fe2+:0,9997
The optimized structures for the crown ethers studied herein in their gorund states were obtained and are shown in Table 2. The dihedral angles were also calculated to analyze the planarity of molecules. The ring-contracted crown ether B5 has the biggest dihedral angle, ØSOOS = 42.51° and indicates the most distorted structures for series of compounds. The results indicate that when a methylene between two adjacent oxygen atoms of a crown ether is reduced, the rigidity of the crown ring is increased and the structure of the crown ether shows a drastic change in the atoms’ disorder. However, compared to the conventional crown ethers B2 and B3, the ring-contracted crown ethers all depict a relatively good oxygen planarity because the crown ether rings became more soft and relaxed for the changing of the methylene-chain length between sulfur and oxygen atoms. The calculated real vibrational frequencies of B1, B2, B3, B4, B5, and B6 at B3LYP/6-31G(d) level show that the molecules are located as a minimum on potential energy surfaces.
The highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of molecules are quite important to define its reactivity. Fukui et al. were the first to recognize this. EHOMO is often called with the electron donating ability of the molecules [42]. EHOMO depicts that the molecular ability in donating electrons to appropriate acceptor molecules with low energy, empty molecular orbital. On the contrary. ELUMO indicates the ability of the molecule to accept electrons. The lower value of ELUMO, indicates that the molecule would accept electrons. Hence, concerning the value of the energy gap, ΔE(ELUMO–EHOMO), higher values of ΔE will provide lower reactivity to a molecule. Lower values of ΔE will indicate the higher reactivity of the molecules, because the energy to remove an electron from the HOMO to the LOMO orbital will be low [43,44,45,46]. Frontier molecular orbital diagrams and energies of B1, B2, B3, B4, B5, and B6, such as ELUMO, EHOMO, and ΔE (in eV) estimated by the B3LYP/6-31G(d) levels are represented in Table 2.

Table 2.
Frontier molecular Orbital Diagrams and Energies of I and II by the B3LYP/6-31G(d) (isovalue: 0.02).
| HOMO | LUMO | ΔE | |
|---|---|---|---|
| B1 | 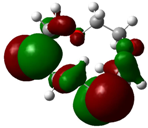 | 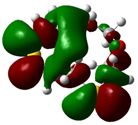 | |
| E (eV) | −0.219 | 0.030 | 0.249 |
| B2 | 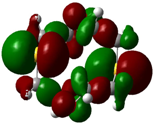 |  | |
| E (eV) | −0.209 | 0.042 | 0.251 |
| B3 | 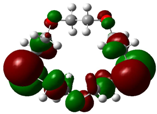 | 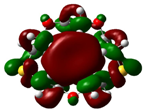 | |
| E (eV) | −0.228 | 0.024 | 0.249 |
| B4 | 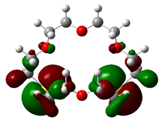 | 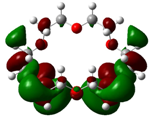 | |
| E (eV) | −0.202 | 0.053 | 0.255 |
| B5 | 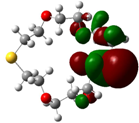 | 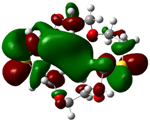 | |
| E (eV) | −0.217 | 0.031 | 0.248 |
| B6 | 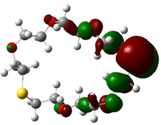 | 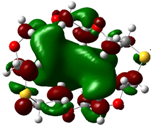 | |
| E (eV) | −0.229 | 0.019 | 0.248 |
The values of EHOMO show the ranking: B4 > B2 > B5 > B1 > B3 > B6 for this property. In addition, the values of ΔE show this order: B4 > B2 > B1 ≥ B3 > B5 ≥ B6. As can be seen from the HOMO and LUMO pictures in Table 2, the majority of HOMO and LUMO are found on the donor atoms in the crown ether ring, suggesting the high electron-donating ability of heteroatoms.
Table 3 shows the atomic charges from natural population analyses (NPA) for B1, B2, B3, B4, B5, and B6. The calculated charges on the sulfur atoms are considerable more positive than that on oxygen atoms. Results primarily indicate the role of delocalized oxygen charges on the torsional barrier of gauche oxyethylene units of crown ethers which could optimize charge densities and therefore the preferred conformations. This also influenced the polarization balances of the rest of atoms in a macromolecule governing the power of macrocycle-cation interactions.

Table 3.
NBO Charges on Oxygen and Sulfur Atoms of B1, B2, B3, B4, B5, and B6.
| Complex | Charges on Oxygen atoms | Charges on Sulfur atoms |
|---|---|---|
| B1 | −0.591 / −0.599 | 0.196 / 0.199 |
| B2 | −0.591 / −0.591 | 0.186 / 0.186 |
| B3 | −0.578 / −0.579 / −0.579 | 0.207 / 0.207 |
| B4 | −0.573 / −0.573 / −0.576 / −0.583 | 0.227 / 0.227 |
| B5 | −0.580 / −0.581 / −0.584 / −0.600 | 0.201 / 0.211 |
| B6 | −0.575 / −0.580 / −0.580 / −0.582 / −0.582 | 0.210 / 0.214 |
3. Experimental
3.1. General
The starting materials triethyleneglycoldiol, diethyleneglycoldiol, triethyleneglycol dichloride, diethyleneglycol dichloride, triethyleneglycol ditosylate diethyleneglycol ditosylate, ethylene ditosylate, Cs2CO3 and thiourea were purchased from Aldrich or Merck. IR spectra were recorded on a Perkin Elmer BX 2 FTIR spectrophotometer using KBr pellets. 1H-NMR spectra were recorded on a Varian 400 MHz spectrometer in CDCl3 and chemical shifts are reported relative to Me4Si used as an internal standard. Mass spectra were obtained using a Shimadzu GS-MS-QP2010 spectrometer. Melting points were measured on an Elektotermal 9200 apparatus.
3.1.1. The Synthesis of Dithiols
Triethyleneglycoldithiol from triethylene glycol (Method A): triethyleneglycol (24 mL, 0.18 mol) and thiourea (30 g, 0.39 mol) were heated in HCl (150 mL) for 40 hours under reflux. Then, the reaction mixture was left to cool to room temperature. A 3.3 M aqueous KOH (300 mL) was added carefully to the reaction mixture. The solution was left to cool to room temperature, and then organic products were extracted into ether (2 × 150 mL). Combined extracts were dried over MgSO4, the solids were filtered and the solvent was evaporated under reduced pressure to give a crude material as liquid. Upon distillation (5 mm Hg, 140 °C) the title product (12.02 g, 36%) was obtained as a yellow liquid. 1H-NMR (δ, CDCl3): 1.55 ppm (2H, t, SH), 2.73 ppm (4H, t, -CH2SH), δ 3.65 ppm (4H, s, ‑OCH2CH2O-) 3.75 ppm (4H, t, OCH2CH2SH); FT-IR (ν cm−1, KBr): 2553 (S-H), 1113 (C-O-C).
From trietyleneglycol dicloride: (Method B): triethyleneglycol dichloride (25 mL, 0.15 mol) and thiourea (30 g, 0.39 mol) were heated in ethanol (150 mL) for 50 hours under reflux. Then, the reaction was left to cool to room temperature. A 1.75 M aqueous KOH (200 mL) was added carefully to the reaction mixture. The solution was left to cool to room temperature; organic products were extracted into ether (2 × 100 mL). Combined extracts were dried over MgSO4 the solids were filtered off and the solvent was evaporated under reduced pressure to give a crude material as liquid. Upon distillation (5 mmHg, 140 °C) the title product (8.92 g, 30%) was obtained as yellow liquid. FT-IR (ν cm−1, KBr): 2555 (S-H), 1115 (C-O-C).
Dietyleneglycoledithiols (by Method B). Starting with diethyleneglycol dichloride (20 mL, 0.17 mol), thiourea (30 g, 0.39 mol) and KOH (20 gr, 0.35 mol) the title product (9.03 g, 36%) was obtained as a clear liquid upon distillation (4 mm-Hg, 143 °C). 1H-NMR (δ, CDCl3): 1.55 (2H, t, SH), 2.65 (4H, t, -CH2SH), 3.55 (4H, t, -OCH2CH2SH). FT-IR (ν cm−1, KBr): 2557 (S-H), 1110 (C-O-C).
3.1.2. The Synthesis of Oxo-thiocrown Ethers
General procedure: ditosylate/dichloride was added to a suspension of dithiol (1.1 equiv.) and Cs2CO3 (5.0 equiv.) in acetonitrile (250 mL). The mixture was then heated under reflux for 24 h under a nitrogen atmosphere. The reaction was left to cool to room temperature and then the precipitates were filtered. The solvent was evaporated under reduced pressure to give a crude material as solid. Distilled water (100 mL) was added and organics were extracted into benzene-chloroform (10:1 v/v). Combined extracts were dried over MgSO4 and then evaporated under reduced pressure. Purification by column chromatography (benzene/ethyl acetate; 1:10 v/v) afforded the title compounds.
1,4-Dithio-7,10-dioxocyclododecane (B1): Starting with ethylene glycol ditosylate (1.57 g, 4.24 mmol), triethyleneglycol dithiol (0.84 g, 4.66 mmol) and Cs2CO3 (7 g, 21.2 mmol) the title compound (0,65 g, 73%) was obtained as thick oil. 1H-NMR (δ, CDCl3): 2.78 (8H, t, -SCH2-CH2O-), 3.00 (4H, s, ‑SCH2CH2S-), 3.60 (4H, s, -OCH2CH2O-), 3.78 (4H, t, -OCH2CH2S-); FT-IR (ν cm−1, KBr): 1177 (C-O-C), 684 (C-S-C). GC-MS: (m/z) M+: 208.34 (100%).
1,7-Dithio-4,10-dioxocycloododecane (B2): Starting with diethylene glycol dichlorides (2.34 mL, 0.02 mol), diethyleneglycol dithiol (2.76 g, 0.02 mol) and Cs2CO3 (35 g, 0.105 mol) the title compound (1.97 g, 45%) was obtained as a thick oil. 1H NMR (δ, CDCl3): 2.90 (8H, t, -SCH2-CH2O-), 3.79 (4H, t, -OCH2CH2S-). FT-IR (ν cm−1, KBr): 1111 (C-O-C), 661 (C-S-C). GC-MS: (m/z) M+: 208.34 (100%).
1,7-Dithio-4,10,13-trioxocyclopentadecane (B3): Starting with diethylene glycol ditosylate (1.75 gr, 4.24 mmol), triethyleneglycoldithiol (1.26 g, 6.99 mmol) and Cs2CO3 (7 g, 21.2 mmol) the title compound (0,88 g, 48%) was obtained as yellow crystal. Melting point: 63–64 °C. 1H-NMR (δ, CDCl3): 2.95 (8H, t, -SCH2-), 3.65 (4H, s, -OCH2CH2O-), 3.75 (8H, t, -OCH2CH2S-). FT-IR (ν cm−1, KBr): 1176 (C-O-C), 665 (C-S-C). GC-MS: (m/z) M+: 252.40 (100%).
1,7-Dithio-4,10,13,16-tetraoxocyclooctadecane (B4): Starting with tetraethyleneglycol dichloride (1.95 mL, 0.01 mol), diethyleneglycol dithiol (1.38 g, 0.01 mol) and Cs2CO3 (17.1 g, 0.05 mol) the title compound (0,24 g, 8%) was obtained as a thick oil. 1H-NMR (δ, CDCl3): 2.80 (8H, t, -SCH2-), 3.65 (8H, t, -OCH2CH2O-), 3.75 (8H, t, -OCH2CH2S-). FT-IR (ν cm−1, KBr): 1115 (C-O-C), 665 (C-S-C) GC-MS: (m/z) M+: 208.34 (100%).
1,10-Dithio-4,7,13,16-tetraoxocyclooctadecane (B5): Starting with triethyleneglycol ditosylate (2.91 g, 6.36 mmol), triethyleneglycol dithiol (1.26 g, 6.99 mmol) and Cs2CO3 (10.30 g, 31.8 mmol) the title compound (0.27 g, 14 %) was obtained as a yellowish solid. When triethyleneglycol dichloride was used, the yield was improved to 38%. Melting point: 93–94 °C. 1H-NMR (δ, CDCl3): 2.80 (8H, t, ‑SCH2-), 3.60 (8H, t, -OCH2CH2O-), 3.75 (8H, t, -OCH2CH2S-). FT-IR (ν cm−1, KBr): 1176, 1114 (C-O-C), 663 (C-S-C). GC-MS: (m/z) M+: 296.45 (100%).
1,10-Dithio-4,7,13,16-pentaoxocyclooctadecane (B6): Starting with tetraethylene glycol dichloride (1.95 mL, 0.01 mol), triethyleneglycol dithiol (1,82 g, 0.01 mol) and Cs2CO3 (17.1 gr, 0.05 mol) the title compound (0.66 g, 19%) was obtained as a thick oil. 1H-NMR (δ, CDCl3): 2.75 ppm (8H, t, ‑SCH2-CH2O-), 3.54 ppm (12H, p, -OCH2CH2O-), 3.69 ppm (48, t, -OCH2CH2S-). FT-IR (ν, cm−1, KBr): 1119 (C-O-C), 665 (C-S-C). GC-MS: (m/z) M+: 340.50 (100%).
3.2. Conductimetric Method
Merck grade alkali halides (NaCl and KCl) were recrystallized three times from a conductivimetric grade water-ethanol mixture and Merck grade AgNO3, CaCl2, MgCI2, ZnCl2 and FeSO4) were used directly. All of the salts were heated below their decomposition temperature at reduced pressure to remove traces of water in the crystal structure. Bi-distilled water was redistilled from alkaline permanganate. All solutions were prepared in dry glassware and transferred into the pre-dried conductivity cell.
All conductances were measured at 25 ± 0.05 °C. The measuring equipment consisted of a glass vessel (Ingold type) with an external jacket connected to a thermostatted water-bath (25 ± 0.05 °C) and a conductivity cell (Cole Parmer 19050-66) with a conductometer (Suntex Model SC-170).
The cell constant was determined to be 0.769 cm−1 at 25 ± 0.05 °C by measuring the conductivity of aqueous KCl solutions of different concentrations [21,25]. A value of the molar conductivity of the pure metal ion solution (ΛMA) was obtained at the appropriate electrolyte concentration before adding any ligand.
4. Conclusions
In conclusion, various macrocyclic hosts with diverse affinity towards the metal ions and other funtions have been readily synthesized by changing the combinations of the oxygen and sulfur atoms forming the ring framework. The synthesized thiocrown ethers exhibited remarkably high binding constants in metal ion transport experiments [21,24,26].
The synthesized macrocyclic ethers exhibit different binding ability orders towards the examined metal ions. Although the effect of various synthesized thiocrown ethers on the transport ability of the studied metal cations was not investigated systematically, preliminary results show that thiocrown ethers may possess an advantage over crown ethers containing only oxygen atoms in the cyclic framework for metal binding, because the introduction of a sulfur atom into a crown ring gives rise to a favorable entropic change upon complexation with the metal ions. The results strongly suggested that the binding results from the synergistic coordination with polarization balance of the oxygen and sulfur atoms to the metal ions.
As a result, we have demonstrated that a complementary theoretical and experimental approach can provide important information on oxo-thiacrown ethers complexation with various metal ions (Ag+, Ca+2, K+, Na+, Mg+2, Zn+2 and Fe+2), essential elements in a wide variety of processes in biological systems. The position of sulfur atom in the crown ether ring plays an important role in determining the ethers’ properties, as it can not only change the cavity size, but may also cause a disorder of the atom arrangement. Less-symmetrical crown ethers show remarkable recognition and binding properties towards specific metal cations compared to the highly-symmetrical crown ethers (Table 1, Table 2, and Table 3). It is believed that both of them will be widely applied in the future.
Acknowledgments
The authors would like to thank Ümit Çakır for the evaluation of complexation data and Akın Azizoğlu for the theoretical calculations.
- Sample Availa1bility: Samples of all compounds are available from the authors.
References and Notes
- Melson, G.A. Coordination Chemistry of Macrocyclic Compounds; Plenum Publishing Corp.: New York, NY, USA, 1979. [Google Scholar]
- Formanowskii, A.A.; Mikhura, I.V. Synthesis of Macrocyclic Compounds. In Macrocyclic Compounds in Analytical Chemistry; Zolotov, Y.A., Ed.; Wiley- Interscience: New York, NY, USA, 1997; pp. 5–39. [Google Scholar]
- Chartres, J.D.; Davies, M.S.; Lindoy, L.F.; Meehan, G.V.; Wei, G. Macrocyclic ligand design: The interaction of selected transition and post-transition metal ions with a 14-membered N2S2-donor macrocycle. Inorg. Chem. Commun. 2006, 9, 751–754. [Google Scholar] [CrossRef]
- Groth, A.M.; Lindoy, L.F.; Meehan, G.V. New linked macrocyclic systems derived from selectively protected S2N2 macrocycles. J. Chem. Soc. Perkin Trans. 1996, 1, 1553–1558. [Google Scholar]
- Lindoy, L.F.; Baldwin, D.S. Ligand design for selective metal-ion transport through liquid membranes. Pure Appl. Chem. 1989, 61, 909–914. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, M.; Ikeda, T.; Ikeda, K.; Ando, H.; Shibutani, Y.; Yajima, S.; Kimura, K. Synthesis and metal-ion binding properties of monoazathiacrown ethers. J. Org. Chem. 2001, 66, 7008–7012. [Google Scholar]
- Alp, H.; Gok, H.Z.; Kantekin, H.; Ocak, Ü. Synthesis and metal ion binding properties of thiaaza crown macrocycles. J. Hazard. Mater. 2008, 159, 519–522. [Google Scholar] [CrossRef]
- Bernardo, M.M.; Heeg, M.J.; Schroeder, R.R.; Ochrymowycz, L. Comparison of the influence of saturated nitrogen and sulfur donor atoms on the properties of copper(II/I)-macrocyclic polyamino polythiaether ligand complexes: Redox potentials and protonation and stability constants of CuIL species and new structural data. Inorg. Chem. 1992, 31, 191–198. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; McAuley, A. Synthesis of an N2S-cyclodecane macrocycle and its nickel(II) complex. J. Chem. Soc. Dalton Trans. 1992, 2967–2970. [Google Scholar] [CrossRef]
- Blake, A.J.; Reid, G.; Schroder, M. Synthesis and electrochemistry of nickel and cobalt complexes of mixed thia-aza crown ethers: single-crystal structures of [Ni([18]aneN2S4)][PF6]2·0.33H2O and [CO([18]aneN2S4)][PF6]3·3H2O ([18]aneN2S4=1,4,10,13-tetrathia-7,16-diazacyclooctadecane). J. Chem. Soc. Dalton Trans. 1994, 3291–3297. [Google Scholar]
- Dalley, N.K.; Larson, S.B.; Smith, J.S.; Matheson, K.L.; Izatt, R.M.; Christensen, J.J. The crystal structures of 10-oxa-1,4,7-trithiacyclododecane, 7,10,13-trioxa-1,4-dithiacyclopentadecane, 7,10,13,16-tetraoxa-1,4-dithiacyclooctadecane and 4,7,13,16-tetraoxa-1,10-dithiacycloocta-decane. J. Heterocycl. Chem. 1981, 18, 463–467. [Google Scholar] [CrossRef]
- Adhikary, B.; Lucas, C.R. Copper(II) Complexes of N2S3 Ligands Involving Aromatic Nitrogen and Thioether Donors and Having High Redox Potentials. Inorg. Chem. 1994, 33, 1376–1381. [Google Scholar] [CrossRef]
- Izatt, R.M.; Pawlak, K.; Bradshaw, J.S.; Bruening, R.L. Thermodynamic and kinetic data for macrocycle interaction with cations, anions, and neutral molecules. Chem. Rev. 1995, 95, 2529–2586. [Google Scholar] [CrossRef]
- Lucas, C.R.; Liang, W.; Miller, D.O.; Bridson, J.N. Metal complexes of 1-oxa-4,7-dithiacyclononane. Inorg. Chem. 1997, 36, 4508–4513. [Google Scholar] [CrossRef]
- Pedersen, C.J. Cyclic polyethers and their complexes with metal salts. J. Am. Chem. Soc. 1965, 89, 7017–7036. [Google Scholar] [CrossRef]
- Pedersen, C.J.; Frensdorff, H.K. Macrocyclic polyethers and their complexes. Angew. Chem. Int. Ed. Eng. 1972, 11, 16. [Google Scholar] [CrossRef]
- Vendilo, A.G.; Djigailo, D.I.; Smirnova, S.V.; Torocheshnikova, I.I.; Popov, K.I.; Krasovsky, V.G.; Pletnev, I.V. 18-Crown-6 and Dibenzo-18-crown-6 Assisted Extraction of Cesium from Water into Room Temperature Ionic Liquids and Its Correlation with Stability Constants for Cesium Complexes. Molecules 2009, 14, 5001–5016. [Google Scholar] [CrossRef]
- Hogen-Esch, T.E.; Smid, J. Conductivities and thermodynamics of dissociation of fluorenyl salts and their complexes with dimethyl-dibenzo-18-crown-6. J. Phys. Chem. 1975, 79, 233–238. [Google Scholar] [CrossRef]
- Hopkins, H.P.; Norman, A.B. Conductance and infrared studies on acetonitrile solutions containing crown ethers and alkali metal salts. J. Phys. Chem. 1980, 84, 309–314. [Google Scholar] [CrossRef]
- Evans, D.F.; Wellington, S.L.; Nads, J.A.; Cussler, E.L. The conductance of cyclic polyether-cation complexes. J. Solution Chem. 1972, 1, 499–506. [Google Scholar] [CrossRef]
- Çiçek, B.; Çakır, Ü.; Erk, Ç. The determination of crown-cation complexation behavior in dioxane/water mixtures by conductometric studies. Polym. Adv. Tecnol. 1998, 9, 831–836. [Google Scholar] [CrossRef]
- Rossotti, F.J.C.; Rossotti, H. The Determination of Stability Constants and Other Equilibrium Constants in Solution; McGraw-Hill Book Company, Inc.: New York, NY, USA, 1961. [Google Scholar]
- Inoue, Y.; Hakushi, T.; Liv, Y. Action binding by macrocycles. In Conductometric Behavior of Cation-Macrocycle Complexes in Solutions; Takeda, Y., Ed.; Marcel Dekker: New York, NY, USA, 1990; pp. 133–178. [Google Scholar]
- Cicek, B.; Cakir, U.; Azizoglu, A. The associations of macrocyclic ethers with cations in 1,4-dioxane/ water mixtures; potentiometric Na+ and K+ binding measurements and computational study. J. Incl. Phenom. Macrocycl. Chem. 2011. [Google Scholar] [CrossRef]
- Lind, J.E.; Zwolnikand, J.J.; Fuoss, R.M. Calibration of conductance cells at 25° with aqueous solutions of potassium Chloride. J. Am. Chem. Soc. 1959, 81, 1557–1559. [Google Scholar] [CrossRef]
- Ugras, H.I.; Cakir, U.; Azizoglu, A.; Kılıc, T.; Erk, C. Experimental, theoretical and biological activity study on the acyl substituted benzo-18-crown-6, dibenzo-18-crown-6, and dibenzo-24-crown-8. J. Incl. Phenom. Macrocycl. Chem. 2006, 55, 159–165. [Google Scholar] [CrossRef]
- Toman, P.; Makrlik, E.; Vanura, P. A combined experimental and theoretical study on the complexation of the ammonium ion with benzo-18-crown-6. Monatsh. Chem. 2010, 141, 301–304. [Google Scholar] [CrossRef]
- Hancock, R. Molecular mechanics calculations and metal recognation. Acc. Chem. Res. 1990, 23, 253–257. [Google Scholar] [CrossRef]
- Shargh, D.N.; Hosseini, M.M.; Ohaninan, T. Configurational and Conformational Properties of 1,3,7,9-Tetraphospha-Cyclododeca-1,2,7,8- tetraene: An Ab Initio Study and NBO Analysis. Phosphorus Sulfur. 2008, 183, 2410–2420. [Google Scholar] [CrossRef]
- Azizoglu, A.; Yildiz, C.B. Ring-opening mechanism of lithium bromosilacyclopropylidenoids to silaallenes. Organometallics 2010, 29, 6739–6743. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, X.; Yi, S.; Wang, N.; Peng, Y. DFT study of the carbon- and nitrogen-pivot lariat crown ethers and their complexes with alkali metal cations: Na+, K+. J. Comp. Chem. 2009, 30, 2674–2683. [Google Scholar] [CrossRef]
- Yuan, M.; Xueye, W.; Xin, J.; Ling, Y.; Cuihuan, R. DFT study for a seria of less-symmetrical crown ethers and their complexes with alkali metal cations. Chin. J. Chem. 2010, 28, 1835–1843. [Google Scholar] [CrossRef]
- Makrlik, E.; Toman, P.; Vanura, P. A combined extraction and DFT study on the complexation of the silver cation with dibenzo-18-crown-6. Monatsh. Chem. 2011, 142, 137–140. [Google Scholar] [CrossRef]
- Didier, S.; Gaudel-Siri, A.; Pons, J.M.; Liotard, D.; Rajzmann, M. Reaction mechanism studies made simple using simulated annealing. Potential energy surface exploration. J. Mol. Struct. (Theochem) 2002, 588, 71–78. [Google Scholar] [CrossRef]
- Khohlova, S.S.; Lebedev, N.G.; Bondarev, S.L.; Knyukshto, V.N.; Turban, A.A.; Mikhailova, V.A.; Ivanov, A.I. Electronic structure of laser dye DCM and its derivatives. Int. J. Quant. Chem. 2005, 104, 189–196. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, version C02; Gaussian, Inc.: Pittsburgh, PA, USA, 2003. [Google Scholar]
- Azizoglu, A.; Demirkol, O.; Kilic, T.; Yildiz, Y.K. Incorporation of an allene unit into 1,4-dihydronapthalene: Generation of 1,2-benzo-1,4,5-cycloheptatriene and its dimerization. Tetrahedron 2007, 63, 2409–2413. [Google Scholar] [CrossRef]
- Kilbas, B.; Azizoglu, A.; Balci, M. Edo- and exo-configured cyclopropylidenes incorporated into the norbornadiene skeleton: Generation, rearrangement to allenes, and the effect of remote substituents on carbene stability. J. Org. Chem. 2009, 74, 7075–7083. [Google Scholar] [CrossRef]
- Kurtaran, R.; Odabaşıoğlu, S.; Azizoğlu, A.; Kara, H.; Atakol, O. Experimental and computational study on [2,6-bis(3,5-dimethyl-n-pyrazoyl)pyridine]- (dithiocyanato)mercury (II). Polyhedron 2007, 26, 5069–5074. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. the role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the colle-salvetti correlation- energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar]
- Fukui, K.; Yonezawa, T.; Shingu, H. A molecular orbital theory of reactivity in aromatic hydrocarbons. J. Chem. Phys. 1952, 20, 722–725. [Google Scholar] [CrossRef]
- Fleming, I. Frontier Orbitals and Organic Chemical Reactions; Wiley: London, UK, 1976. [Google Scholar]
- Azizoglu, A. Quantum chemical ınvestigation of monostanna[n]cyclacenes. Struct. Chem. 2003, 14, 575–580. [Google Scholar] [CrossRef]
- Odabasioglu, S.; Kurtaran, R.; Azizoglu, A.; Kara, H.; Oz, S.; Atakol, O. Experimental and computational study on [2, 6-bis(3, 5-dimethyl-N-pyrazolyl) pyridine]-(dithiocyanato)mercury (II). Cent. Eur. J. Chem. 2009, 7, 402–409. [Google Scholar] [CrossRef]
- Turker, L.; Azizoglu, A. The Effect of boron substitution on cyclacenes. J. Mol. Struct. (Theochem) 2001, 535, 151–157. [Google Scholar] [CrossRef]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
