Mn(III)-Initiated Facile Oxygenation of Heterocyclic 1,3-Dicarbonyl Compounds
Abstract
:1. Introduction

2. Results and Discussion
2.1. Aerobic Oxidation of 4-Alkyl-substituted Pyrazolidine-3,5-diones 1a–h

| Entry | 1 | Oxidant | 1:oxidant b | Time/h | Product/yield (%) c |
|---|---|---|---|---|---|
| 1 | 1a | Mn(OAc)3 | 1:0.1 | 2 | 2a (95) |
| 2 | 1b | Mn(OAc)3 | 1:0.1 | 2 | 2b (95) |
| 3 | 1c | Mn(OAc)3 | 1:0.1 | 2 | 2c (99) |
| 4 | 1d | none | - | 3 | Recovery of 1d (100) |
| 5 | 1d | Cu(OAc)2 | 1:0.2 | 15 | Recovery of 1d (94) |
| 6 d | 1d | CAN e | 1:0.1 | 1 | 2d (40) |
| 7 | 1d | Mn(OAc)3 | 1:0.1 | 2 | 2d (97) |
| 8 f | 1d | Mn(OAc)3 | 1:0.1 | 2 | 2d (96) |
| 9 | 1e | Mn(OAc)3 | 1:0.1 | 2 | 2e (99) |
| 10 | 1f | Mn(OAc)3 | 1:0.1 | 10 min | 2f (95) |
| 11 | 1g | Mn(OAc)3 | 1:0.1 | 2 | 2g (97) |
| 12 | 1h | Mn(OAc)3 | 1:0.1 | 2 | 2h (99) |
2.2. Aerobic Oxidation of 3-Alkyl-substituted Pyrrolidine-2,4-diones 3a–p
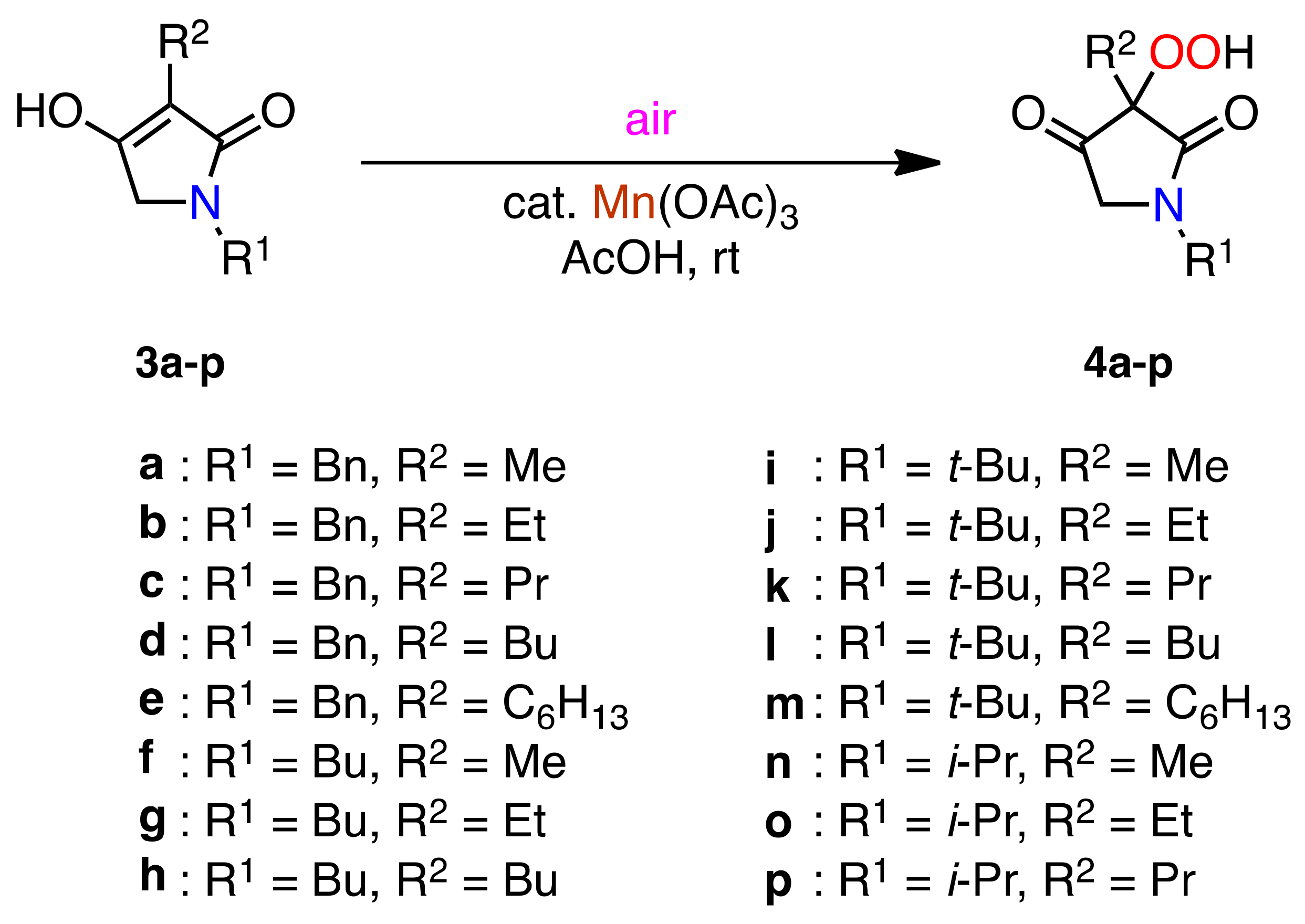
| Entry | 3 | 3:Mn(OAc)3 b | Time/h | Product/yield (%) c |
|---|---|---|---|---|
| 1 | 3a | 1:1 | 2 | 4a (65) |
| 2 | 3a | 1:0.1 | 14 | 4a (68) |
| 3 | 3a | 1:0.1 | 2 | 4a (94) |
| 4 | 3b | 1:0.1 | 2 | 4b (95) |
| 5 | 3c | 1:0.1 | 2 | 4c (98) |
| 6 | 3d | 1:0.1 | 2 | 4d (93) |
| 7 | 3e | 1:0.1 | 2 | 4e (96) |
| 8 | 3f | 1:0.1 | 1.5 | 4f (91) |
| 9 | 3g | 1:0.1 | 1.5 | 4g (90) |
| 10 | 3h | 1:0.1 | 1.5 | 4h (95) |
| 11 | 3i | 1:0.1 | 1.5 | 4i (90) |
| 12 | 3j | 1:0.1 | 1.5 | 4j (96) |
| 13 | 3k | 1:0.1 | 1.5 | 4k (99) |
| 14 | 3l | 1:0.1 | 1.5 | 4l (99) |
| 15 | 3m | 1:0.1 | 1.5 | 4m (98) |
| 16 | 3n | 1:0.1 | 1.5 | 4n (95) |
| 17 | 3o | 1:0.1 | 1.5 | 4o (97) |
| 18 | 3p | 1:0.1 | 1.5 | 4p (98) |
2.3. Aerobic Oxidation of 3-Alkyl-1,5-dimethylbarbituric Acids 5a–e and 3-Butyl-4-hydroxy-2-quinolinone (7)

| Entry | Amide | Amide:Mn(OAc)3 b | Time/h | Product/yield (%) c |
|---|---|---|---|---|
| 1 | 5a | 1:0.1 | 4 | 6a (80) |
| 2 | 5b | 1:0.1 | 2 | 6b (90) |
| 3 | 5c | 1:0.1 | 4 | 6c (94) |
| 4 | 5d | 1:0.1 | 4 | 6d (94) |
| 5 | 5e | 1:0.1 | 2 | 6e (88) |
| 6 | 7 | 1:0.1 | 2 | 8 (57) |
2.4. Mechanism for the Formation of Hydroperoxides 2, 4, 6, and 8

2.5. Conversion of the Hydroperoxides 2 and 6 into the Alcohols 9 and 10

| Entry | Amide | Catalyst | 1:Catalyst b | Solvent | Time/h | Product/yield (%) c | Recovery/% | |
|---|---|---|---|---|---|---|---|---|
| 1 | 1d | Mn(OAc)3 | 1:0.1 | AcOH | 2 | 2d (97) | ||
| 2 | 1d | Mn(OAc)3 | 1:0.1 | AcOH | 12 | 2d (87) | 9d (8) | |
| 3 | 1d | Mn(OAc)3 | 1:0.2 | AcOH | 27 | 2d (52) | 9d (46) | |
| 4 | 1d | Mn(OAc)3 | 1:0.28 | EtOH | 3 | 2d (54) | 9d (41) | |
| 5 | 1d | CANd | 1:1 | MeOH | 1 | 2d (35) | 9d (55) | |
| 6 | 2d | none | AcOH | 23 | 100 | |||
| 7 | 2d | Mn(OAc)3 | 1:0.01 | AcOH | 23 | 9d (47) | 32 | |
| 8e | 2d | Mn(OAc)2 | 1:1 | AcOH | 14 | 9d (25) | 66 | |
| 9 | 2d | CANd | 1:1 | MeOH | 2 | 9d (47) | 33 | |
| 10 | 2c | Mn(OAc)3 | 1:0.01 | AcOH | 17 | 9c (45) | 48 | |
| 11 | 2g | Mn(OAc)3 | 1:0.01 | AcOH | 17 | 9g (40) | 28 | |
| 12 | 5b | Mn(OAc)3 | 1:0.1 | AcOH | 3 | 6b (88) | 10b (7) | |
| 13 | 5b | Mn(OAc)3 | 1:0.1 | AcOH | 5 | 6b (79) | 10b (18) | |
| 14 | 5b | CAN d | 1:1 | MeOH | 0.5 | 6b (47) | 10b (43) | |
| 15 | 6b | none | AcOH | 12 | 100 | |||
| 16 | 6b | Mn(OAc)3 | 1:0.1 | AcOH | 14 | 10b (14) | 88 | |
| 17 | 6b | Mn(OAc)3 | 1:0.4 | AcOH | 23 | 10b (40) | 50 | |

2.6. Application of the Mn(III)-Mediated Oxygenation

| Entry | ROOH | ROOH:Ph3P | Time/h | Product/Yield (%) b |
|---|---|---|---|---|
| 1 | 2a | 1:1 | 3 | 9a (96) |
| 2 | 2b | 1:1 | 3 | 9b (97) |
| 3 | 2c | 1:1 | 3 | 9c (98) |
| 4 | 2d | 1:1 | 3 | 9d (97) |
| 5 | 6b | 1:1 | 3 | 10b (80) |
2.7. Structural Determination of the Hydroperoxides and the Hydroxyl Derivatives

| Hydroperoxide | 13C-OOH/ppm | Alcohol | 13C-OH/ppm | ||
|---|---|---|---|---|---|
| 2a |  | 81.6 | 9a |  | 71.1 |
| 2b |  | 85.8 | 9b |  | 74.7 |
| 2c |  | 85.3 | 9c |  | 74.0 |
| 2d |  | 87.6 | 9d |  | 76.3 |
| 2g |  | 86.7 | 9g |  | 75.8 |
| 6b |  | 87.2 | 10b |  | 76.6 |
3. Experimental
3.1. Measurements
3.2. Materials
3.3. Mn(III)-Initiated Aerobic Oxidation of Heterocyclic 1,3-Dicarbonyl Compounds 1a–h, 3a–p, 5a–e, and 7
3.4. Conversion of the Hydroperoxides 2 and 6 into the Alcohols 9 and 10
3.5. Aerobic Oxidation of 1d, 2d, and 5b Using Ammonium Cerium (IV) Nitrate (CAN)
3.6. Mn(III)-Initiated Aerobic Oxidation of Acyclic Amides 11a,b, ketones 13b, and Esters 13b,15
4. Conclusions
Supplementary Materials
Acknowledgements
References and Notes
- Chen, B.-C.; Zhou, P.; Davis, F.A.; Ciganek, E. a-Hydroxylation of enolates and silyl enol ethers. Org. React. 2003, 62, 1–356. [Google Scholar]
- Vedej, E.; Engler, D.A.; Telschow, J.E. Transition-metal peroxide reactions. Synthesis of α-hydroxycarbonyl compounds from enolates. J. Org. Chem. 1978, 43, 188–196, and references cited therein. [Google Scholar] [CrossRef]
- Abu-Omar, M.M.; Espenson, J.H. Oxidations of cyclic β-diketones catalyzed by methylrhenium trioxide. Organometallics 1996, 15, 3543–3549. [Google Scholar] [CrossRef]
- Heathcock, C.H.; Mahain, C.; Schlecht, M.F.; Utawanit, T. A synthetic approach to the quassinoids. J. Org. Chem. 1984, 49, 3264–3274. [Google Scholar] [CrossRef]
- Davis, F.A.; Clark, C.; Kumar, A.; Chen, B.-C. Asymmetric synthesis of the AB ring segments of daunomycin and 4-demethoxydaunomycin. J. Org. Chem. 1994, 59, 1184–1190. [Google Scholar] [CrossRef]
- Davis, F.A.; Liu, H.; Chen, B.-C.; Zhou, P. Oxidation of 1,3-dicarbonyl compounds using (camphorylsulfonyl)oxaziridines. Tetrahedron 1998, 54, 10481–10492. [Google Scholar] [CrossRef]
- Zhang, W.; Watanabe, K.; Cai, X.; Jung, M.E.; Tang, Y.; Zhan, J. Identifying the minimal enzymes required for anhydrotetracycline biosynthesis. J. Am. Chem. Soc. 2008, 130, 6068–6069. [Google Scholar]
- Giovanelli, E.; Leroux, S.; Moisan, L.; Carreyre, H.; Thuéry, P.; Buisson, D.-A.; Meddour, A.; Coustard, J.-M.; Thibaudeau, S.; Rousseau, B.; et al. On the elucidation of the mechanism of Vinca alkaloid fluorination in superacidic medium. Org. Lett. 2011, 13, 4116–4119. [Google Scholar]
- Büchi, G.; Matsumoto, K.E; Nishimura, H. Total synthesis of (±)-vindorosine. J. Am. Chem. Soc. 1971, 93, 3299–3301. [Google Scholar] [CrossRef]
- Andriamialisoa, R.Z.; Langlois, N.; Langlois, Y. A new efficient total synthesis of vindorosine and vindoline. J. Org. Chem. 1985, 50, 961–967. [Google Scholar] [CrossRef]
- Sasaki, Y.; Kato, D.; Boger, D.L. Asymmetric total synthesis of vindorosine, vindoline, and key vinblastine analogues. J. Am. Chem. Soc. 2010, 132, 13533–13544. [Google Scholar] [CrossRef]
- Rannoux, C.; Roussi, F.; Martin, M.-T.; Guéritte, F. Elaboration of vinblastine hybrids using a reactive in situ generated N-carboxyanhydride. Org. Biomol. Chem. 2011, 9, 4873–4881. [Google Scholar] [CrossRef]
- Zhu, J.; Klunder, J.H.; Zwanenburg, B. Stereospecific total synthesis of (−)-kjellmanianone and a revision of its absolute configuration. Tetrahedron Lett. 1994, 35, 2787–2790. [Google Scholar] [CrossRef]
- Charest, M.G.; Lerner, C.D.; Brubaker, J.D.; Siegel, D.R.; Myers, A.G. A convergent enantioselective route to structurally diverse 6-deoxytetracycline antibiotics. Science 2005, 308, 395–398. [Google Scholar] [CrossRef]
- Mark, G.; Charest, M.G.; Siegel, D.R.; Myers, A.G. Synthesis of (−)-tetracycline. J. Am. Chem. Soc. 2005, 127, 8292–8293. [Google Scholar] [CrossRef]
- Reed, G.A.; Griffin, I.O.; Eling, T.E. Inactivation of prostaglandin H synthase and prostacyclin synthase by phenylbutazone. Requirement for peroxidative metabolism. Mol. Pharmacol. 1985, 27, 109–114. [Google Scholar]
- Vennerstorm, J.L.; Holmes, T.J., Jr. Preparation and evaluation of electrophilic derivatives of phenylbutazone as inhibitors of prostaglandin-H-synthase. J. Med. Chem. 1987, 30, 563–567. [Google Scholar] [CrossRef]
- Mentz, V.P.; Schulz, M.; Kluge, R. Chemische darstellung von phenylbutazon-hydroperoxid und prüfung der substanz auf herzwirksamkeit unter in-vitro- und in-vivo-bedingungen. Arzneim.-Frosch. 1987, 37, 1229–1232. [Google Scholar]
- Rechenberg, H.K.V. Phenylbutazone, 2nd ed; Edward Arnold Ltd.: London, UK, 1962. [Google Scholar]
- Chauhan, S.M.S.; Srinivas, K.A.; Mohapatra, P.P. Oxidation of phenylbutazone with hydrogen peroxide catalyzed by 5,10,15,20-tetraarylporphyrinoiron(III) chlorides in dichloromethane. Ind. J. Chem 1999, 31B, 724–725, and references cited therein. [Google Scholar]
- Demir, A.S.; Jeganathan, A. Selective oxidation of α,β-unsaturated ketones at the α′-position. Synthesis 1992, 235–247. [Google Scholar]
- Vedejs, E.; Engler, D.A.; Telschow, J.E. Transition-metal peroxide reactions. Synthesis of α-hydroxycarbonyl compounds from enolates. J. Org. Chem 1978, 43, 188–196. [Google Scholar] [CrossRef]
- Hubert, A.J.; Starcher, P.S. The Baeyer-Villiger oxidation of alkyl oxocyclohexanecarboxylates. J. Chem. Soc. C 1968, 2500–2502. [Google Scholar]
- Adam, W.; Smerz, A.K. Nickel-catalyzed hydroxylation of 1,3-dicarbonyl compounds by dimethyldioxirane. Tetrahedron 1996, 52, 5799–5804. [Google Scholar] [CrossRef]
- Christoffers, J. Novel manganese-catalyzed α-oxidation of cyclic β-keto esters with molecular oxygen. J. Org. Chem. 1999, 64, 7668–7669. [Google Scholar] [CrossRef]
- Christoffers, J.; Wenner, T. Cerium-catalyzed α-oxidation of β-dicarbonyl compounds with molecular oxygen. SynLett 2002, 119–121. [Google Scholar] [CrossRef]
- Christoffers, J.; Werner, T.; Unger, S.; Frey, W. Preparation of acyloins by cerium-catalyzed, direct hydroxylation of β-dicarbonyl compounds with molecular oxygen. Eur. J. Org. Chem. 2003, 425–431. [Google Scholar]
- Baucherel, X.; Levoirier, E.; Uziel, J.; Juge, S. Monohydroxylation of cyclic and acyclic β-keto esters with molecular oxygen catalyzed by cobalt(II) chloride in neutral conditions. Tetrahedron Lett. 2000, 41, 1385–1387. [Google Scholar] [CrossRef]
- Nair, V.; Nair, L.G.; Mathew, J. Cerium(IV) mediated oxygenation of dialkyl malonates: A novel synthesis of tartronic acid derivatives. Tetrahedron Lett. 1998, 39, 2801–2804. [Google Scholar] [CrossRef]
- Watanabe, T.; Ishikawa, T. Mild air-oxidation of 1,3-dicarbonyl compounds with cesium salts: Novel α-hydroxylation accompanied by partial hydrolysis of malonate derivatives. Tetrahedron Lett. 1999, 40, 7795–7798. [Google Scholar] [CrossRef]
- Nishino, H. The facile synthesis of dihydrofurans by the oxidation of olefins with tris(2,4-pentanedionato)manganese(III). Bull. Chem. Soc. Jpn. 1985, 58, 1922–1927. [Google Scholar] [CrossRef]
- Tategami, S.; Yamada, T.; Nishino, H.; Korp, J.D.; Kurosawa, K. Formation of 1,2-dioxacyclohexanes by the reaction of alkenes with tris(2,4-pentanedionato)manganese(III) or with manganese(III) acetate. Tetrahedron Lett. 1990, 31, 6371–6374. [Google Scholar] [CrossRef]
- Nishino, H.; Tategami, S.; Yamada, T.; Korp, J.D.; Kurosawa, K. Formation of 1, 2-dioxanes by the use of tris (2,4-pentanedionato)-manganese (III) or manganese (III) acetate. Bull. Chem. Soc. Jpn. 1991, 64, 1800–1809. [Google Scholar] [CrossRef]
- Asahi, K.; Nishino, H. Facile endoperoxypropellane synthesis by manganese(III) acetate-mediated aerobic oxidation. Eur. J. Org. Chem. 2008, 2404–2416. [Google Scholar] [CrossRef]
- Tsubusaki, T.; Nishino, H. Formation of 1,2-dioxolanes using Mn(III)-based reaction of various arylacetylenes with 2,4-pentanedione and related reaction. Tetrahedron 2009, 65, 3745–3752. [Google Scholar] [CrossRef]
- Haque, M.A.; Nishino, H. Synthesis of peroxylactones using Mn(III)-catalyzed aerobic oxidation. Heterocycles 2011, 83, 1783–1805. [Google Scholar] [CrossRef]
- Qian, C.-Y.; Nishino, H.; Kurosawa, K. Manganese(II) acetate-mediated double 2-hydroperoxyalkylations of barbituric acid and its derivatives. J. Org. Chem. 1993, 58, 4448–4451. [Google Scholar] [CrossRef]
- Rahman, M.T.; Nishino, H.; Qian, C.-Y. Synthesis of 4,4-bis(2-hydroperoxyalkyl)pyrazolidine-3,5-diones using manganese(III)-catalyzed autoxidation. Tetrahedron Lett. 2003, 44, 5225–5228. [Google Scholar] [CrossRef]
- Rahman, M.T.; Nishino, H. Manganese(III)-based oxidation of 1,2-disubstituted pyrzolidine-3,5-diones in the presence of alkenes. Tetrahedron 2003, 59, 8383–8392. [Google Scholar] [CrossRef]
- Kumabe, R.; Nishino, H. A unique peroxide formation based on the Mn(III)-catalyzed aerobic oxidation. Tetrahedron Lett. 2004, 45, 703–706. [Google Scholar] [CrossRef]
- Haque, M.A.; Nishino, H. Synthesis of peroxylactones using Mn(III)-catalyzed aerobic oxidation. Heterocycles 2011, 83, 1783–1805. [Google Scholar] [CrossRef]
- Rahman, M.T.; Nishino, H. Manganese(III)-catalyzed facile direct hydroperoxidation of some heterocyclic 1,3-dicarbonyl compounds. Org. Lett. 2003, 5, 2887–2890. [Google Scholar] [CrossRef]
- Fugger, J.; Tien, J.M.; Hunsberger, I.M. The preparation of substituted hydrazines. I. Alkylhydrazines via alkylsydnone. J. Am. Chem. Soc. 1955, 77, 1843–1848. [Google Scholar] [CrossRef]
- King, J.A.; McMillan, F.H. The preparation of some α-benzylamino-β,β-dialkoxypropionic acid derivatives. J. Am. Chem. Soc. 1950, 72, 1236–1240. [Google Scholar] [CrossRef]
- Koech, P.K.; Krische, M.J. Catalytic addition of metallo-aldehyde enolates to ketones: A new C−C bond-forming hydrogenation. Org. Lett. 2004, 6, 691–694. [Google Scholar] [CrossRef]
- Speziale, A.J.; Jaworski, E.G. N-Substituted glycinate and alaninate esters. J. Org. Chem. 1960, 25, 728–732. [Google Scholar] [CrossRef]
- Zhu, Y.; Zou, X.; Hu, F.; Yao, C.; Liu, B.; Yang, H. Synthesis and herbicidal evaluation of novel 3-[(α-Hydroxy-substituted)benzylidene]pyrrolidine-2,4-diones. J. Agric. Food Chem. 2005, 53, 9566–9570. [Google Scholar] [CrossRef]
- Chowdhury, F.A.; Nishino, H.; Kurosawa, K. Simple route to azabicyclic peroxides from tetramic acid derivatives usingmanganese(III)-based molecular oxygen trapping reaction. Heterocycles 1999, 51, 575–591. [Google Scholar] [CrossRef]
- Jursic, B.S.; Neumann, D.M. Reductive C-alkylation of barbituric acid derivatives with carbonyl compounds in the presence of platinum and palladium catalysts. Tetrahedron Lett. 2001, 42, 4103–4107. [Google Scholar] [CrossRef]
- Detsi, A.; Bardakos, V.; Markopoulos, J.; Igglessi-Markopoulou, O. Reactions of 2-methyl-3,1-benzoxazin-4-one with active methylene compounds: A new route to 3-substituted 4-hydroxyquinolin-2(1H)-ones. J. Chem. Soc. Perkin Trans. 1 1996, 2909–2913, and references cited therein. [Google Scholar]
- McQuaid, L.A.; Smith, E.C.; Lodge, D.; Pralong, E.; Wikel, J.H.; Calligaro, D.O.; O'Malley, P.J. 3-Phenyl-4-hydroxyquinolin-2(1H)-ones: Potent and selective antagonists at the strychnine-insensitive glycine site on the N-methyl-D-aspartate receptor complex. J. Med. Chem. 1992, 35, 3423–3425. [Google Scholar] [CrossRef]
- Klásek, A.; Mrkvicka, V.; Pevec, A.; Kosmrlj, J. Novel tandem hydration/cyclodehydration of α-thiocyanatoketones to 2-oxo-3-thiazolines. Application to thiazolo[5,4-c]quinoline-2,4(3aH,5H)-dione Synthesis. J. Org. Chem 2004, 69, 5646–5651. [Google Scholar] [CrossRef]
- Ohshima, T.; Sodeoka, M.; Shibasaki, M. Manganese(III)-based oxidative free-radical reaction of α-allyl-β-keto ester with molecular oxygen. Tetrahedron Lett. 1993, 34, 8509–8512. [Google Scholar]
- Lamarque, L.; Méou, A.; Brun, P. Mn(OAc)3-promoted hydroxylation of α-carbomethoxy-γ-lactones by molecular oxygen. Can. J. Chem. 2000, 78, 128–132. [Google Scholar]
- Lide, D.R. Handbook of Chemistry and Physics; CRC press: Boca Raton, FL, USA, 1994; pp. 22–23. [Google Scholar]
- Ling, K.-Q.; Lee, Y.; Macikenas, D.; Protasiewicz, J.D.; Sayre, L.M. Copper(II)-mediated autoxidation of tert-butylresorcinols. J. Org. Chem 2003, 68, 1358–1366, and references cited therein. [Google Scholar]
- Bredereck, H.; Bauer, G. Hydroperoxide cyclischer β-diketone. JustusLiebigs Ann. Chem. 1970, 739, 117–120. [Google Scholar] [CrossRef]
- Tona, M.; Guardiola, M.; Fajari, L.; Messeguer, A. A study on the mechanism and scope of the radical-mediated oxidation of arylacetoacetates. Tetrahedron 1995, 51, 10041–10052. [Google Scholar] [CrossRef]
- Nair, V.; Nair, L.G.; Mathew, S. Cerium(IV) mediated oxygenation of dialkyl malonates: A novel synthesis of tartronic acid derivatives. Tetrahedron Lett. 1998, 39, 2801–2804. [Google Scholar] [CrossRef]
- Neumann, B.; Müller, S.C.; Hauser, M.J.B.; Steinbock, O.; Simoyi, R.H.; Dalal, N.S. Identification and kinetics study of the peroxymalonyl radical in the aerobic oxidation of malonic acid by cerium(IV). J. Am. Chem. Soc. 1995, 117, 6372–6373. [Google Scholar]
- Yoshioka, M.; Nishioka, T.; Hasegawa, T. Dye-sensitized photooxidation of 6-acyl- and 6-carboalkoxybenzocycloalken-5-ones: Reaction of singlet oxygen with enolic 1,3-dicarbonyl compounds. J. Org. Chem. 1993, 58, 278–281. [Google Scholar] [CrossRef]
- Christoffers, J.; Wenner, T. Cerium-catalyzed α-oxidation of β-dicarbonyl compounds with molecular oxygen. SynLett 2002, 119–121. [Google Scholar] [CrossRef]
- Christoffers, J.; Werner, T.; Unger, S.; Frey, W. Preparation of acyloins by cerium-catalyzed, direct hydroxylation of β-dicarbonyl compounds with molecular oxygen. Eur. J. Org. Chem. 2003, 425–431. [Google Scholar]
- Ye, J.-H.; Xue, J.; Ling, K.-Q.; Xu, J.-H. Ceric ammonium nitrate (CAN) mediated novel dimerizations of 4-hydroxyquinolin-2(1H)-ones: The first example of reactions of oxygen-centered radicals from 1,3-dicarbonyl compounds. Tetrahedron Lett. 1999, 40, 1365–1368. [Google Scholar] [CrossRef]
- Hiatt, R.; Irwin, K.C. Homolytic decompositions of hydroperoxides. V. Thermal decompositions. J. Org. Chem. 1968, 33, 1436–1441. [Google Scholar] [CrossRef]
- Stannett, V.; Mesrobian, R.B. The kinetics of the decomposition of tertiary hydroperoxides in solvents. J. Am. Chem. Soc. 1950, 72, 4125–4130. [Google Scholar] [CrossRef]
- Boyaci, F.G.; Takaç, S.; Özdamar, T.H. Effects of process parameters on the kinetics of the decomposition of 2-isopropylnaphthalene hydroperoxide into 2-naphthol and acetone. Rev. Chem. Engl. 2000, 16, 249–299. [Google Scholar]
- Dannley, R.L.; Jalics, G. The decomposition of silyl hydroperoxides. J. Org. Chem. 1965, 30, 3848–3851. [Google Scholar] [CrossRef]
- Richardson, W.H. Metal ion decomposition of hydroperoxides. IV. Kinetics and products of copper salt catalyzed decomnposition of t-butyl hydroperxide. J. Am. Chem. Soc. 1966, 88, 975–979. [Google Scholar] [CrossRef]
- Sheldon, R.A.; Kochi, J.K. Metal Catalyzed Oxidations in Organic Chemistry; Academic Press: New York, NY, USA, 1981. [Google Scholar]
- Hirano, K.; Sakaguchi, S.; Ishii, Y. Radical addition of ethers to alkenes under dioxygen catalyzed by N-hydroxyphthalimide (NHPI)/Co(OAc)2. Tetrahedron Lett. 2002, 43, 3617–3620. [Google Scholar] [CrossRef]
- Olah, G.A.; Parker, D.G.; Yoneda, N.; Pelizza, F. Oxyfunctionalization of hydrocarbons. 1. Protolytic cleavage-rearrangement reactions of tertiary alkyl hydroperoxides with magic acid. J. Am. Chem. Soc. 1976, 98, 2245–2250. [Google Scholar] [CrossRef]
- Andrulis, P.J., Jr.; Dewar, M.J.S.; Dietz, R.; Hunt, R.L. Aromatic oxidation by electron transfer. I. Oxidations of p-methoxytoluene. J. Am. Chem. Soc. 1966, 88, 5473–5478. [Google Scholar] [CrossRef]
- Heiba, E.I.; Dessau, R.M.; Koehl, W.J., Jr. Oxidation by metal salts. III. Reaction of manganic acetate with aromatic hydrocarbons and the reactivity of the carboxymethyl radical. J. Am. Chem. Soc. 1969, 91, 138–145. [Google Scholar] [CrossRef]
- Fugger, J.; Tien, J.M.; Hunsberger, I.M. The preparation of substituted hydrazines. I. Alkylhydrazines via alkylsydnone. J. Am. Chem. Soc. 1955, 77, 1843–1848. [Google Scholar] [CrossRef]
- King, J.A.; McMillan, F.H. The preparation of some α-benzylamino-β,β-dialkoxypropionic acid derivatives. J. Am. Chem. Soc. 1950, 72, 1236–1240. [Google Scholar] [CrossRef]
- Koech, P.K.; Krische, M.J. Catalytic addition of metallo-aldehyde enolates to ketones: A new C−C bond-forming hydrogenation. Org. Lett. 2004, 6, 691–694. [Google Scholar] [CrossRef]
- Speziale, A.J.; Jaworski, E.G. N-Substituted glycinate and alaninate esters. J. Org. Chem. 1960, 25, 728–732. [Google Scholar] [CrossRef]
- Zhu, Y.; Zou, X.; Hu, F.; Yao, C.; Liu, B.; Yang, H. Synthesis and herbicidal evaluation of novel 3-[(α-hydroxy-substituted)benzylidene]pyrrolidine-2,4-diones. J. Agric. Food Chem. 2005, 53, 9566–9570. [Google Scholar]
- Lee, V.J.; Branfman, A.R.; Herrin, T.R.; Rinehart, K.L. Synthesis of 3-dienoyl tetramic acids related to streptolydigin and tirandamycin. J. Am. Chem. Soc. 1978, 100, 4225–4236. [Google Scholar] [CrossRef]
- Mallinger, A.; Nadal, B.; Chopin, N.; Gall, T.L. One-pot synthesis of 3-aryltetramic acids. Eur. J. Org. Chem. 2010, 1142–1148. [Google Scholar]
- Nemoto, H.; Kubota, Y.; Yamamoto, Y. Development of a new acyl anion equivalent for the preparation of masked activated esters, and their use to prepare a dipeptide. J. Org. Chem. 1990, 55, 4515–4516. [Google Scholar] [CrossRef]
- Martínez, R.; Clara-Sosa, A.; Apan, M.T.R. Synthesis and cytotoxic evaluation of new (4,5,6,7-tetrahydro-indol-1-yl)-3-R-propionic acids and propionic acid ethyl esters generated by molecular mimicry. Bioorg. Med. Chem. 2007, 15, 3912–3918. [Google Scholar] [CrossRef]
- Sample Availability: Samples are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rahman, M.T.; Haque, M.A.; Igarashi, H.; Nishino, H. Mn(III)-Initiated Facile Oxygenation of Heterocyclic 1,3-Dicarbonyl Compounds. Molecules 2011, 16, 9562-9581. https://doi.org/10.3390/molecules16119562
Rahman MT, Haque MA, Igarashi H, Nishino H. Mn(III)-Initiated Facile Oxygenation of Heterocyclic 1,3-Dicarbonyl Compounds. Molecules. 2011; 16(11):9562-9581. https://doi.org/10.3390/molecules16119562
Chicago/Turabian StyleRahman, Md. Taifur, Md. Aminul Haque, Hikaru Igarashi, and Hiroshi Nishino. 2011. "Mn(III)-Initiated Facile Oxygenation of Heterocyclic 1,3-Dicarbonyl Compounds" Molecules 16, no. 11: 9562-9581. https://doi.org/10.3390/molecules16119562
APA StyleRahman, M. T., Haque, M. A., Igarashi, H., & Nishino, H. (2011). Mn(III)-Initiated Facile Oxygenation of Heterocyclic 1,3-Dicarbonyl Compounds. Molecules, 16(11), 9562-9581. https://doi.org/10.3390/molecules16119562





