Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters
Abstract
:1. Introduction
2. Results and Discussion
3. Experimental
3.1. General
3.2. Procedure for the preparation of methyl-4-nitrobenzoate (1a)
3.3. Procedure for the preparation of 4-nitrobenzoic acid hydrazide (2a)
3.4. Procedure for the preparation of 5-(4-nitrophenyl)-1,3,4-oxadiazole-2-thiol (3a)
3.5. Procedure for the preparation of 5-(4-nitrophenyl)-4-amino-1,2,4-triazole-3-thiol (4a)
3.6. Procedure for the preparation of 5-(4-methoxybenzyl)-1,3,4-oxadiazole-2-ylnaphthalene-1-carbothioate (5a)
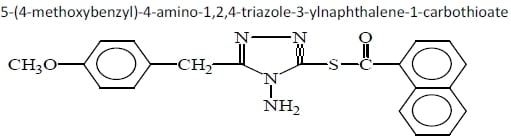
3.7. Preparation for the preparation of 5-(4-methoxybenzyl)-4-amino-1,2,4-triazole-3-ylnaphthalene-1-carbothioate (6a).
3.8. Antifungal evaluation
4. Conclusions
Acknowledgements
References
- Ikízler, A.; Gümüs, F.; Ozden, S.; Abbasoğlu, U. Biological activities of some 1,2,4-triazoles and 1,2,4-triazolin-5-ones. Pharmazie 1989, 44, 506–507. [Google Scholar] [PubMed]
- Vinícius, R.C.; Paula, A.A.; Helena, C.C.; Carlos, R.R.; Alessandro, K.J.; Vitor, F.F.; Maria, C.B.V.S.; Fernanda da, C.S.; Laura, A.M.; Thaisa, S.D.; Carla, C.; Eládio, F.S.; André, L.F.; Anna, C.C. Synthesis, biological, and theoretical evaluations of new 1,2,3-triazoles against the hemolytic profile of the Lachesis muta snake venom. Bioorg. Med.Chem. 2009, 17, 7429–7434. [Google Scholar]
- Prasad, A.; Ramalingam, R.J.; Rao, A.B.; Diwan, P.V.; Sattur, P.B. Synthesis and biological evaluation of 3-aryloxyalkyl-6-aryl-7H-s-triazolo[3,4-b][1,3,4]thiadiazines. Eur. J. Med. Chem. 1989, 24, 199–201. [Google Scholar] [CrossRef]
- El-masry, A.H.; Fahmy, H.H.; Ali, S.H.; Waheed, A. Synthesis and Antimicrobial Activity of Some New Benzimidazole Derivatives. Molecules 2000, 5, 1429–1438. [Google Scholar] [CrossRef]
- Orabi, A.S.; Moneim, M.A.; El-Din Salem, E.; El-Din Abdel-Fattah, M. Synthesis and Physicochemical Studies of Some Antimicrobial Active Triazole Metal Complexe. Polish J. Chem. 2000, 74, 1675–1683. [Google Scholar]
- Almasirad, A.; Tabatabai, S.A.; Faizi, M.; Kebriaeezadeh, A.; Mehrabi, N.; Dalvandi, A.; Shafiee, A. Synthesis and anticonvulsant activity of new 2-substituted-5- [2-(2-fluorophenoxy)phenyl]-1,3,4-oxadiazoles and 1,2,4-triazoles. Bioorg. Med. Chem Lett. 2004, 14, 6057–6059. [Google Scholar] [CrossRef] [PubMed]
- George, T.; Mehta, D.V.; Tahilramani, R.; Davvid, J.; Talwalker, P.K. Synthesis of some s-triazoles with potential analgesic and antiinflammatory activities. J. Med. Chem. 1971, 14, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Vivona, N.; Caronna, T. hotoinduced Molecular Rearrangements. The Photochemistry of Some 1,2,4-Oxadiazoles in the Presence of Nitrogen Nucleophiles. Formation of 1,2,4-Triazoles, Indazoles, and Benzimidazoles. J. Org. Chem. 1996, 61, 8397–8401. [Google Scholar] [CrossRef]
- Yoo, B.R.; Suk, M.Y.; Man, Y.; Hong, Y.S.G.; Jung, I.N. Synthesis and Fungicidal Activity of 1-[(1H-1, 2, 4-triazol-1-yl) alkyl]-1-silacyclohexanes. Bull. Korean Chem. Soc. 1998, 19, 358–362. [Google Scholar]
- Paulvannan, K.; Chen, T.; Hale, R. An Improved Synthesis of 1,2,4-Triazoles using Ag2CO3. Tetrahedron 2000, 56, 8071–8076. [Google Scholar] [CrossRef]
- Catarzi, D.; Colotta, V.; Varano, F.; Cecchi, L.; Filacchioni, G.; Galli, A.; Costagli, C.; Carla, V. 7-Chloro-4,5-dihydro-8-(1,2,4-triazol-4-yl)-4-oxo-1,2,4-triazolo[1,5-a] quinoxal. J. Med.Chem. 2000, 43, 3824–3826. [Google Scholar] [CrossRef]
- Batchelor, D.V.; Beal, D.M.; Brown, T.B.; Ellis, D.; Gordon, D.W.; Johnson, P.S.; Mason, H.J.; Ralph, M.J.; Underwood, T.J.; Wheeler, S. Synthesis of 3-N,N-Dialkylamino-1,2,4-triazoles from S-methylisothioureas and acyl hydrazides. Synlett 2008, 10, 2421–2424. [Google Scholar]
- Huntsman, H.; Balsells, J. New Method for the General Synthesis of [1,2,4]Triazolo[1,5-a]pyridines. Eur. J. Org. Chem. 2005, 20, 3761–3765. [Google Scholar] [CrossRef]
- Alan, R.; Katritzky, A.R.; Zhang, M.Q.D.F.G.; Griffith, M.C.; Karen Watson, K. Synthesis of 1,2,4-Triazole-Functionalized Solid Support and Its Use in the Solid-Phase Synthesis of Trisubstituted 1,2,4-Triazoles. Org. Lett. 1999, 1, 1189–1191. [Google Scholar]
- Hasan, A.; Gapil, S.; Khan, I. Synthesis, Characterization and Antifungal Activity of Some New 5-Substituted-1,3,4-oxadiazol- 2-Thiol. Asian J. Chem. 2010. In Press. [Google Scholar]
- Hasan, A.; Gapil, S.; Khan, I. Synthesis and characterization of some new 5-substituted-4-amino-1,2,4-triazole -3-thiols. J. Heterocycl. Chem. 2010. In Press. [Google Scholar]
- Choudhary, M.I.; Dur-e-Shahwar, Z.; Parveen, A.; Jabbar, I.A.; Atta-ur-Rahman. Antifungal steroidal lactones from Withania coagulance. Phytochemistry 1995, 40, 1243–1246. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds are available from the authors. |



| Comp. | Protons [1H-δ(ppm), Multiplicity, J(Hz)] | Other protons | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ar–H | NH2 | ||||||||||
| H-1 | H-2 | H-3 | H-4 | H-1′ | H-2′ | H-3′ | H-4′ | H-5′ | |||
| 6a | 7.72 1H,dd 1.2,4.6 | 8.03 1H,dd 2.5,7.2 | 8.01 1H,dd 2.4,7.1 | 7.73 1H,dd 2.3,4.4 | 7.76 1H,m | 7.42 1H,m | 7.90 1H,m | 7.88 1H,m | 7.55 1H,m | 4.32 2H,s | – |
| 6b | 7.60 1H,dd 3.1,4.2 | 7.77 1H,dd 3.2,4.1 | 7.04 1H,dd 2.5,6.6 | 7.45 1H,dd 4.2,3.1 | 7.88 1H,m | 7.79 1H,m | 7.62 1H,m | 8.25 1H,m | 7.21 1H,m | 3.95 2H,s | – |
| 6c | 7.48 1H,m | 7.32 1H,m | 7.22 1H,m | 7.97 1H,m | 7.65 1H,dd 3.1,6.7 | 7.591H,dd1H,m | 7.69 1H,dd 2.5,4.7 | 7.98 1H,dd 3.1,6.8 | 7.45 1H,dd 2.8,6.5 | 4.122H,s | – |
| 6d | 7.12 1H,m | 7.22 1H,dd 2.4,6.7 | 7.08 1H,m | 7.22 1H,dd 2.4,6.7 | – | – | – | – | – | 4.33 2H,s | 2.88 4H,t 3.2 2H,t 2.1,1.5 10H,m |
| 6e | 7.14 1H,m | 7.47 1H,m | 7.12 1H,m | 7.67 1H,m | 7.97 1H,dd 2.4,6.5 | 7.58 1H,dd 3.1,4.5 | 7.77 1H,m | 7.67 1H,m | – | 4.16 2H,s | 2.90 4H,t 2.8 |
| 6f | 7.47 1H,dd 3.2,6.2 | 7.27 1H,dd 2.1,7.2 | 6.97 1H,dd 3.2,7.3 | 7.47 1H,dd 3.4,6.2 | – | – | – | – | – | 3.94 2H,s | 2.50 2H,t 3.2,1.52 10H,m |
| 6g | 6.50 1H,m | 6.87 1H,m | 6.50 1H,m | 6.87 1H,m | 7.76 1H,dd 2.4,6.2 | 7.42 1H,dd 3.5,6.5 | 7.73 1H,m | 7.46 1H,m | 7.55 1H,m | 3.83 2H,s | 2.50 2H,s 3.56 3H,s |
| 6h | 6.52 1H,m | 6.77 1H,m | 7.14 1H,m | 7.02 1H,m | – | – | – | – | – | 4.45 2H,s | 2.5 2H,t 3.2,1.60 12H,m |
| 6i | 7.12 1H,dd 2.3,6.4 | 7.27 1H,dd 3.2,7.4 | 7.12 1H,dd 2.3,6.4 | 7.77 1H,m | 7.67 1H,m | 7.85 1H,m | 7.58 1H,m | 7.82 1H,m | 7.25 1H,m | 4.26 2H,s | – |
| Carbon No. | 13C-δ(ppm), Compounds | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 6a | 6b | 6c | 6d | 6e | 6f | 6g | 6h | 6i | |
| 1 | 129.41 | 128.40 | 130.05 | 28.36 | 30.33 | 130.06 | 30.60 | 130.46 | 133.06 |
| 2 | 128.40 | 127.41 | 126.41 | 30.18 | 32.71 | 127.25 | 55.90 | 125.34 | 127.45 |
| 3 | 116.52 | 155.50 | 116.24 | 127.21 | 130.43 | 152.44 | 130.02 | 116.73 | 153.44 |
| 4 | 115.52 | 116.62 | 115.24 | 126.33 | 118.23 | 127.25 | 127.05 | 118.62 | 125.21 |
| 5 | 116.31 | 118.62 | 116.37 | 127.41 | 117.23 | 126.31 | 116.65 | 116.73 | 127.40 |
| 6 | 130.43 | 127.42 | 126.21 | 116.24 | 115.48 | 117.21 | 158.03 | 125.23 | 117.67 |
| 7 | 160.42 | 162.61 | 166.04 | 118.44 | 118.23 | 161.37 | 166.04 | 159.68 | 163.23 |
| 8 | 171.40 | 174.65 | 176.23 | 116.26 | 125.63 | 170.68 | 175.10 | 168.77 | 177.45 |
| 9 | 182.31 | 180.37 | 184.32 | 166.23 | 159.21 | 182.04 | 180.01 | 184.66 | 181.08 |
| 10 | 135.61 | 136.23 | 134.05 | 175.90 | 170.62 | 54.34 | 116.65 | 53.62 | 136.38 |
| 11 | 127.12 | 129.12 | 130.73 | 182.64 | 183.47 | 44.31 | 127.05 | 44.02 | 127.21 |
| 12 | 120.43 | 122.31 | 126.32 | 45.46 | 134.24 | 33.39 | 134.50 | 34.71 | 116.90 |
| 13 | 122.41 | 125.71 | 125.61 | 33.67 | 128.40 | 24.36 | 127.12 | 29.01 | 126.64 |
| 14 | 125.32 | 128.81 | 126.67 | 26.34 | 129.65 | 25.62 | 125.62 | 25.72 | 128.03 |
| 15 | 126.32 | 127.50 | 130.73 | 25.80 | 126.31 | 20.22 | 120.50 | 25.69 | 125.64 |
| 16 | 128.43 | 130.64 | – | 20.21 | 130.52 | – | 119.42 | 20.02 | – |
| 17 | 129.42 | 132.80 | – | 18.44 | 127.43 | – | 129.31 | 18.33 | – |
| 18 | 130.21 | 126.21 | – | 22.37 | – | – | 131.61 | – | – |
| 19 | 129.66 | 129.44 | – | – | – | – | 130.71 | – | – |
| 20 | – | – | – | – | – | – | 123.63 | – | – |
| Sample No. | Structure of 5-substituted-4-amino-1,2,4-triazole-thioester | Fungal strains/ Inhibition (%) | |||
|---|---|---|---|---|---|
| Aspergillus flavus | Mucor species | Aspergillus niger | Aspergillus flavus | ||
| 6a |  | 96.22 | 87.66 | 74.79 | 97.20 |
| 6b |  | 84.12 | 100.00 | 81.20 | 100.00 |
| 6c |  | 69.00 | 80.90 | 75.20 | 87.00 |
| 6d |  | 81.00 | 78.36 | 59.19 | 93.60 |
| 6e |  | 71.00 | 45.89 | 69.99 | 73.00 |
| 6f |  | 27.00 | 15.58 | 24.39 | 75.00 |
| 6g |  | 100.00 | 88.64 | 98.20 | 88.00 |
| 6h |  | 54.00 | 21.22 | 20.00 | 52.00 |
| 6i |  | 50.00 | 19.48 | 51.99 | 88.00 |
| Terbinafine standard drug |  | 100.00 | 90.00 | 110.80 | 98.40 |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hasan, A.; Thomas, N.F.; Gapil, S. Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters. Molecules 2011, 16, 1297-1309. https://doi.org/10.3390/molecules16021297
Hasan A, Thomas NF, Gapil S. Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters. Molecules. 2011; 16(2):1297-1309. https://doi.org/10.3390/molecules16021297
Chicago/Turabian StyleHasan, Aurangzeb, Noel Francis Thomas, and Shelly Gapil. 2011. "Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters" Molecules 16, no. 2: 1297-1309. https://doi.org/10.3390/molecules16021297
APA StyleHasan, A., Thomas, N. F., & Gapil, S. (2011). Synthesis, Characterization and Antifungal Evaluation of 5-Substituted-4-Amino-1,2,4-Triazole-3-Thioesters. Molecules, 16(2), 1297-1309. https://doi.org/10.3390/molecules16021297