An Allelochemical from Myrica gale with Strong Phytotoxic Activity against Highly Invasive Fallopia x bohemica Taxa
Abstract
:1. Introduction
2. Results and Discussion
2.1. Allelopathic potential of M. gale fruit exudates against F. x bohemica
2.2. Identification of an allelochemical from M. gale fruit exudates
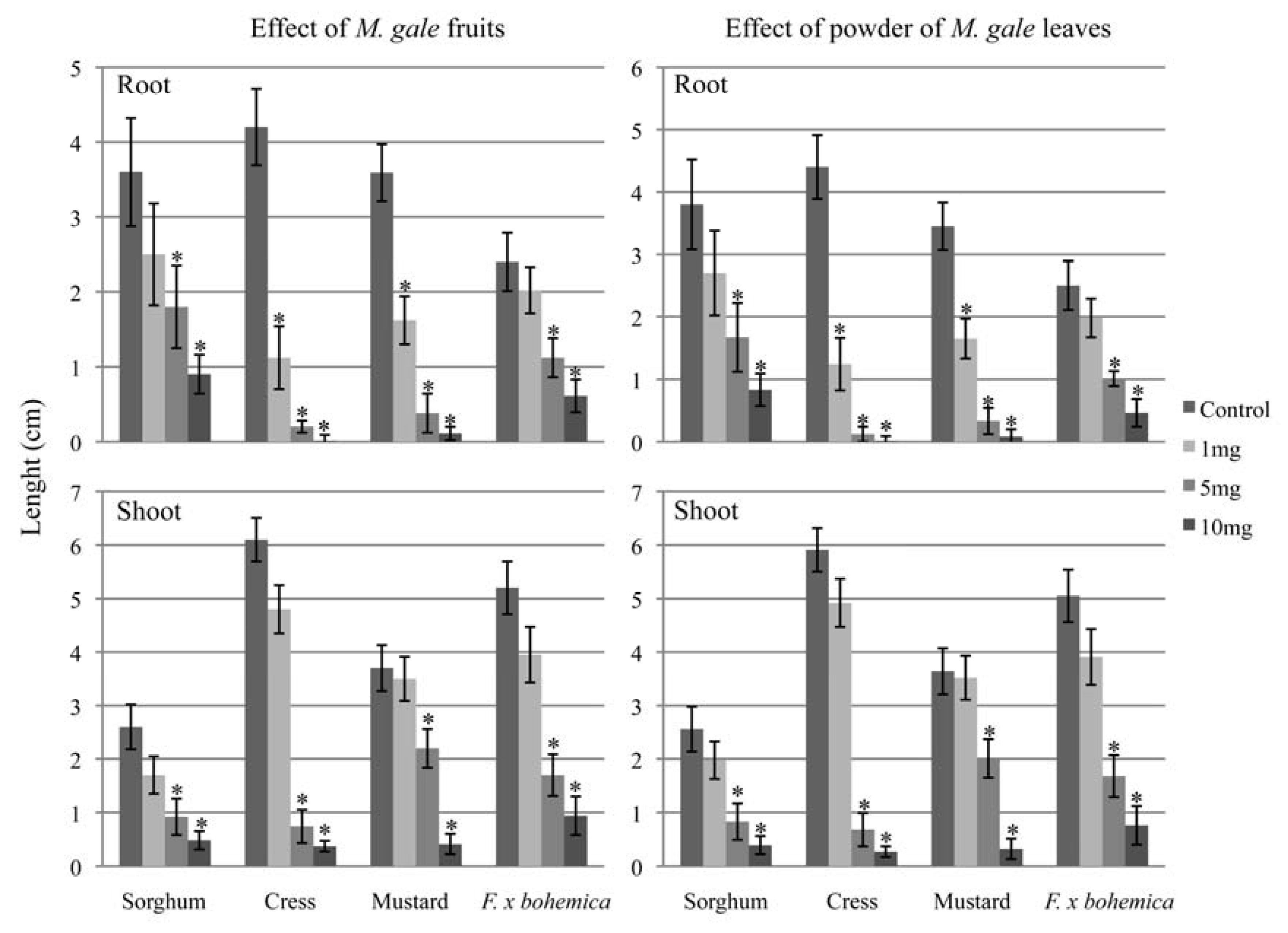
2.3. Phytotoxicity of whole fruits and leaves of M. gale
3. Experimental
3.1. General
3.2. Plant material
3.3. Phytotoxicity assays
3.4. Extraction and isolation
4. Conclusions
Acknowledgments
References
- Vitousek, P.M.; D’Antonio, C.M.; Loope, L.L.; Westbrooks, R. Biological invasions as global environmental change. Am. Sci. 1996, 84, 468–478. [Google Scholar]
- Braithwaite, R.W.; Lonsdale, W.M.; Estbergs, J.A. Alien vegetation and native biota in tropical Australia: the impact of Mimosa pigra. Biol. Conservat. 1989, 48, 189–210. [Google Scholar] [CrossRef]
- Alvarez, M.E.; Cushman, J.H. Community-level consequences of a plant invasion: effects on three habitats in coastal California. Ecol. Appl. 2002, 12, 1434–1444. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Walker, L.R.; Whiteaker, L.D.; Mueller-Dombois, D.; Matson, P.A. Biological Invasion by Myrica faya Alters Ecosystem Development in Hawaii. Science 1987, 238, 802–804. [Google Scholar] [CrossRef] [PubMed]
- D’Antonio, C.M.; Vitousek, P.M. Biological invasions by exotic grasses, the grass/fire cycle, and global change. Annu. Rev. Ecol. Systemat. 1992, 23, 63–87. [Google Scholar] [CrossRef]
- Belnap, J.; Phillips, S.L. Soil biota in an ungrazed grassland: response to annual grass (Bromus tectorum) invasion. Ecol. Appl. 2001, 11, 1261–1275. [Google Scholar] [CrossRef]
- Asner, G.; Beatty, S. Effects of an African grass invasion on Hawaiian shrubland nitrogen biogeochemistry. Plant Soil 1996, 186, 205–211. [Google Scholar] [CrossRef]
- Blank, R.R.; Young, J.A. Influence of the Exotic Invasive Crucifer, Lepidium Latifolium, on Soil Properties and Elemental Cycling. Soil Sci. 2002, 167, 821–829. [Google Scholar] [CrossRef]
- Dassonville, N.; Vanderhoeven, S.; Vanparys, V.; Hayez, M.; Gruber, W.; Meerts, P. Impacts of alien invasive plants on soil nutrients are correlated with initial site conditions in NW Europe. Oecologia 2008, 157, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Godefroid, S. A propos de l’extension spectaculaire de Fallopia japonica, F. sachalinensis, Buddleja davidii et Senecio inaequidens en Région bruxelloise. Dumortiera 1996, 63, 9–16. [Google Scholar]
- Pyšek, P.; Mandák, B.; Francírková, T.; Prach, K. Persistence of stout clonal herbs as invaders in the landscape: A field test of historical records. In Plant Invasions: Species Ecology and Ecosystem Management; Brundu, G., Brock, J., Camarda, I., Child, L., Wade, M., Eds.; Backhuys: Leiden, The Netherlands, 2001. [Google Scholar]
- Weber, E. Invasive Plant Species of the World: A Reference Guide to Environmental Weeds; CABI Publishing: Wallingford, UK, 2003. [Google Scholar]
- Bailey, J.; Bímová, K.; Mandák, B. Asexual spread versus sexual reproduction and evolution in Japanese Knotweed <i>s.l.</i> sets the stage for the “Battle of the Clones”. Biol. Invasions 2009, 11, 1189–1203. [Google Scholar]
- Dassonville, N.; Vanderhoeven, S.; Gruber, W.; Meerts, P. Invasion by Fallopia japonica increases topsoil mineral nutrient concentrations. Ecoscience 2007, 14, 230–240. [Google Scholar] [CrossRef]
- Gerber, E.; Krebs, C.; Murrell, C.; Moretti, M.; Rocklin, R.; Schaffner, U. Exotic invasive knotweeds (Fallopia spp.) negatively affect native plant and invertebrate assemblages in European riparian habitats. Biol. Conservat. 2008, 141, 646–654. [Google Scholar] [CrossRef]
- Urgenson, L.S.; Reichard, S.H.; Halpern, C.B. Community and ecosystem consequences of giant knotweed (Polygonum sachalinense) invasion into riparian forests of western Washington, USA. Biol. Conservat. 2009, 142, 1536–1541. [Google Scholar] [CrossRef]
- Lecerf, A.; Patfield, D.; Boich; Anatole; Riipinen, M.P.; Chauvet, E.; Dobson, M. Stream ecosystems respond to riparian invasion by Japanese knotweed (Fallopia japonica). Canadian J. Fish. Aquat. Sci. 2007, 64, 1273–1283. [Google Scholar] [CrossRef]
- Dawson, F.H.; Holland, D. The distribution in bankside habitats of three alien invasive plants in the U.K. in relation to the development of control strategies. Hydrobiologia 1999, 415, 193–201. [Google Scholar] [CrossRef]
- Bímová, K.; Mandák, B.; Kašparová, I. How does Reynoutria invasion fit the various theories of invasibility? J. Veg. Sci. 2004, 15, 495–504. [Google Scholar] [CrossRef]
- Bailey, J.; Wisskirchen, R. The distribution and origins of Fallopia × bohemica (Polygonaceae) in Europe. Nord. J. Bot. 2004, 24, 173–199. [Google Scholar] [CrossRef]
- Tiebre, M.-S.; Bizoux, J.-P.; Hardy, O.J.; Bailey, J.P.; Mahy, G. Hybridization and morphogenetic variation in the invasive alien Fallopia (Polygonaceae) complex in Belgium. Am. J. Bot. 2007, 94, 1900–1910. [Google Scholar] [CrossRef] [PubMed]
- Bais, H.P.; Vepachedu, R.; Gilroy, S.; Callaway, R.M.; Vivanco, J.M. Allelopathy and Exotic Plant Invasion: From Molecules and Genes to Species Interactions. Science 2003, 301, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Callaway, R.M.; Ridenour, W.M. Novel weapons: Invasive success and the evolution of increased competitive ability. Front. Ecol. Environ. 2004, 2, 436–443. [Google Scholar] [CrossRef]
- Callaway, R.M.; Aschehoug, E.T. Invasive Plants Versus Their New and Old Neighbors: A Mechanism for Exotic Invasion. Science 2000, 290, 521–523. [Google Scholar] [CrossRef] [PubMed]
- Cappuccino, N.; Arnason, J.T. Novel chemistry of invasive exotic plants. Biol. Lett. 2006, 2, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Bertrand, C.; Cochinaire, A.; Chanut, A.; Bellvert, F.; Popovici, J.; Comte, G.; Piola, F. From allelopathy to agrochemistry: A new approach for the valorisation of invasive plants. Planta Med. 2008, 74, 1134. [Google Scholar] [CrossRef]
- Vrchotová, N.; Šerá, B. Allelopathic properties of knotweed rhizome extracts. Plant Soil Environ. 2008, 54, 301–303. [Google Scholar] [CrossRef]
- Fan, P.; Hostettmann, K.; Lou, H. Allelochemicals of the invasive neophyte Polygonum cuspidatum Sieb. & Zucc. (Polygonaceae). Chemoecology 2010, 20, 223–227. [Google Scholar]
- Konstantinidou-Doltsinis, S.; Markellou, E.; Kasselaki, A.M.; Fanouraki, M.; Koumaki, C.; Schmitt, A.; Liopa-Tsakalidis, A.; Malathrakis, N. Efficacy of Milsana®, a Formulated Plant Extract from Reynoutria sachalinensis, against Powdery Mildew of Tomato (Leveillula taurica). Biocontrol 2006, 51, 375–392. [Google Scholar] [CrossRef]
- Wurms, K.; Labbé, C.; Benhamou, N.; Bélanger, R.R. Effects of Milsana and Benzothiadiazole on the Ultrastructure of Powdery Mildew Haustoria on Cucumber. Phytopathology 1999, 89, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, A. Monographie des Myricaceae; anatomie et histologie, organographie, classification et description des espèces, distribution géographique. Mem. Soc. Nat. Sci. Nat. Math. Cher. 1901, 32, 85–340. [Google Scholar]
- Svoboda, K.P.; Inglis, A.; Hampson, J.; Galambosi, B.; Asakawa, Y. Biomass production, essential oil yield and composition of Myrica gale L. harvested from wild populations in Scotland and Finland. Flavour Frag. J. 1998, 13, 367–372. [Google Scholar] [CrossRef]
- Popovici, J.; Bertrand, C.; Bagnarol, E.; Fernandez, M.P.; Comte, G. Chemical composition of essential oil and headspace-solid microextracts from fruits of Myrica gale L. and antifungal activity. Nat. Prod. Res. 2008, 22, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Popovici, J.; Comte, G.; Bagnarol, E.; Alloisio, N.; Fournier, P.; Bellvert, F.; Bertrand, C.; Fernandez, M.P. Differential effects of rare specific flavonoids on compatible and incompatible strains in the Myrica gale-Frankia actinorhizal symbiosis. Appl. Environ. Microb. 2010, 76, 2451–2460. [Google Scholar] [CrossRef] [PubMed]
- Malterud, K.E. C-methylated dihydrochalcones from Myrica gale fruit exudate. Acta Pharm. Nord. 1992, 4, 65–68. [Google Scholar]
- Tolliver, K.S.; Colley, D.M.; Young, D.R. Inhibitory effects of Myrica cerifera on Pinus taeda. Am. Midl. Nat. 1995, 133, 256–263. [Google Scholar] [CrossRef]
- Malterud, K.E.; Anthonsen, T.; Lorentzen, G.B. Two new C-methylated flavonoids from Myrica gale. Phytochemistry 1977, 16, 1805–1809. [Google Scholar] [CrossRef]
- Dayan, F.E.; Duke, S.O.; Sauldubois, A.; Singh, N.; McCurdy, C.; Cantrell, C. p-Hydroxyphenylpyruvate dioxygenase is a herbicidal target site for [beta]-triketones from Leptospermum scoparium. Phytochemistry 2007, 68, 2004–2014. [Google Scholar] [CrossRef] [PubMed]
- Dayan, F.E.; Singh, N.; McCurdy, C.R.; Godfrey, C.A.; Larsen, L.; Weavers, R.T.; Van Klink, J.W.; Perry, N.B. β-Triketone Inhibitors of Plant p-Hydroxyphenylpyruvate Dioxygenase: Modeling and Comparative Molecular Field Analysis of Their Interactions. J. Agr. Food Chem. 2009, 57, 5194–5200. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.L.; Knudsen, C.G.; Michaely, W.J.; Chin, H.L.; Nguyen, N.H.; Carter, C.G.; Cromartie, T.H.; Lake, B.H.; Shribbs, J.M.; Fraser, T. The structure–activity relationships of the triketone class of HPPD herbicides. Pestic. Sci. 1998, 54, 377–384. [Google Scholar] [CrossRef]
- Ruifed, S.; Puijalon, S.; Viricel, M.R.; Piola, F. Achene buoyancy and germinability of the terrestrial invasive Fallopia x bohemica in aquatic environment: A new vector of dispersion? Ecoscience 2011, in press. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compound myrigalone A is available from the authors. |



| Treatment | Sorghum | Cress | Mustard | F. x bohemica |
|---|---|---|---|---|
| Control | 90% | 100% | 93% | 88% |
| 1mg | 87% | 100% | 94% | 90% |
| 5mg | 80% | 7% | 37% | 70% |
| 10mg | 70% | 1% | 24% | 70% |
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Popovici, J.; Bertrand, C.; Jacquemoud, D.; Bellvert, F.; Fernandez, M.P.; Comte, G.; Piola, F. An Allelochemical from Myrica gale with Strong Phytotoxic Activity against Highly Invasive Fallopia x bohemica Taxa. Molecules 2011, 16, 2323-2333. https://doi.org/10.3390/molecules16032323
Popovici J, Bertrand C, Jacquemoud D, Bellvert F, Fernandez MP, Comte G, Piola F. An Allelochemical from Myrica gale with Strong Phytotoxic Activity against Highly Invasive Fallopia x bohemica Taxa. Molecules. 2011; 16(3):2323-2333. https://doi.org/10.3390/molecules16032323
Chicago/Turabian StylePopovici, Jean, Cedric Bertrand, Dominique Jacquemoud, Floriant Bellvert, Maria P. Fernandez, Gilles Comte, and Florence Piola. 2011. "An Allelochemical from Myrica gale with Strong Phytotoxic Activity against Highly Invasive Fallopia x bohemica Taxa" Molecules 16, no. 3: 2323-2333. https://doi.org/10.3390/molecules16032323
APA StylePopovici, J., Bertrand, C., Jacquemoud, D., Bellvert, F., Fernandez, M. P., Comte, G., & Piola, F. (2011). An Allelochemical from Myrica gale with Strong Phytotoxic Activity against Highly Invasive Fallopia x bohemica Taxa. Molecules, 16(3), 2323-2333. https://doi.org/10.3390/molecules16032323






