Effect of Lectins from Diocleinae Subtribe against Oral Streptococci
Abstract
:1. Introduction
2. Results and Discussion
 ) 0.9% NaCl (
) 0.9% NaCl (  ) Lectin 500 µg/mL.
) Lectin 500 µg/mL.
 ) 0.9% NaCl (
) 0.9% NaCl (  ) Lectin 500 µg/mL.
) Lectin 500 µg/mL.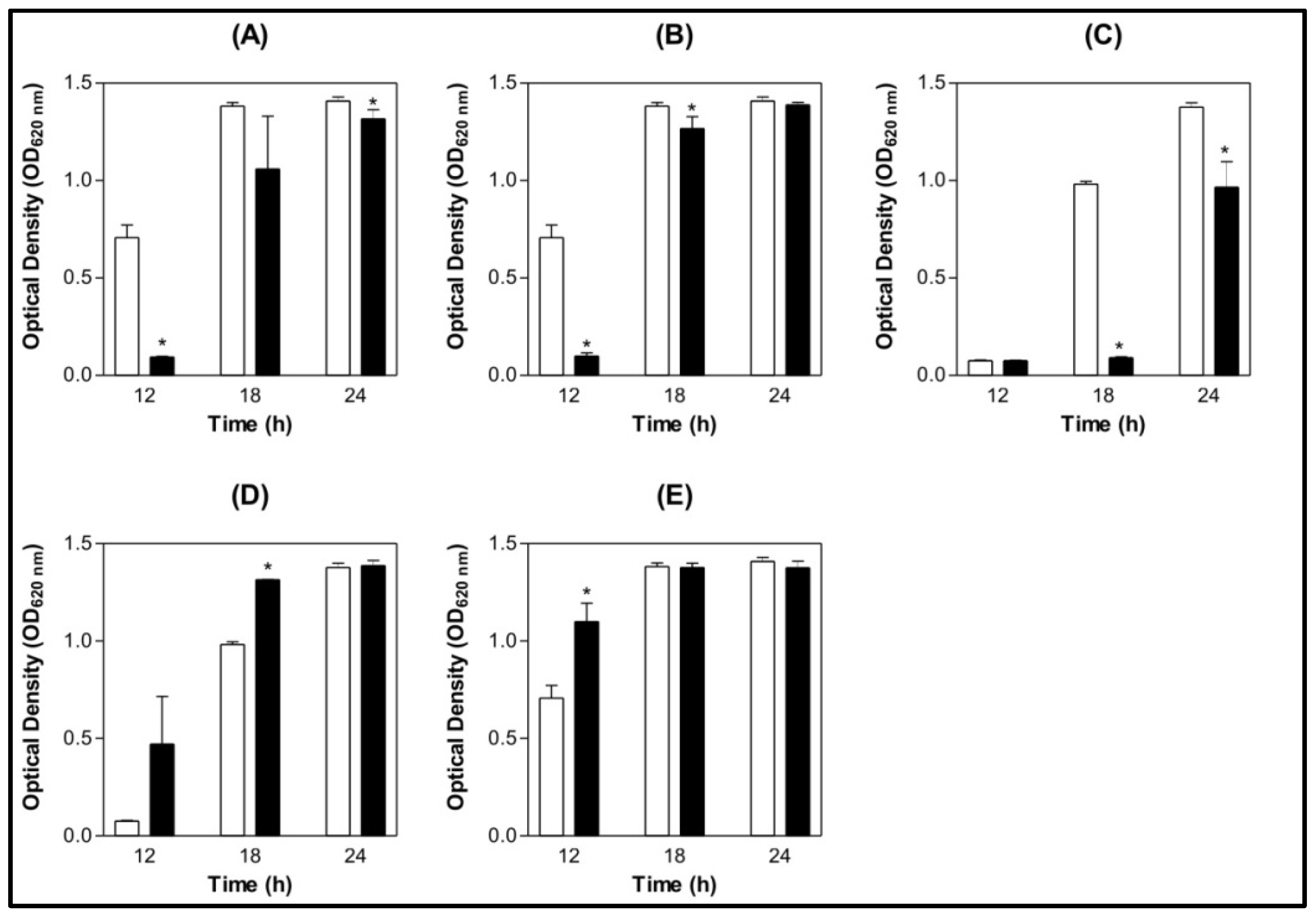
 ) 0.9% NaCl (
) 0.9% NaCl (  ) Lectin 500 µg/mL.
) Lectin 500 µg/mL.
 ) 0.9% NaCl (
) 0.9% NaCl (  ) Lectin 500 µg/mL.
) Lectin 500 µg/mL.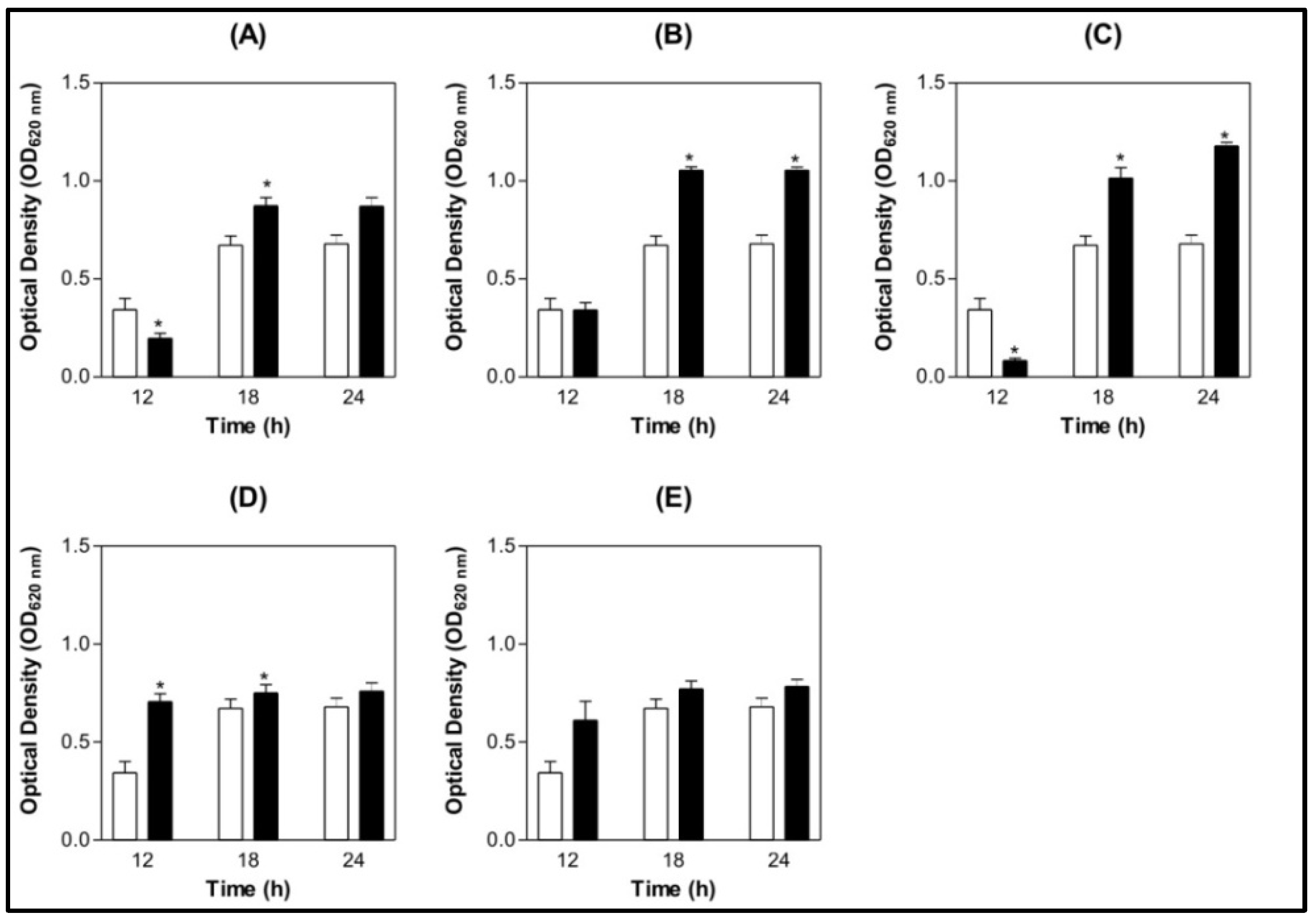
 ) BSA 200 µg/mL (
) BSA 200 µg/mL (  ) Lectin 100 µg/mL (
) Lectin 100 µg/mL (  ) Lectin 200 µg/mL.
) Lectin 200 µg/mL.
 ) BSA 200 µg/mL (
) BSA 200 µg/mL (  ) Lectin 100 µg/mL (
) Lectin 100 µg/mL (  ) Lectin 200 µg/mL.
) Lectin 200 µg/mL.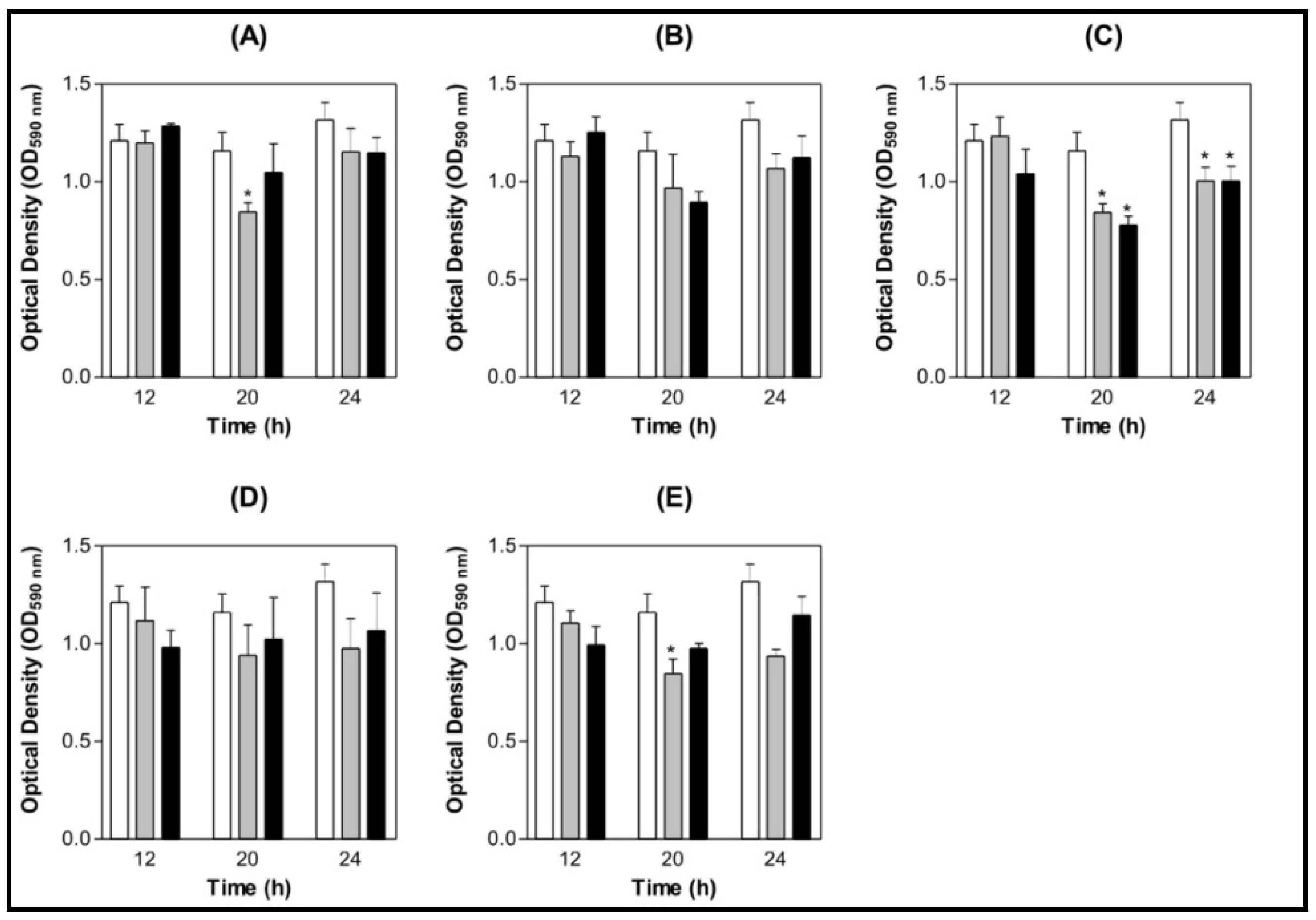
 ) BSA 200 µg/mL (
) BSA 200 µg/mL (  ) Lectin 100 µg/mL (
) Lectin 100 µg/mL (  ) Lectin 200 µg/mL.
) Lectin 200 µg/mL.
 ) BSA 200 µg/mL (
) BSA 200 µg/mL (  ) Lectin 100 µg/mL (
) Lectin 100 µg/mL (  ) Lectin 200 µg/mL.
) Lectin 200 µg/mL.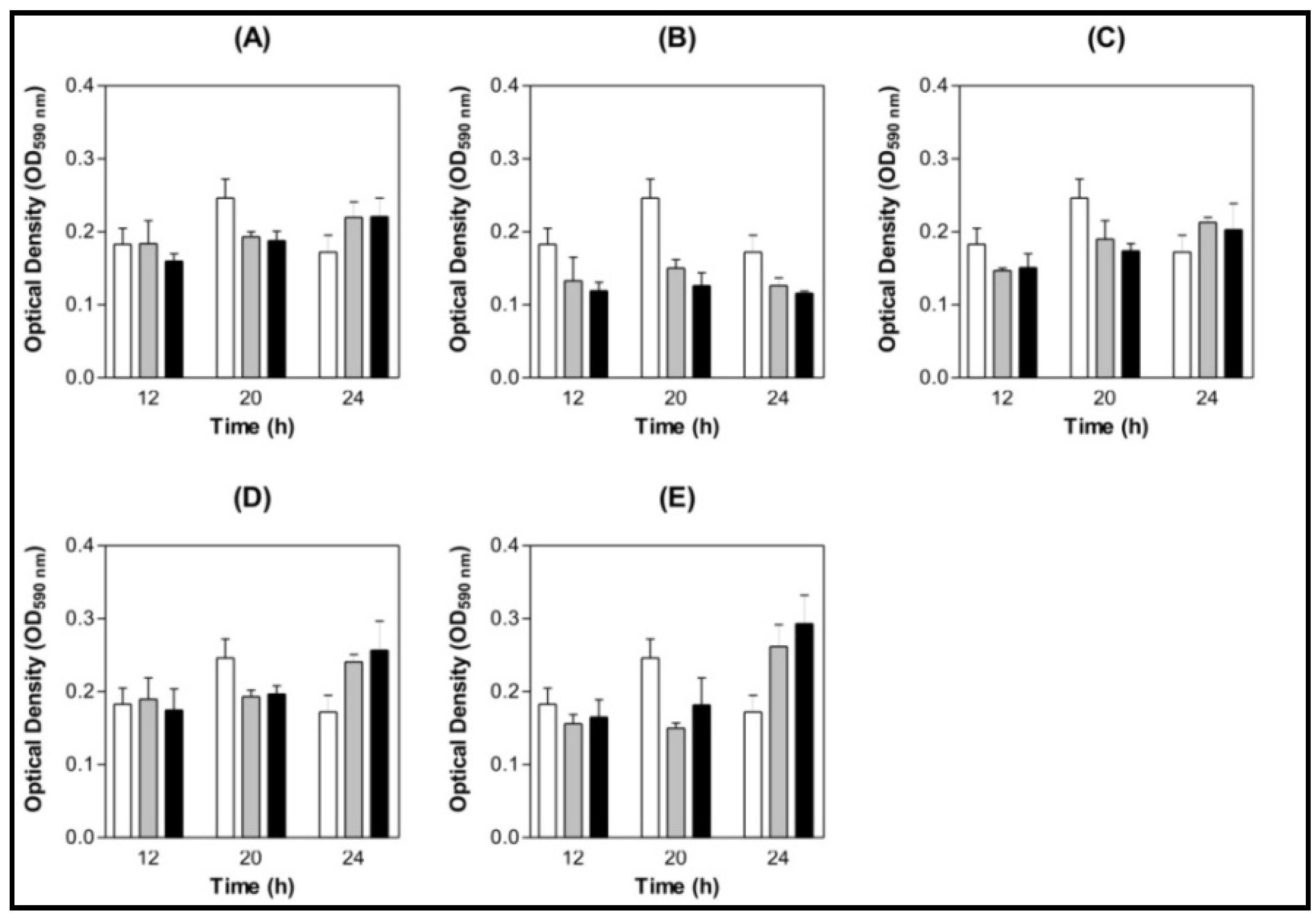
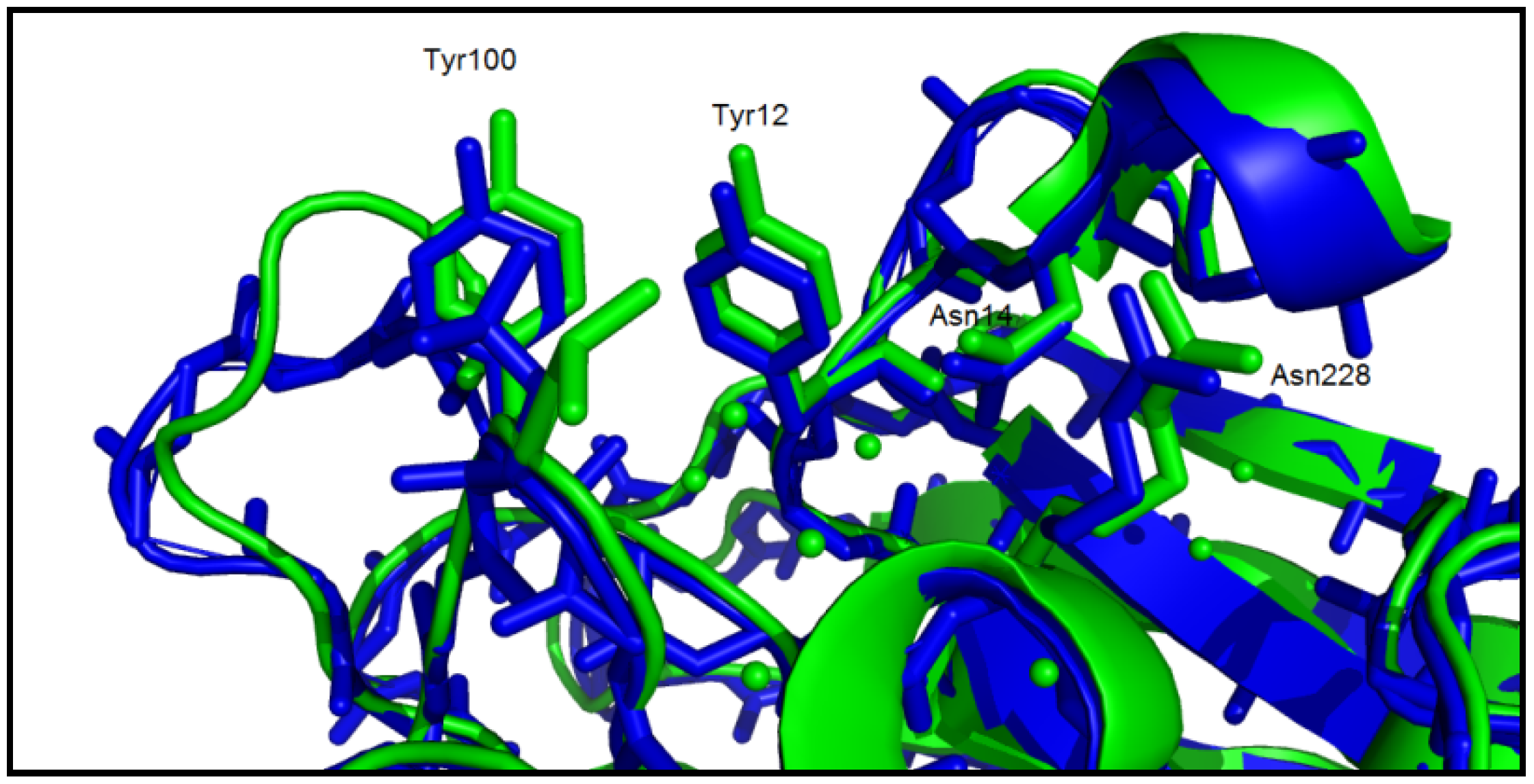
3. Experimental
3.1. Bacterial Strains and Growth Conditions
3.2. Lectin Purification
3.3. Effect of Lectins on Bacterial Growth
3.4. Effect of Lectins on S. mutans and S. oralis Biofilm Formation on Saliva-coated Surface
3.4.1. Saliva Processing
3.4.2. Biofilm Assay
3.5. Structure/Function Analysis
3.6. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Marsh, P.D. Dental plaque: Biological significance of a biofilm and community life-style. J. Clin. Periodontol. 2005, 32, 7–15. [Google Scholar] [CrossRef]
- Karunakaran, E.; Biggs, C. Mechanisms of Bacillus cereus biofilm formation: An investigation of the physicochemical characteristics of cell surfaces and extracellular proteins. Appl. Microbiol. Biotechnol. 2010, in press. [Google Scholar]
- Bowden, G.H.W.; Hamilton, I.R. Survival of oral bacteria. Crit. Rev. Oral Biol. Med. 1998, 9, 54–85. [Google Scholar] [CrossRef]
- Hall-Stoodley, L.; Costerton, J.W.; Stoodley, P. Bacterial biofilms: From the natural environment to infectious diseases. Nat. Rev. Microbiol. 2004, 2, 95–108. [Google Scholar] [CrossRef]
- Oppermann, R.V.; Haas, A.N.; Villoria, G.E.; Primo, L.G.; Serra-Negra, J.M.; Ferreira, E.F.; Pannuti, C.M. Proposal for the teaching of the chemical control of supragingival biofilm. Braz. Oral Res. 2010, 1, 33–36. [Google Scholar]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a biofilm: The significance of pH in health and caries. Compend. Contin. Educ. Dent. 2009, 30, 76–78. [Google Scholar]
- Fitzgerald, R.J.; Keyes, P.H. Demonstration of the etiologic role of streptococci in experimental caries in the hamster. J. Am. Dent. Assoc. 1960, 61, 9–19. [Google Scholar]
- Kuramitsu, H.K. Virulence properties of oral bacteria: Impact of molecular biology. Curr. Issues Mol. Biol. 2001, 3, 35–36. [Google Scholar]
- Kara, D.; Luppens, S.B.I.; van Marle, J.; Ozok, R.; ten Cate, J.M. Microstructural differences between single-species and dual species biofilm of Streptococcus mutans and Veillonella parvula before and after exposure to chlorhexidine. FEMS Microbiol. Lett. 2007, 271, 90–97. [Google Scholar] [CrossRef]
- Wilson, M. Bacterial biofilms and human disease. Sci. Prog. 2001, 84, 235–254. [Google Scholar] [CrossRef]
- Marsh, P.D. Are dental diseases examples of ecological catastrophes? Microbiology 2003, 149, 279–294. [Google Scholar] [CrossRef]
- Shemesh, M.; Tam, A.; Steinberg, D. Differential gene expression profiling of Streptococcus mutans cultured under biofilm and planktonic conditions. Microbiology 2007, 153, 1307–1317. [Google Scholar] [CrossRef]
- O’toole, G.; Kaplan, H.B.; Kolter, R. Biofilm formation as a microbial development. Annu. Rev. Microbiol. 2000, 54, 49–79. [Google Scholar] [CrossRef]
- Orstavik, D.; Kraus, F.W.; Henshaw, C. In vitro attachment of streptococci to the tooth surface. Infect. Immun. 1974, 9, 794–800. [Google Scholar]
- Sharon, N.; Ofek, I. Safe as mother's milk: Carbohydrates as future anti-adhesion drugs for bacterial diseases. Glycoconj. J. 2000, 17, 659–664. [Google Scholar] [CrossRef]
- Bies, C.; Lehr, C.; Woodley, J.F. Lectin-mediated drug targeting: History and applications. Adv. Drug Deliv. Rev. 2004, 56, 425–435. [Google Scholar] [CrossRef]
- Imberty, A.; Varrot, A. Microbial recognition of human cell surface glycoconjugates. Curr. Opin. Struct. Biol. 2008, 18, 567–576. [Google Scholar] [CrossRef]
- Vydryakova, G.A.; Bondar, V.S. Location of lectin exhibiting specificity for N-acetyl-D-galactosamine in cells of the symbiotic marine bacteria Photobacterium phosphoreum. Biochem. Biophys. 2008, 420, 155–157. [Google Scholar]
- Wayman, A.M.; Chen, W.; McEver, R.P.; Zhu, C. Triphasic force dependence of E-selectin/ligand dissociation governs cell rolling under flow. Biophys. J. 2010, 99, 1166–1174. [Google Scholar] [CrossRef]
- Sharon, N. Lectins: Carbohydrate-specific reagents and biological recognition molecules. J. Biol. Chem. 2007, 282, 2753–2764. [Google Scholar] [CrossRef]
- Chandra, N.R.; Prabu, M.M.; Suguna, K.; Vijayan, M. Structural similarity and functional diversity in proteins containing the legume lectin fold. Protein Eng. 2001, 14, 857–866. [Google Scholar] [CrossRef]
- Srinivas, V.R.; Reddy, G.B.; Ahmad, N.; Swaminathan, C.P.; Mitra, N.; Surolia, A. Legume lectin family, the ‘‘natural mutants of the quaternary state”, provide insights into the relationship between protein stability and oligomerization. Biochim. Biophys. Acta 2001, 1527, 102–111. [Google Scholar] [CrossRef]
- Sanz-Aparicio, J.; Hermoso, J.; Graneiro, T.B.; Calvete, J.J.; Cavada, B.S. The crystal structure of Canavalia brasiliensis lectin suggests a correlation between its quaternary conformation and its distinct biological properties from concanavalin A. FEBS Lett. 1997, 405, 114–118. [Google Scholar] [CrossRef]
- Calvete, J.J.; Thole, H.H.; Raida, M.; Urbanke, C.; Romero, A.; Grangeiro, T.B.; Ramos, M.V.; Almeida da Rocha, I.M.; Guimarães, F.N.; Cavada, B.S. Molecular characterization and crystallization of Diocleinae lectins. Biochim. Biophys. Acta 1999, 1430, 367–375. [Google Scholar] [CrossRef]
- Teixeira, E.H.; Napimoga, M.H.; Carneiro, V.A.; de Oliveira, T.M.; Cunha, R.M.; Havt, A.; Martins, J.L.; Pinto, V.P.; Gonçalves, R.B.; Cavada, B.S. In vitro inhibition of Streptococci binding to enamel acquired pellicle by plant lectins. J. Appl. Microbiol. 2006, 101, 111–116. [Google Scholar] [CrossRef]
- Balls, A.K.; Hale, W.S.; Harris, T.H. A crystalline protein obtained from a lipoprotein of wheat flour. Cereal Chem. 1942, 19, 279–288. [Google Scholar]
- Etzler, M.E. Distribution and function of plant lectins. In The Lectins. Properties, Function, and Applications in Biology and Medicine; Liener, I.E., Sharon, N., Goldstein, I.J., Eds.; Academic Press: Orlando, FL, USA, 1986; pp. 371–435. [Google Scholar]
- De Bolle, M.F.; Osborn, R.W.; Goderis, I.J.; Noe, L.; Acland, D.; Hart, C.A.; Torrekens, S.; van Leuven, F.; Broekart, N.F. Antimicrobial properties from Mirablis jalapa and Amaranthus caudalus: Expression, processing, localization and biological activity in transgenic tobacco. Plant Mol. Biol. 1996, 31, 993–1008. [Google Scholar] [CrossRef]
- Liao, W.R.; Lin, J.Y.; Shieh, W.Y.; Jeng, W.L.; Huang, R. Antibiotic activity of lectinsfrom marine algae against marine vibrios. J. Ind. Microbiol. Biotechnol. 2003, 30, 433–439. [Google Scholar] [CrossRef]
- Santi-Gadelha, T.; Gadelha, C.A.A.; Aragão, K.S.; Oliveira, C.C.; Mota, M.R.L.; Gomes, R.C.; Pires, A.F.; Toyama, M.H.; Toyama, D.O.; de Alencar, N.M.N.; David Neil Criddle, D.N.; Ana, A.M.S.; Cavada, B.S. Purification and biological effects of Araucaria angustifolia (Araucariaceae) seed lectin. Biochem. Biophys. Res. Commun. 2006, 350, 1050–1055. [Google Scholar] [CrossRef]
- Oliveira, M.R.; Napimoga, M.H.; Cogo, K.; Gonçalves, R.B.; Macedo, M.L.; Freire, M.G.; Groppo, F.C. Inhibition of bacterial adherence to saliva-coated through plant lectins. J. Oral Sci. 2007, 49, 141–145. [Google Scholar] [CrossRef]
- Oliveira, M.D.L.; Andrade, C.A.S.; Santos-Magalhães, N.S.; Coelho, L.C.B.B.; Teixeira, J.A.; Carneiro-da-Cunha, M.G.; Correia, M.T.S. Purification of a lectin from Eugenia uniflora L. seeds and its potential antibacterial activity. Lett. Appl. Microbiol. 2008, 46, 371–376. [Google Scholar] [CrossRef]
- Terras, F.R.G.; Schoofs, H.M.E.; Thevissen, K.; Osborn, R.W.; Vanderleyden, J.; Cammue, B.P.A.; Broekaert, W.F. Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oilseed rape 2s albumins and by barley trypsin inhibitors. Plant Physiol. 1993, 103, 1311–1319. [Google Scholar]
- Calderon, A.M.; Buck, G.; Doyle, R.J. Lectin-microorganism Complexes. In Lectins: Biology, Biochemistry, Clinical Biochemistry; van Driessche, E., Beckmans, S., Bog-Hanse, T.C., Eds.; TEXTOP: Hellerup, Denmark, 1997; Volume 12, ISBN number 87-984583-0-2. [Google Scholar]
- Kaszuba, M.; Lyle, I.G.; Jones, M.N. The targeting of lectin bearing liposomes to skin associated bacteria. Colloid. Surf. B Biointerfaces 1995, 4, 151–158. [Google Scholar] [CrossRef]
- van der Waaij, D.; Berghuis de Vries, J.M.; van der Wees, J.E.C. Colonisation resistance of the digestive tract in conventional and antibiotic-treated mice. J. Hyg. 1971, 69, 405–411. [Google Scholar] [CrossRef]
- Caglar, E.; Kargul, B.; Tanboga, I. Bacteriotherapy and probiotics' role on oral health. Oral Dis. 2005, 11, 131–137. [Google Scholar] [CrossRef]
- Hamilton, I.R. Ecological Basis for Dental Caries in Oral Bacterial Ecology: The Molecular Basis; Kuramitsu, H.K., Ellen, R.P., Eds.; Horizon Scientific Press: Norfolk, UK, 2000; pp. 219–274. [Google Scholar]
- Nyvad, B.; Kilian, M. Microflora associated with experimental root surface caries in humans. Infect. Immun. 1990, 58, 1628–1633. [Google Scholar]
- Do, T.; Jolley, K.A.; Maiden, M.C.J.; Gilbert, S.C.; Douglas, C.; Wade, W.G.; Beighton, D. Population structure of Streptococcus oralis. Microbiology 2009, 155, 2593–2602. [Google Scholar] [CrossRef]
- Marsh, P.D. Sugar, fluoride, pH and microbial homeostasis in dental plaque. Proc. Finn. Dent. Soc. 1991, 87, 515–525. [Google Scholar]
- He, X.; Lux, R.; Kuramitsu, H.K.; Anderson, M.H.; Shi, W. Achieving probiotic effects via modulating oral microbial ecology. Adv. Dent. Res. 2009, 21, 53–56. [Google Scholar] [CrossRef]
- Islam, B.; Khan, S.N.; Naeem, A.; Sharma, V.; Khan, A.U. Novel effect of plant lectinson the inhibition of Streptococcus mutans biofilm formation on saliva-coated surface. J. Appl. Microbiol. 2009, 106, 1682–1689. [Google Scholar] [CrossRef]
- Liljemark, W.F.; Bloomquist, C.G.; Germaine, G.R. Effect of bacterial aggregation on theadhrence of oral streptococci to hydroxiapatite. Infect. Immun. 1981, 31, 935–941. [Google Scholar]
- Chia, J.S.; Chang, L.Y.; Shun, C.T.; Chang, Y.Y.; Tsay, Y.G.; Chen, J.Y. A 60-kilodalton immunodominant glycoprotein is essential for cell wall integrity and the maintenance of cell shape in Streptococcus mutans. Infect. Immun. 2001, 69, 6987–6998. [Google Scholar] [CrossRef]
- Delatorre, P.; Rocha, B.A.M.; Souza, E.P.; Oliveira, T.M.; Bezerra, G.A.; Moreno, F.B.; Freitas, B.T.; Santi-Gadelha, T.A.; Sampaio, H.; De Azevedo Junior, W.F.; Cavada, B.S. Structure of a lectin from Canavalia gladiata seeds: New structural insights for old molecules. BMC Struct. Biol. 2007, 7, 52. [Google Scholar] [CrossRef]
- Bezerra, G.A.; Oliveira, T.M.; Moreno, F.B.; de Souza, E.P.; da Rocha, B.A.; Benevides, R.G.; Delatorre, P.; de Azevedo, W.F., Jr.; Cavada, B.S. Structural analysis of Canavalia maritima and Canavalia gladiate lectins complexed with different dimannosides: New insights into the understanding of the structure-biological activity relationship in legume lectins. J. Struct. Biol. 2007, 160, 168–176. [Google Scholar] [CrossRef]
- Byers, H.L.; Homer, K.A.; Tarelli, E.; Beighton, D. N-acetylneuraminic acid transport by Streptococcus oralis strain AR3. J. Med. Microbiol. 1999, 48, 375–381. [Google Scholar] [CrossRef]
- Gadelha, C.A.A.; Moreno, F.B.M.B.; Santi-Gadelha, T.; Cajazeiras, J.B.; Rocha, B.A.M.; Assreuy, A.M.S.; Mota, M.R.L.; Pinto, N.V.; Meireles, A.V.P.; Freitas, B.T.; Canduri, F.; Souza, E.P.; Delatorre, P.; Criddle, D.N.; De Azevedo, W.F., Jr.; Cavada, B.S. Native crystal structure of a nitric oxide-releasing lectin from the seeds of Canavalia maritima. J. Struct. Biol. 2005, 152, 185–194. [Google Scholar] [CrossRef]
- Wu, A.M. Carbohydrate structural units in glycoproteins and polysaccharides as important ligands for Gal and GalNAc reactive lectins. J. Biomed. Sci. 2003, 10, 676–688. [Google Scholar] [CrossRef]
- Wu, A.M.; Lisowska, E.; Duk, M.; Yang, Z. Lectins as tools in glycoconjugate research. Glycoconj. J. 2009, 26, 899–913. [Google Scholar] [CrossRef]
- Sumner, J.B.; Howell, S.F. The identification of the hemagglutinin of the jack bean with concanavalin A. J. Bacteriol. 1936, 32, 227–237. [Google Scholar]
- Moreira, R.A.; Cavada, B.S. Lectinfrom Canavalia brasiliensis (Mart.). Isolation, characterisation and behaviour during germination. Biol. Plant. 1984, 26, 113–120. [Google Scholar] [CrossRef]
- Perez, G.; Perez, C.; Cavada, B.S.; Moreira, R.; Richardson, M. Comparison of the amino acid sequences of the lectins from seeds of Dioclea lehmanni and Canavalia maritima. Phytochemistry 1991, 30, 2619–2621. [Google Scholar] [CrossRef]
- Moreno, F.B.M.B.; Delatorre, P.; Freitas, B.T.; Rocha, B.A.M.; Souza, E.P.; Faco, F.; Canduri, F.; Cardoso, A.L.H.; Freire, V.N.; Lima, J.L.; et al. Crystallization and preliminary X-ray diffraction analysis of the lectin from Canavalia gladiata seeds. Acta Cryst. 2004, 60, 1493–1495. [Google Scholar]
- Moura, T.R.; Bezerra, G.A.; Bezerra, M.J.B.; Teixera, C.S.; Bezerra, E.H.S.; Benevides, R.G.; Rocha, B.A.M.; Souza, L.A.G.; Delatorre, P.; Nagano, C.S.; Cavada, B.S. Crystallization and preliminary X-ray diffraction analysis of the lectin from Canavalia boliviana Piper seeds. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 2009, 65, 213–215. [Google Scholar] [CrossRef]
- Guggenheim, B.; Giertsen, W.; Schupbach, P.; Shapiro, S. Validation of an in vitro biofilm model of supragingival plaque. J. Dent. Res. 2001, 80, 363–370. [Google Scholar] [CrossRef]
- O’Toole, G.A.; Kolter, R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol. Microbiol. 1998, 30, 295–304. [Google Scholar] [CrossRef]
- DeLano, W.L. The PyMOL Molecular Graphics System; DeLano Scientific: San Carlos, CA, USA, 2002. [Google Scholar]
- Sample Availability: Contact the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Cavalcante, T.T.A.; Anderson Matias da Rocha, B.; Alves Carneiro, V.; Vassiliepe Sousa Arruda, F.; Fernandes do Nascimento, A.S.; Cardoso Sá, N.; Do Nascimento, K.S.; Sousa Cavada, B.; Holanda Teixeira, E. Effect of Lectins from Diocleinae Subtribe against Oral Streptococci. Molecules 2011, 16, 3530-3543. https://doi.org/10.3390/molecules16053530
Cavalcante TTA, Anderson Matias da Rocha B, Alves Carneiro V, Vassiliepe Sousa Arruda F, Fernandes do Nascimento AS, Cardoso Sá N, Do Nascimento KS, Sousa Cavada B, Holanda Teixeira E. Effect of Lectins from Diocleinae Subtribe against Oral Streptococci. Molecules. 2011; 16(5):3530-3543. https://doi.org/10.3390/molecules16053530
Chicago/Turabian StyleCavalcante, Theodora Thays Arruda, Bruno Anderson Matias da Rocha, Victor Alves Carneiro, Francisco Vassiliepe Sousa Arruda, Antônia Sâmia Fernandes do Nascimento, Nairley Cardoso Sá, Kyria Santiago Do Nascimento, Benildo Sousa Cavada, and Edson Holanda Teixeira. 2011. "Effect of Lectins from Diocleinae Subtribe against Oral Streptococci" Molecules 16, no. 5: 3530-3543. https://doi.org/10.3390/molecules16053530
APA StyleCavalcante, T. T. A., Anderson Matias da Rocha, B., Alves Carneiro, V., Vassiliepe Sousa Arruda, F., Fernandes do Nascimento, A. S., Cardoso Sá, N., Do Nascimento, K. S., Sousa Cavada, B., & Holanda Teixeira, E. (2011). Effect of Lectins from Diocleinae Subtribe against Oral Streptococci. Molecules, 16(5), 3530-3543. https://doi.org/10.3390/molecules16053530



