Synthesis, Properties Characterization and Applications of Various Organobismuth Compounds
Abstract
:1. Introduction
2. Synthesis and Property Characterization of Various Organobismuth Compounds
| Compounds | Characterization Methods | Potential Applications | References |
|---|---|---|---|
| BiAr3 and BiAr3L2 | NMR, X-ray diffraction | Reagent | [25] |
| Bu4N[PhBiX2Y] | IR, FAB− MS, X-ray crystallography | Lewis acid | [35] |
| (Biphenyl-2,2’-ylene)phenylbismuth diacetate | NMR | Reagent | [36] |
| Ladder-type organobismuth compounds | GC-MS, NMR, X-ray crystallography | Lewis acid | [40] |
| Ar3Bi=NCOR | NMR, IR, FABMS, X-ray crystallography | Reagent | [42] |
| Tris[ortho-chloromethylphenyl]bismuthane | Reagent | [43] | |
| Ph4BiF | X-ray diffraction | Reagent | [47] |
| (4-CH3C6H4SO2NHCH2CO2)2BiAr3 | Elemental analysis, IR, NMR, MS | Antitumor activity | [16] |
| Organobismuth chloride and triphenylgermylpropionate | NMR, IR, elemental analysis | Antiproliferative activity | [15] |
| Resin-bound triarylbismuthanes | NMR | Reagent | [50] |
| Cyclopropylbismuth | NMR, IR, MS | Reagent | [52] |
| Ar3Bi(OAc)2 and Ar3BiCl2 | NMR | Reagent | [9] |
| [tBuN(CH2C6H4)2Bi]+[B(C6F5)4]− | NMR | Catalyst | [28] |
| New dibismuthanes | NMR, X-ray crystallography | Reagent | [54] |
| Borate ester coordinated organobismuth | NMR, elemental analysis | Reagent | [55] |
| [2,6-Mes2-4-R-C6H2BiX2]2 | NMR, IR, ESI-MS, MS | [23] | |
| [S(CH2C6H4)2Bi(OH2)]+[ClO4]− | NMR, X-ray diffraction | Catalyst | [58] |
| [S(CH2C6H4)2Bi(OH2)]+[OSO2C8F17]− | NMR, X-ray diffraction, TG-DSC analysis, Hammett indicator | Catalyst | [59] |
| C6H11N(CH2C6H4)2BiBF4 | X-ray analysis, TG-DSC analysis | Catalyst | [60] |
| Silyl-substituted bismuth | NMR, X-ray analysis | Reagent | [62] |
| [Ar1Ar2Ar3Ar4Bi+][X−] | NMR | Reagent | [63] |
| Water-soluble non-ionic triarylbismuthanes | NMR, IR, elemental analysis | X-ray contrast media | [18] |
| Organobismuth rings (RBi)3 and (RBi)4 | NMR, X-ray analysis | [19] | |
| [(Me2Bi)3(Tm tBu)2]+[Me2BiCl2]− | X-ray diffraction | Reagent | [65] |
| [2,6-(Me2NCH2)2C6H3]BiX2 | NMR, X-ray diffraction | [26] | |
| BiVR3(O2CR’)2 | NMR, X-ray diffraction, elemental analysis | Reagent | [67] |
2.1. Organobismuth(III) and Organobismuth(V) Complexes

2.2. Mixed Halophenylbismuthates(III)
2.3. Pentavalent Biphenyl-2,2’-ylenebismuth Derivatives

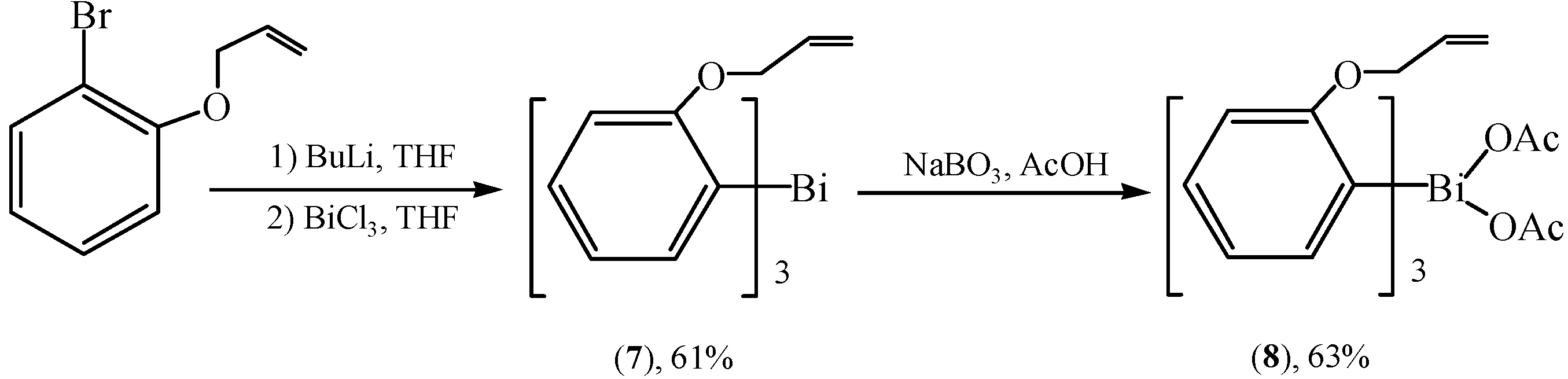
2.4. Ladder-Type Organobismuth Compounds

2.5. (Acylimino)triaryl-λ5-bismuthanes
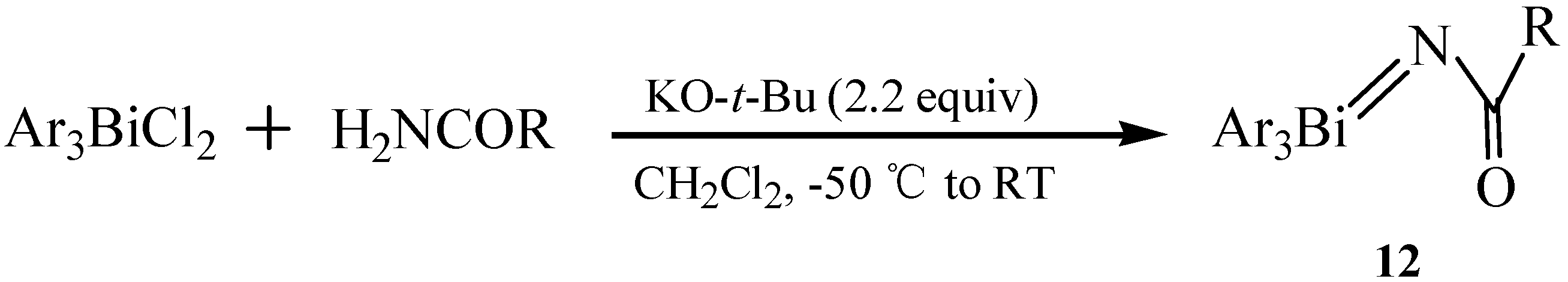
2.6. Tris[ortho-chloromethylphenyl]bismuthane

2.7. Fluorotetraphenylbismuth
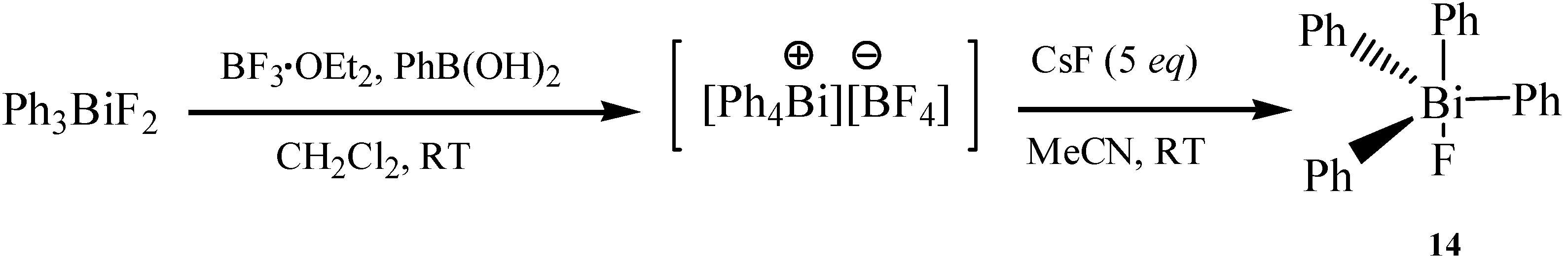
2.8. Triarylbismuth(V) Di(N-p-toluenesulfonyl)aminoacetates
2.9. Cyclic Organobismuth(III) Chlorides and their Triphenylgermylpropionate Derivatives


2.10. Resin-Bound Triaryl Bismuthanes



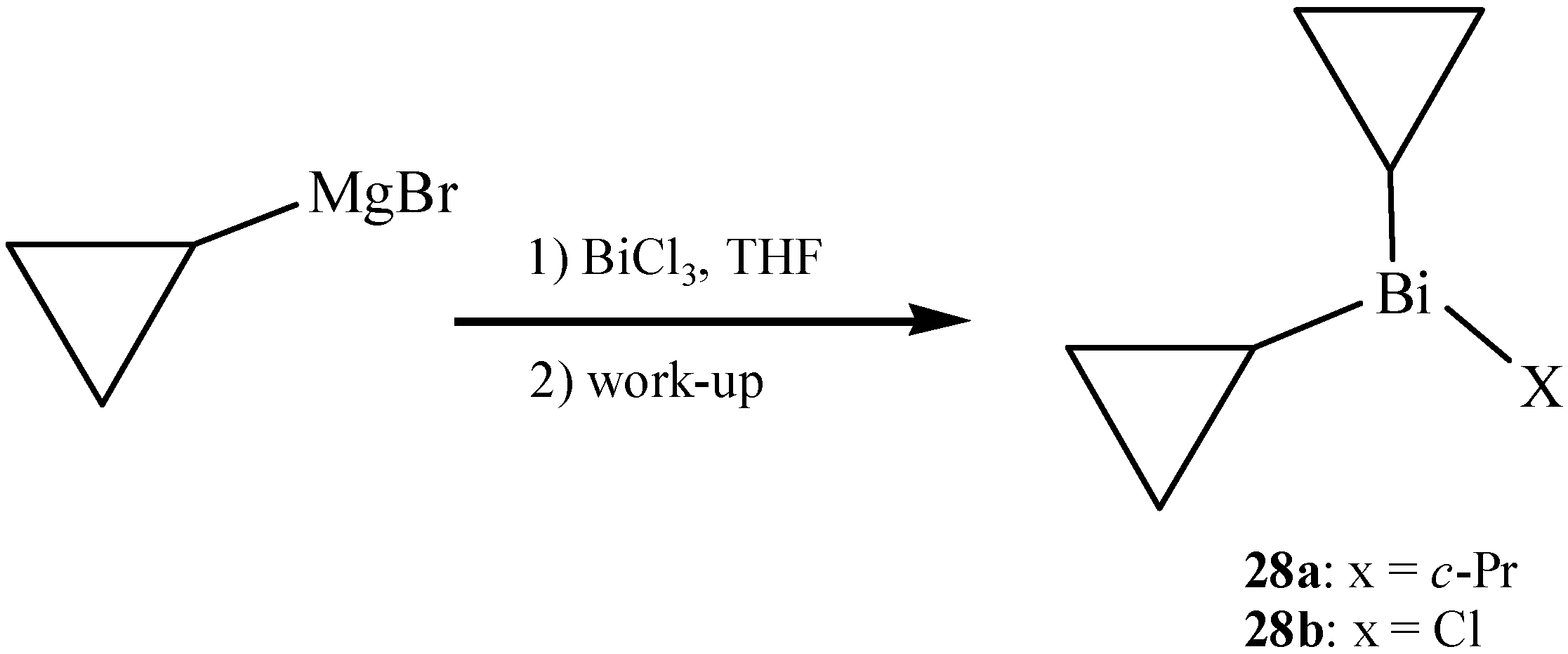
2.11. Cyclopropylbismuth
2.12. Tris(polymethoxyphenyl)bismuth Derivatives

2.13. Cationic Organobismuth Complex and Its Coordination Complexes
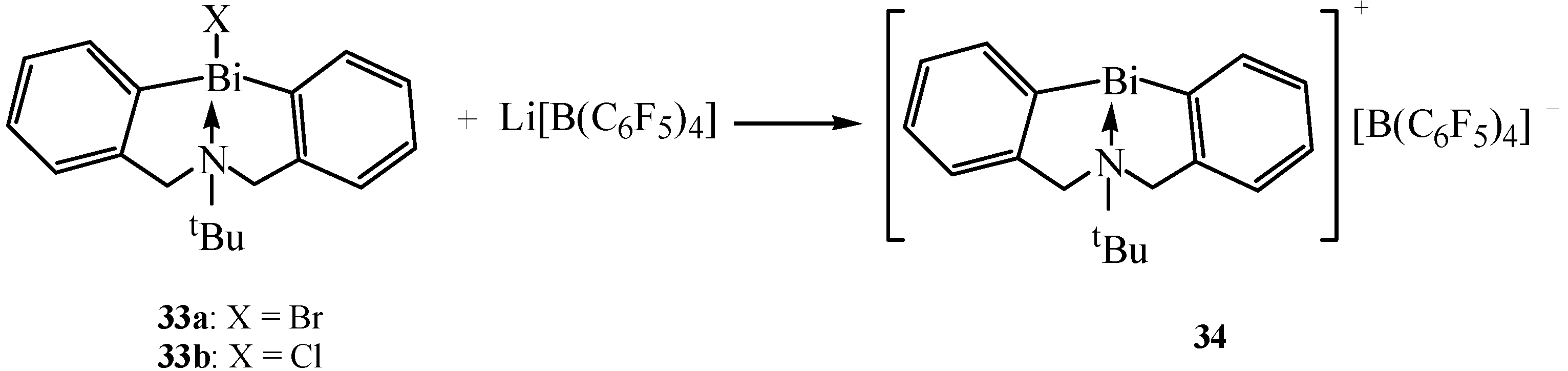
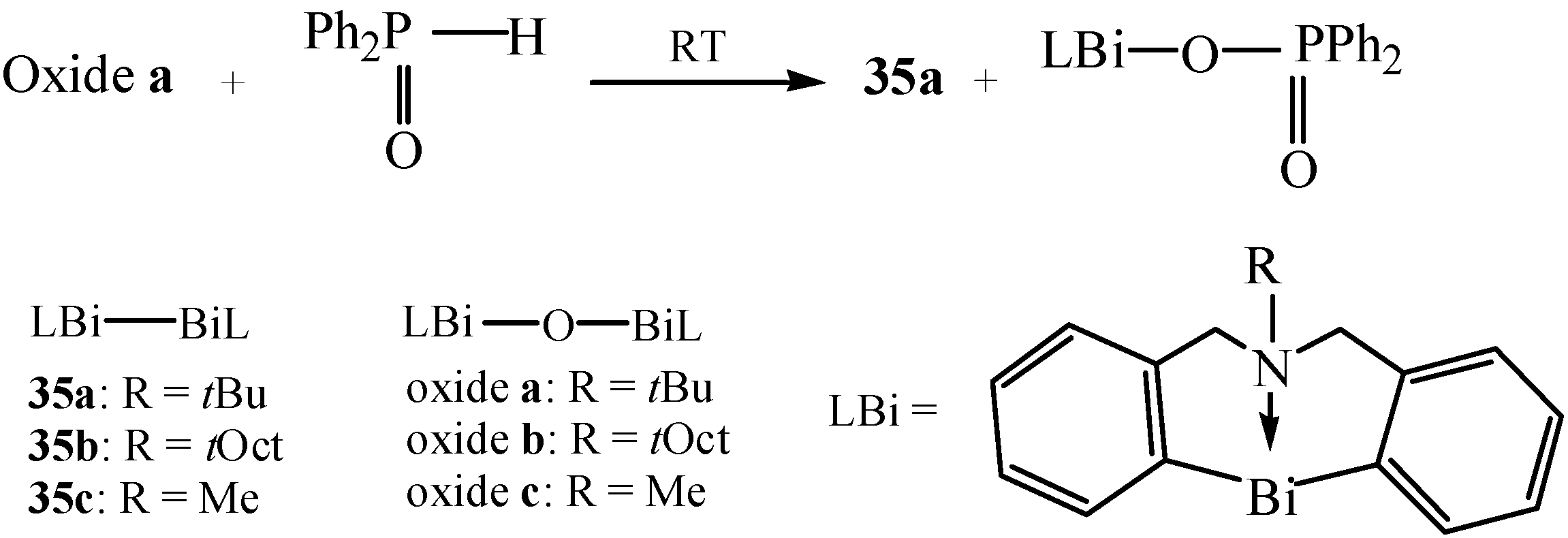
2.14. Borate Ester Coordinated Organobismuth Compounds

2.15. Monoorganobismuth(III) Compounds

2.16. Air-Stable Organobismuth Compounds


2.17. Silyl-Substituted Bismuth Compounds

2.18. Other Special Organobismuth Compounds
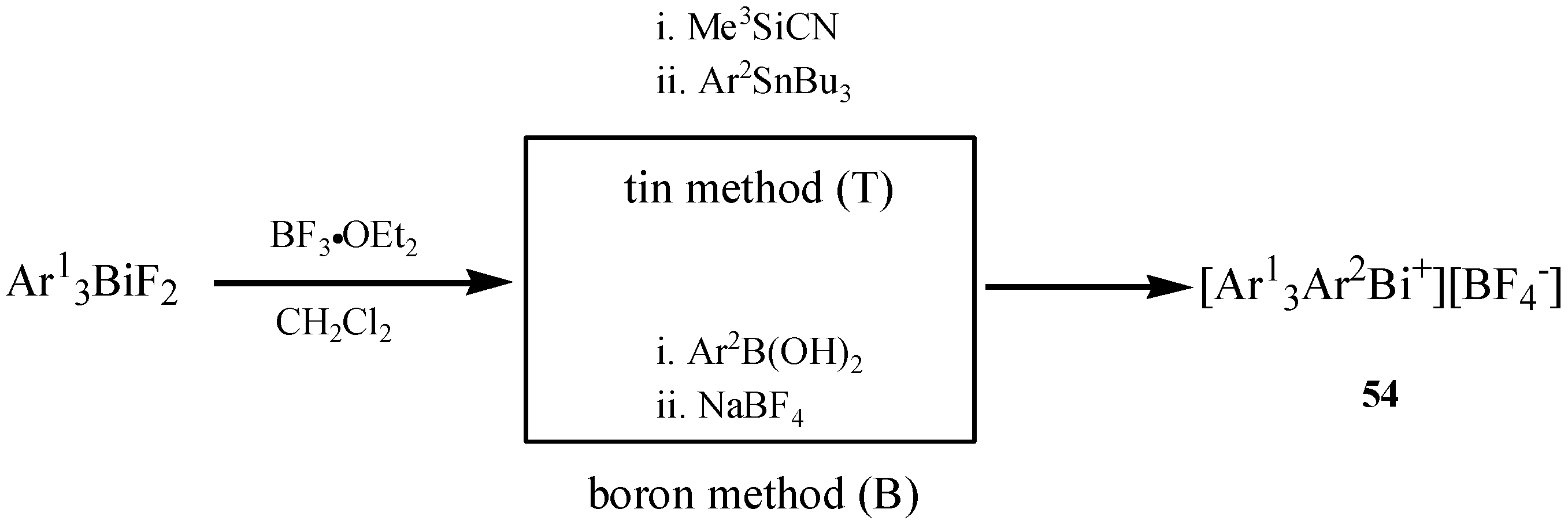


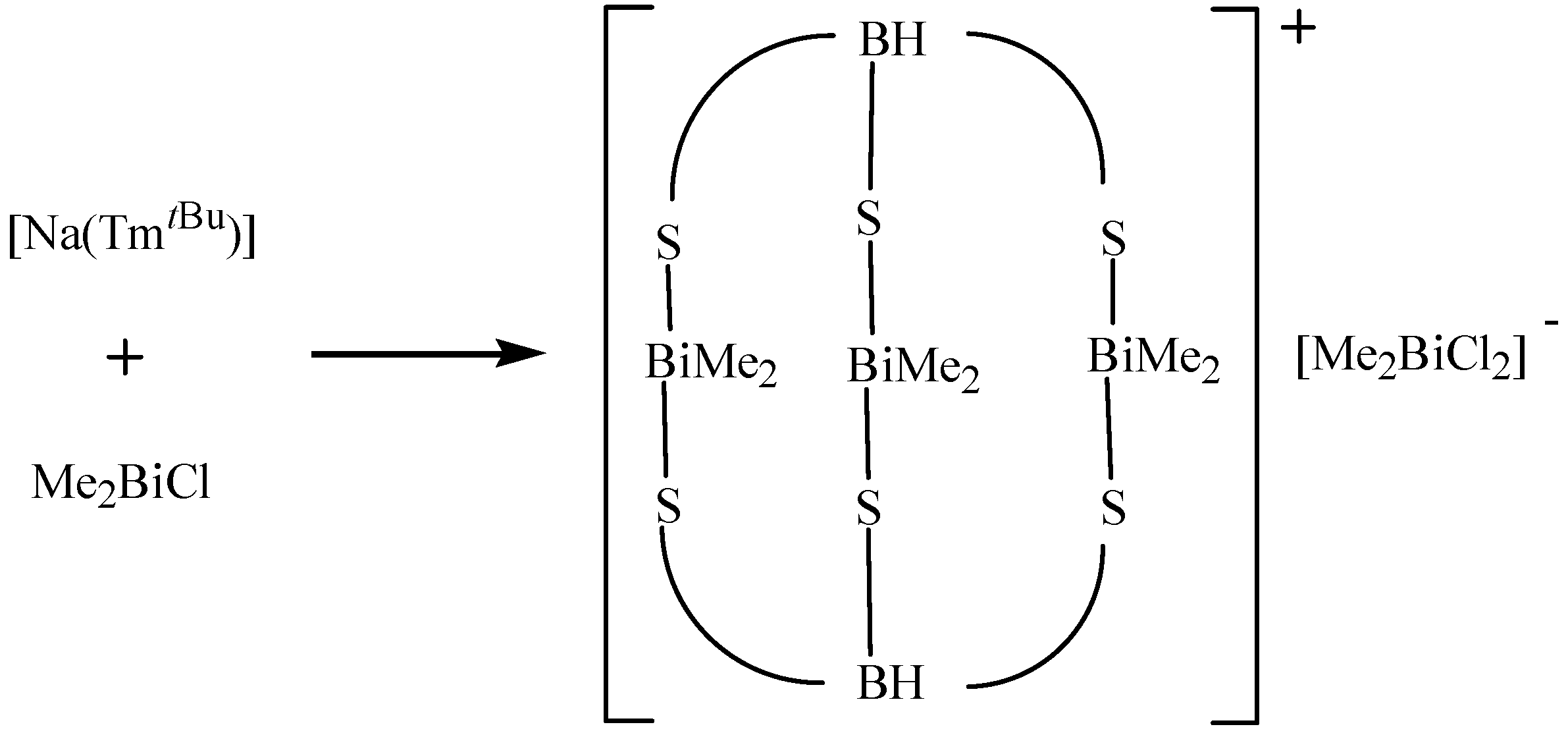
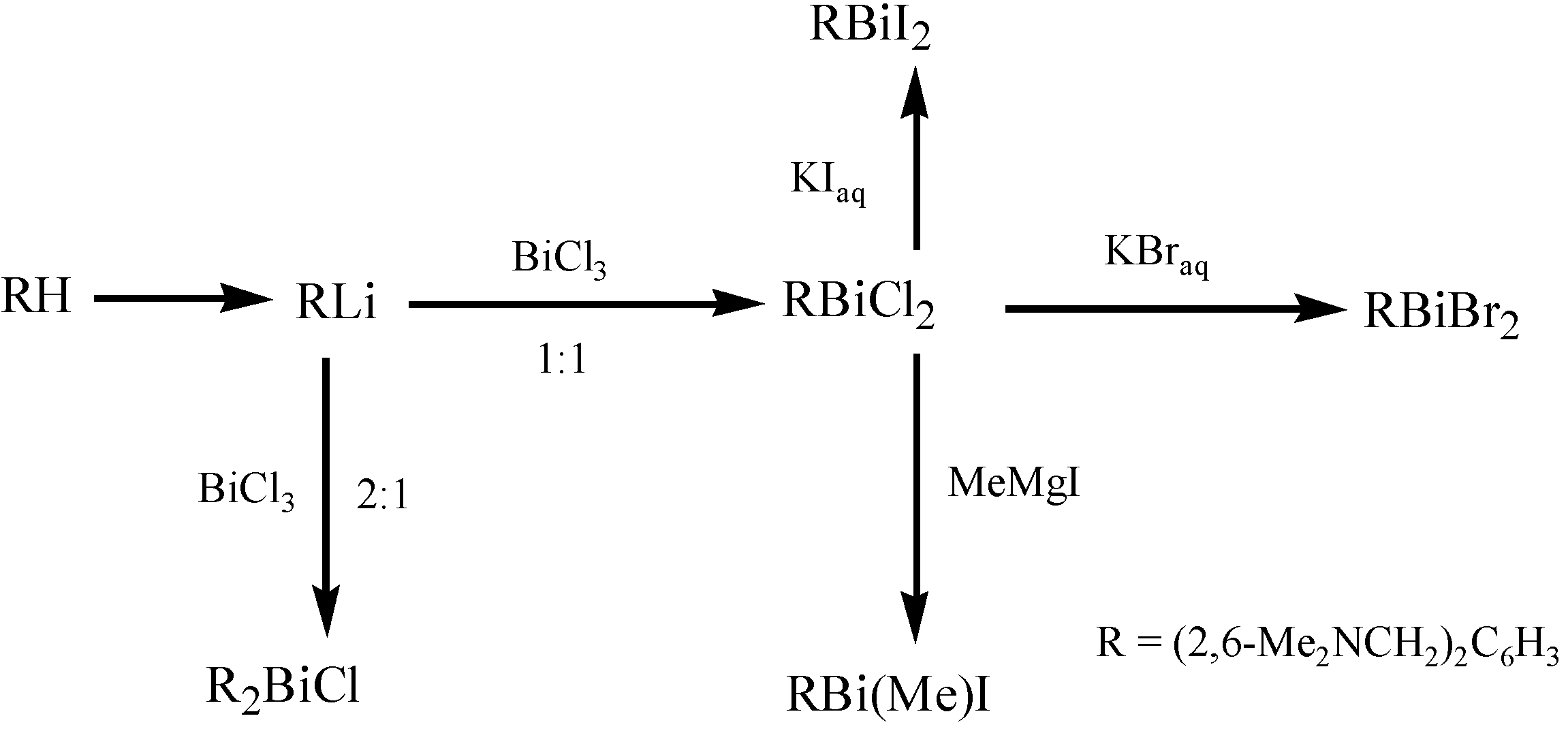
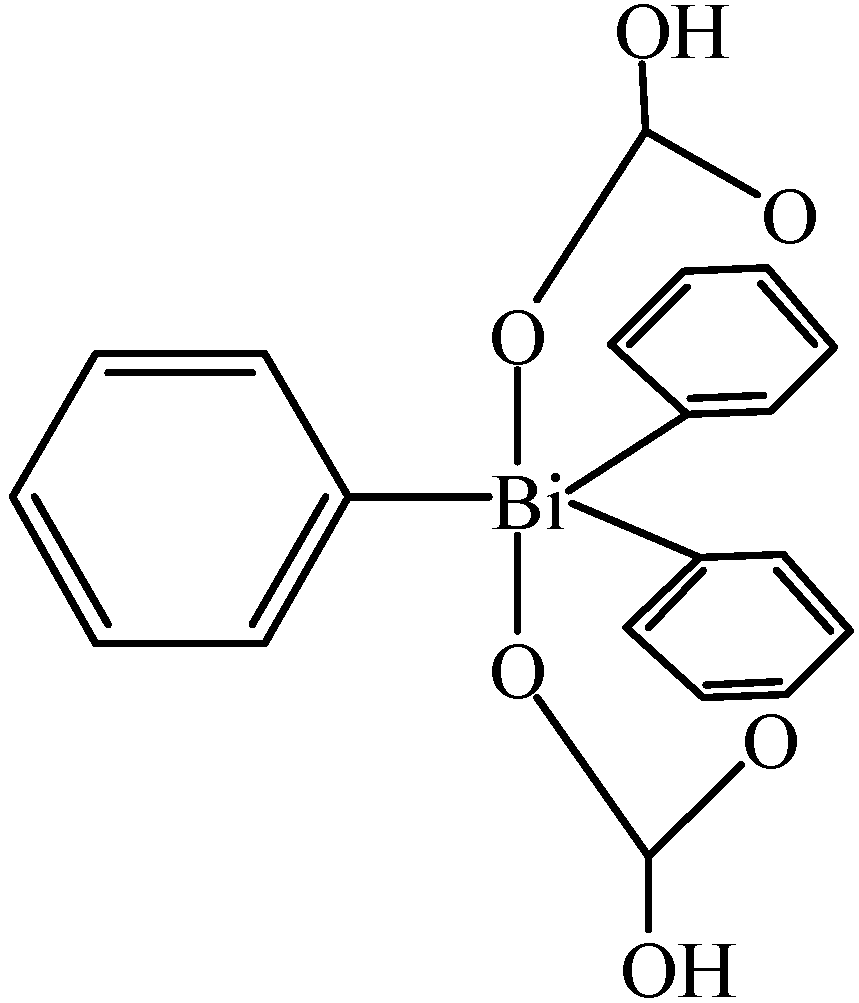
3. Applications of Organobismuth Reagents in Organic Synthesis
3.1. Arylation Reactions
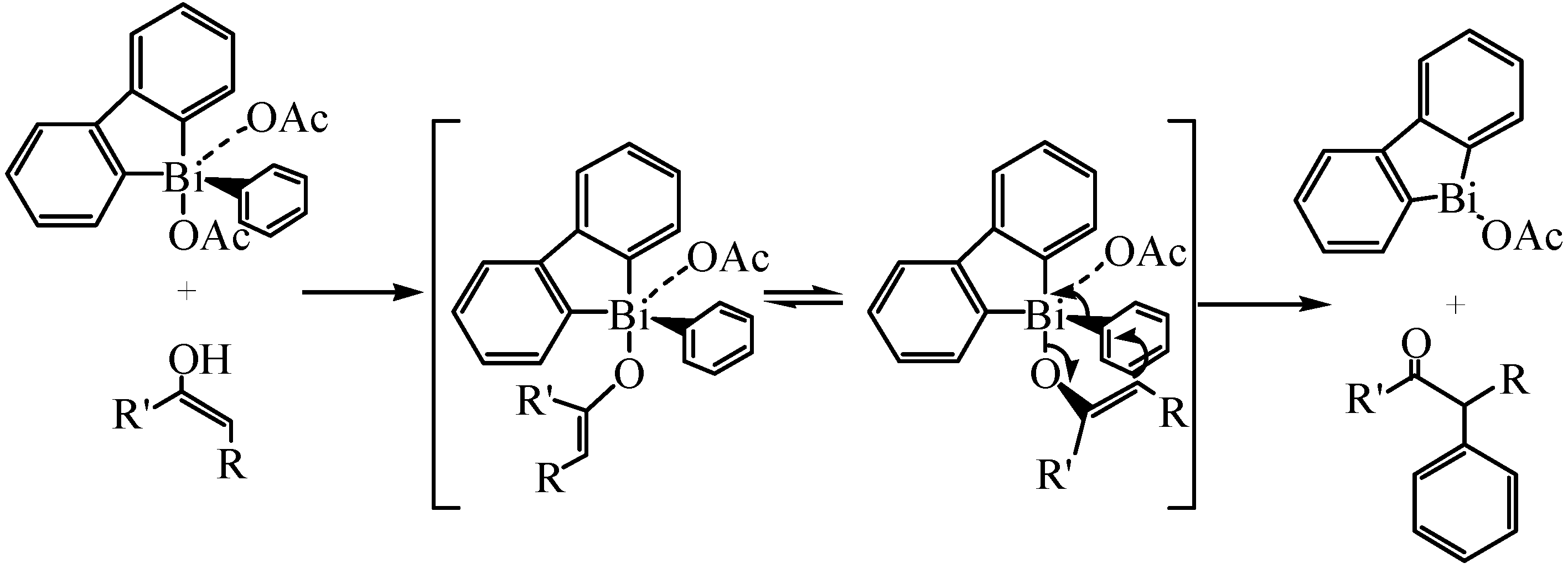
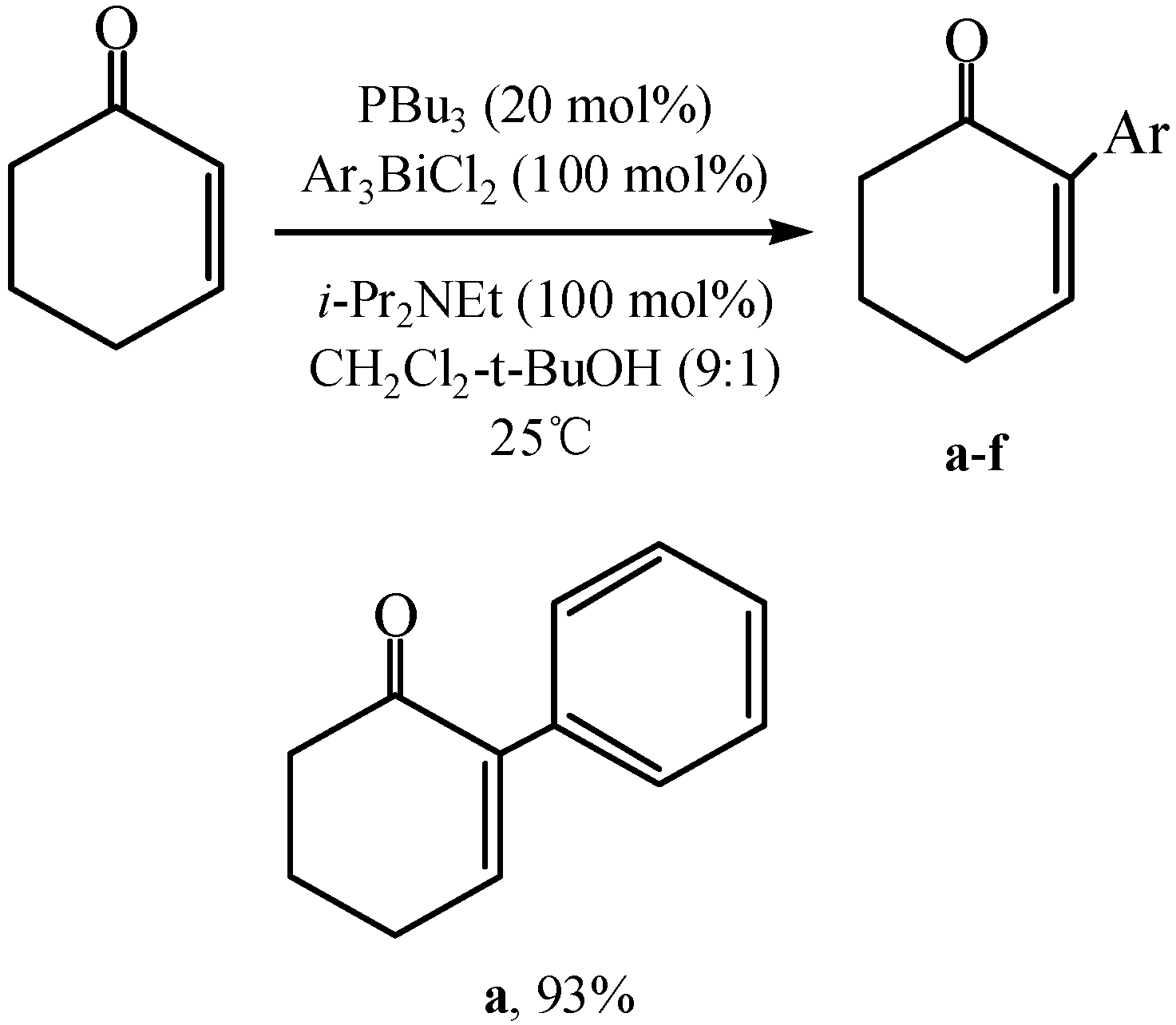


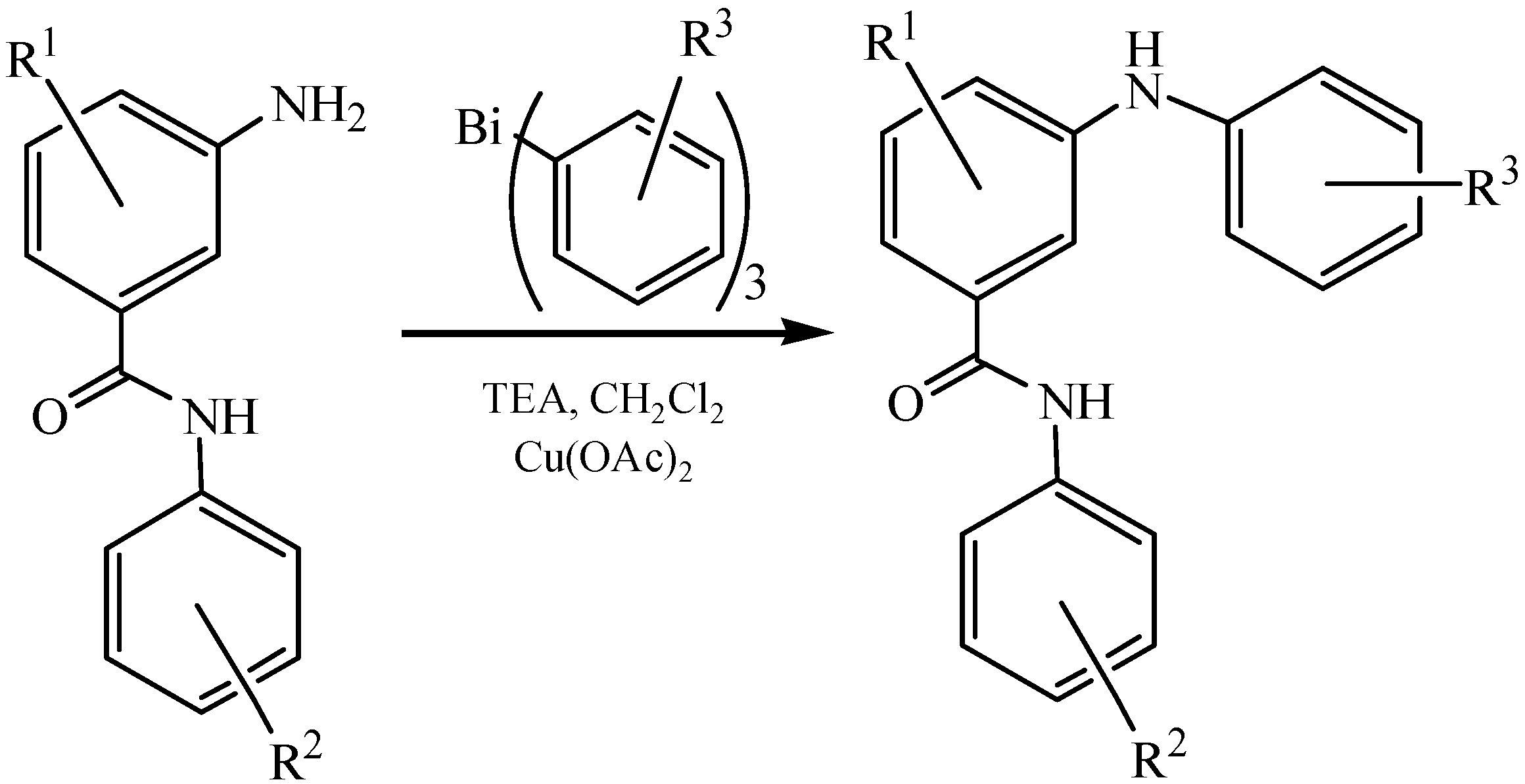
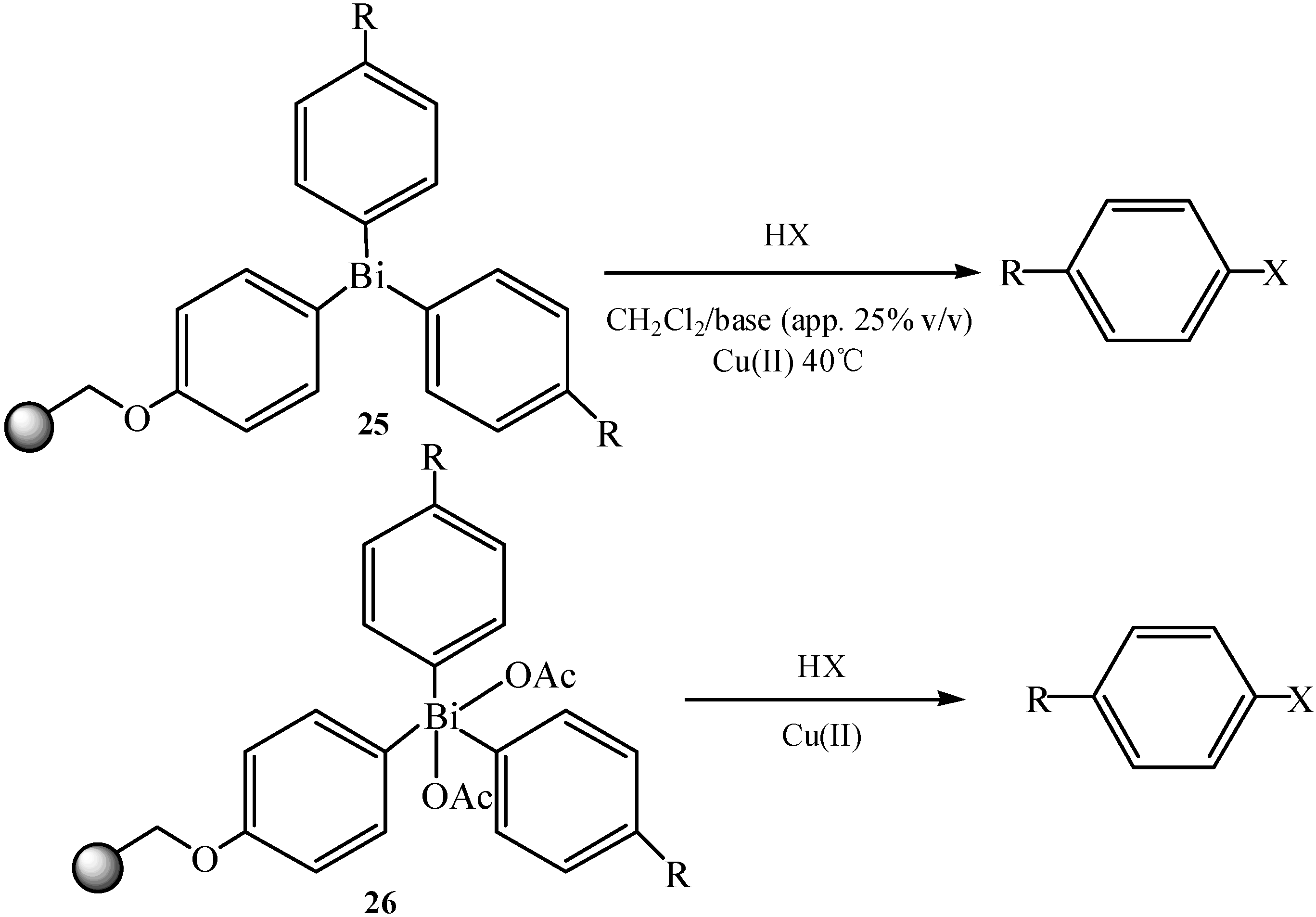
3.2. Cross-coupling Reactions
| Reagent | Reaction | Catalyst | Yield, % (highest) | References |
|---|---|---|---|---|
| Organobismuth dialkoxides | Cross-coupling with aryl and vinyl triflates | Pd(PPh3)4 | 99 | [84] |
| Organobismuth dialkoxides | Cross-coupling with aryl bromides and iodides | Pd(PPh3)4 | 99 | [81] |
| Triarylbismuths | Cross-coupling with aryl halides and triflates | PdCl2/PPh3 | 96 | [1] |
| Triarylbismuths | Cross-coupling with α,β-unsaturated acyl chlorides | PdCl2/PPh3 | 91 | [10] |
| Triarylbismuths | Cross-coupling with allylic carbonates | PdCl2(PPh3)2 | 90 | [85] |
| Resin-boundtriarylbismuthanes | Suzuki cross-coupling with aryl boronic acids | Pd2dba3 andtri- tert-butylphosphane | 83 | [51] |
| Triarylbismuths | Cross-coupling with aryl bromides or iodides | polystyrene-supported PdII | 94 | [82] |
| Triarylbismuths | Multi-coupling with vinylic iodides | PdCl2(PPh3)2 | 85 | [83] |
| Triarylbismuths | Domino coupling with 1,1-dibromo-1-alkenes | Pd(PPh3)4 | 88 | [86] |
| Triarylbismuths | Multi-coupling with bromide and chloride derivatives of Baylis–Hillman adducts | Pd2dba3 | 91 | [87] |

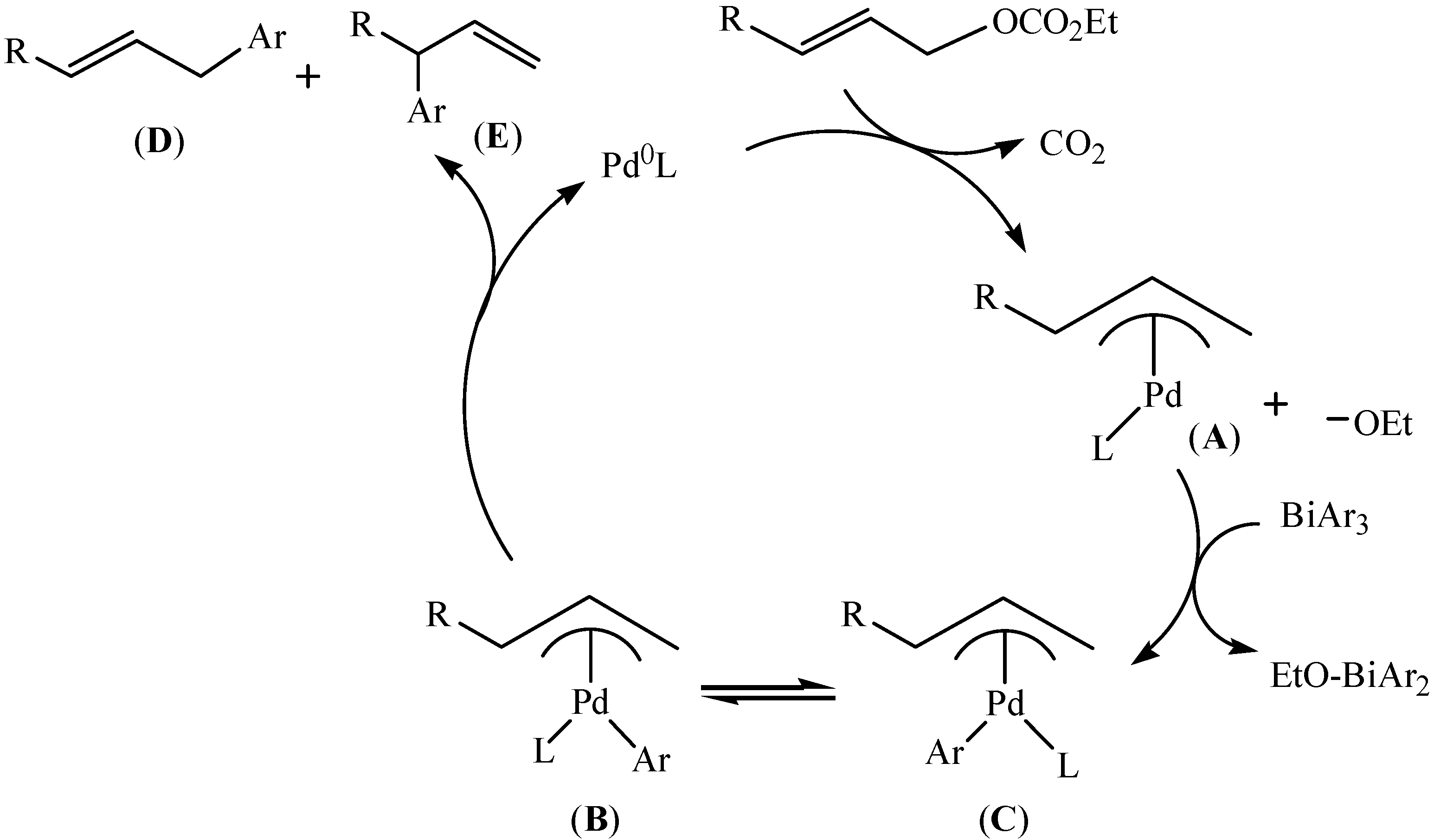


3.3. Asymmetric Synthesis

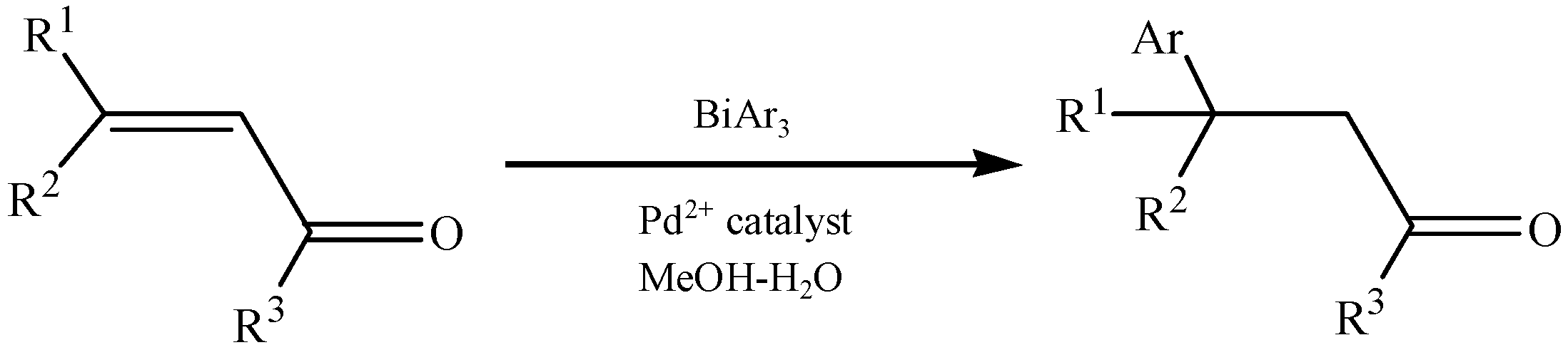
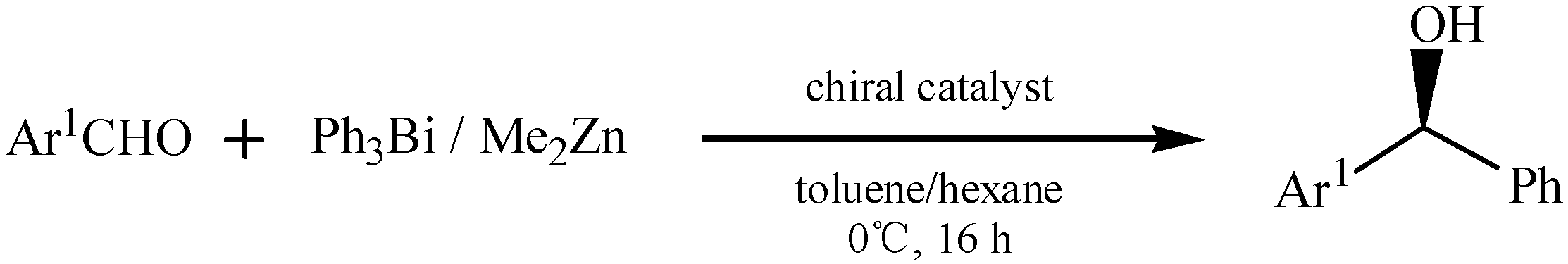
3.4. Other Special Reactions

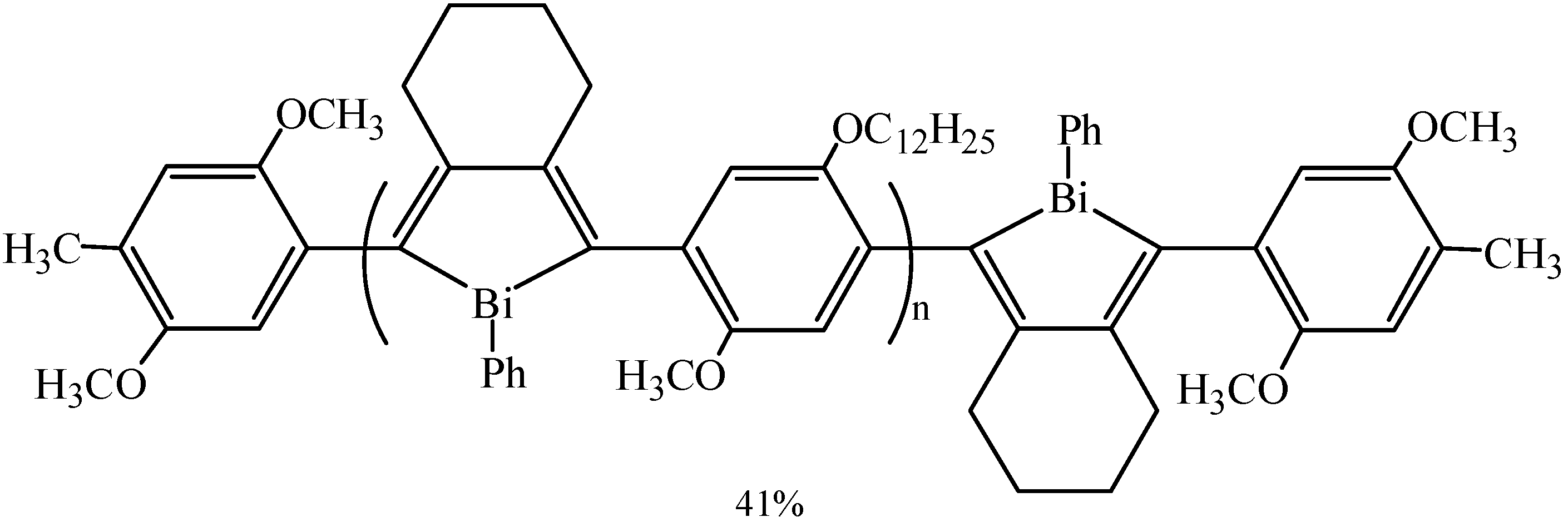

4. Applications of Organobismuth Catalysts in Organic Synthesis
4.1. Cationic Organobismuth Complexes

4.2. Air-Stable Organobismuth Compounds


4.3. Carbon Dioxide Fixation by Organobismuths


5. Conclusions
Acknowledgements
References
- Rao, M.L.N.; Jadhav, D.N.; Banerjee, D. A new palladium catalyzed protocol for atom-efficient cross-coupling reactions of triarylbismuths with aryl halides and triflates. Tetrahedron 2008, 64, 5762–5772. [Google Scholar] [CrossRef]
- Chovancová, M.; Jambor, R.; Růžička, A.; Jirásko, R.; Císařová, I.; Dostál, L. Synthesis, structure, and reactivity of intramolecularly coordinated organoantimony and organobismuth sulfides. Organometallics 2009, 28, 1934–1941. [Google Scholar]
- Dostál, L.; Novák, P.; Jambor, R.; Růžička, A.; Císařová, I.; Jirásko, R.; Holeček, J. Synthesis and structural study of organoantimony(III) and organobismuth(III) triflates and cations containing O,C,O-pincer type ligands. Organometallics 2007, 26, 2911–2917. [Google Scholar]
- Kyne, R.E.; Ryan, M.C.; Kliman, L.T.; Morken, J.P. Allylation of nitrosobenzene with pinacol allylboronates. A regioselective complement to peroxide oxidation. Org. Lett. 2010, 12, 3796–3799. [Google Scholar] [CrossRef]
- El-Newehy, M.H.; Al-Deyab, S.S.; Al-Hazmi, A.M.A. Reactivity ratios for organotin copolymer systems. Molecules 2010, 15, 2749–2758. [Google Scholar]
- Saxena, K.; Bisaria, C.S.; Saxena, A.K. Synthesis and characterization of some novel silicon esters and their application as lubricant base stock solution. Appl. Organometal. Chem. 2009, 23, 535–540. [Google Scholar]
- Opris, L.M.; Preda, A.M.; Varga, R.A.; Breunig, H.J.; Silvestru, C. Synthesis and characterization of hypervalent organoantimony(III) compounds containing the [2-(Me2NCH2)C6H4]2Sb fragment. Eur. J. Inorg. Chem. 2009, 2009, 1187–1193. [Google Scholar]
- Elliott, G.I.; Konopelski, J.P. Arylation with organolead and organobismuth reagents. Tetrahedron 2001, 57, 5683–5705. [Google Scholar] [CrossRef]
- Finet, J.-P.; Fedorov, A.Y. Tris(polymethoxyphenyl)bismuth derivatives: Synthesis and reactivity. J. Organometal. Chem. 2006, 691, 2386–2393. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Venkatesh, V.; Jadhav, D.N. A palladium catalyzed atom-efficient cross-coupling reactivity of triarylbismuths with α,β-unsaturated acyl chlorides. J. Organometal. Chem. 2008, 693, 2494–2498. [Google Scholar]
- Spafford, M.J.; Christensen, J.E.; Huddle, M.G.; Lacey, J.R.; Mohan, R.S. Environmentally friendly organic synthesis using bismuth compounds. Bismuth trifluoromethanesulfonate-catalyzed allylation of dioxolanes. Aust. J. Chem. 2008, 61, 419–421. [Google Scholar] [CrossRef]
- Khosropour, A.R.; Mohammadpoor-Baltork, I.; Ghorbankhani, H. Bi(TFA)3 immobilized in [nbpy]FeCl4: An efficient catalyst system for the one-pot synthesis of 4,6-diarylpyrimidin-2(1H)-ones. Catal. Commun. 2006, 7, 713–716. [Google Scholar]
- Ménard, C.; Doris, E.; Mioskowski, C. Ph3BiCO3: A mild reagent for in situ oxidation of urazoles to triazolinediones. Tetrahedron Lett. 2003, 44, 6591–6593. [Google Scholar] [CrossRef]
- Kirija, N.V.; Pasenoka, S.V.; Yagupolskiia, Y.L.; Tyrrab, W.; Naumann, D. Bi(CF3)3/Cu(OCOCH3)2—A new system for the synthesis of 2-trifluoromethylcycloalkan-1-ones, trifluoromethylanilines and phenyl(trifluoromethyl)sulfane. J. Fluorine Chem. 2000, 106, 217–221. [Google Scholar] [CrossRef]
- Zhang, X.W.; Xia, J.; Yan, H.W.; Luo, S.L.; Yin, S.F.; Au, C.T.; Wong, W.Y. Synthesis, structure, and in vitro antiproliferative activity of cyclic hypervalent organobismuth(III) chlorides and their triphenylgermylpropionate derivatives. J. Organometal. Chem. 2009, 694, 3019–3026. [Google Scholar] [CrossRef]
- Yu, L.; Ma, Y.Q.; Wang, G.C.; Song, H.B.; Wang, H.G.; Li, J.S.; Cui, J.R.; Wang, R.Q. Synthesis, characterization and cytotoxicity of some triarylbismuth(V) di(N-p-toluenesulfonyl) aminoacetates and the crystal structure of (4-CH3C6H4SO2NHCH2CO2)2Bi(C6H4Cl-4)3. Appl. Organometal. Chem. 2004, 18, 187–190. [Google Scholar]
- Postel, M.; Duñach, E. Bismuth derivatives for the oxidation of organic compounds. Coordin. Chem. Rev. 1996, 155, 127–144. [Google Scholar]
- Matano, Y.; Aratani, Y.; Miyamatsu, T.; Kurata, H.; Miyaji, K.; Sasako, S.; Suzuki, H. Water-soluble non-ionic triarylbismuthanes. First synthesis and properties. J. Chem. Soc. Perkin Trans. 1 1998, 2511–2518. [Google Scholar]
- Breunig, H.J.; Rösler, R.; Lork, E. The first organobismuth rings: (RBi)3 and (RBi)4, R = (Me3Si)2CH. Angew. Chem. Int. Ed. 1998, 37, 3175–3177. [Google Scholar] [CrossRef]
- Barucki, H.; Coles, S.J.; Costello, J.F.; Hursthouse, M.B. Upon the intriguing stereoselective formation of organobismuth(V) complexes. Chem. Eur. J. 2003, 9, 2877–2884. [Google Scholar]
- Soran, A.; Breunig, H.J.; Lippolis, V.; Arca, M.; Silvestru, C. Syntheses, solid-state structures, solution behavior of hypervalent organobismuth(III) compounds [2-(Et2NCH2)C6H4]nBiX3–n and DFT characterization of [2-(Me2NCH2)C6H4]nBiX3–n [X = Cl, Br, I; n = 1–3]. J. Organometal. Chem. 2010, 695, 850–862. [Google Scholar]
- Iwata, T.; Miyake, Y.; Nishibayashi, Y.; Uemura, S. Palladium(II) complex-catalysed enantioselective benzoylation of alcohols using carbon monoxide and an organobismuth(V) compound. J. Chem. Soc. Perkin Trans. 1 2002, 1548–1554. [Google Scholar]
- Breunig, H.J.; Haddad, N.; Lork, E.; Mehring, M.; Mügge, C.; Nolde, C.; Raţ, C.I.; Schürmann, M. Novel sterically congested monoorganobismuth(III) compounds: Synthesis, structure, and bismuth-arene π interaction in ArBiXY (X, Y = Br, I, OH, 2,6-Mes2-4-t-Bu-C6H2PHO2). Organometallics 2009, 28, 1202–1211. [Google Scholar]
- Zhang, X.W.; Yin, S.F.; Qiu, R.H.; Xia, J.; Dai, W.L.; Yu, Z.Y.; Au, C.T.; Wong, W.Y. Synthesis and structure of an air-stable hypervalent organobismuth(III) perfluorooctanesulfonate and its use as high-efficiency catalyst for Mannich-type reactions in water. J. Organometal. Chem. 2009, 694, 3559–3564. [Google Scholar]
- Hassan, A.; Breeze, S.R.; Courtenay, S.; Deslippe, C.; Wang, S.N. Organobismuth(III) and organobismuth(V) complexes containing pyridyl and amino functional groups. Syntheses and characterizations of BiIIIAr3 (Ar = p-C6H4(NMe2), p-C6H4CH2(NPri2), p-C6H4[CH2N(2-Py)2]), BiVAr3L2, [BiVAr3Cl]2O, [BiVAr4][PF6], and [BiVAr4]2[Ag2Cl4] (Ar = p-C6H4(NMe2) or p-C6H4[CH2N(2-Py)2], L = Cl, CH3CO2−, CF3CO2−). Organometallics 1996, 15, 5613–5621. [Google Scholar] [CrossRef]
- Soran, A.P.; Silvestru, C.; Breunig, H.J.; Balázs, G.; Green, J.C. Organobismuth(III) dihalides with T-shaped geometry stabilized by intramolecular N→Bi interactions and related diorganobismuth(III) halides. Organometallics 2007, 26, 1196–1203. [Google Scholar]
- Benjamin, S.L.; Karagiannidis, L.; Levason, W.; Reid, G.; Rogers, M.C. Hybrid dibismuthines and distibines: Preparation and properties of antimony and bismuth oxygen, sulfur, and nitrogen donor ligands. Organometallics 2011, 30, 895–904. [Google Scholar]
- Bao, M.; Hayashi, T.; Shimada, S. Cationic organobismuth complex with 5,6,7,12-tetra-hydrodibenz[c,f][1,5]azabismocine framework and its coordination complexes with neutral molecules. Organometallics 2007, 26, 1816–1822. [Google Scholar]
- Gilman, H.; Yale, H.L. Organobismuth compounds. Chem. Rev. 1942, 30, 281–320. [Google Scholar] [CrossRef]
- Finet, J.P. Arylation reactions with organobismuth reagents. Chem. Rev. 1989, 89, 1487–1501. [Google Scholar] [CrossRef]
- Suzuki, H.; Ikegami, T.; Matano, Y. Bismuth in organic transformations. Synthesis-Stuttgart 1997, 249–267. [Google Scholar]
- Zhang, X.W.; Yin, S.F.; Wu, S.S.; Dai, W.L.; Li, W.S.; Zhou, X.P. Organobismuth chemistry in the past decade. Prog. Chem. 2008, 20, 878–886. [Google Scholar]
- Jiang, Q.Y.; Shen, J.; Zhong, G.Q. Synthesis of bismuth(III) complexes and coordination chemistry of bismuth(III). Prog. Chem. 2006, 18, 1634–1645. [Google Scholar]
- Jha, N.K.; Ugal, J.R.; Sharma, P. Mixed halophenylantimonates(III). Indian J. Chem. Sect. A 1993, 32, 71–73. [Google Scholar]
- Sharma, P.; Cabrera, A.; Rosas, N.; Arias, J.L.; Lemus, A.; Sharma, M.; Hernández, S.; Garcia, J.L. Synthesis and crystal structures of mixed halophenylbismuthates(III). Z. Anorg. Allg. Chem. 2000, 626, 921–924. [Google Scholar] [CrossRef]
- Fedorov, A.Y.; Finet, J.-P. Synthesis and reactivity of pentavalent biphenyl-2,2’-ylenebismuth derivatives. J. Chem. Soc. Perkin Trans. 1 2000, 3775–3778. [Google Scholar] [CrossRef]
- Wittig, G.; Hellwinkel, D. Recent synthesis of aromatic compounds of pentavalent antimony and bismuth. Chem. Ber. 1964, 97, 789–793. [Google Scholar] [CrossRef]
- Combe, S.; Finet, J.-P. On the Exclusion of radical species in the ligand coupling reactions with pentavalent triarylbismuth derivatives. Tetrahedron 1999, 55, 3377–3386. [Google Scholar] [CrossRef]
- Yoshida, S.; Yasui, M.; Iwasaki, F.; Yamamoto, Y.; Chen, X.; Akiba, K. Crystal and molecular-structures of bismuth compounds of a spiro-delta-sulfurane type. Acta Crystallogr. Sect. B-Struct. Sci. 1994, 50, 151–157. [Google Scholar]
- Uchiyama, Y.; Kano, N.; Kawashima, T. Synthesis and structure of a novel ladder-type organobismuth compound with bismuth-oxygen interactions. Organometallics 2001, 20, 2440–2442. [Google Scholar]
- Yamamoto, Y.; Chen, X.; Akiba, K. Synthesis and crystal-structure of intramolecularly coordinated organobismuth compounds and edge inversion at trivalent bismuth. J. Am. Chem. Soc. 1992, 114, 7906–7907. [Google Scholar] [CrossRef]
- Matano, Y.; Nomura, H.; Suzuki, H.; Shiro, M.; Nakano, H. Synthesis, structure, and reactions of (acylimino)triaryl-λ5-bismuthanes: First comparative study of the (acylimino)pnictorane series. J. Am. Chem. Soc. 2001, 123, 10954–10965. [Google Scholar] [CrossRef]
- Bolshakov, A.V.; Ganina, O.G.; Shavirin, A.S.; Kurskii, Y.A.; Finet, J.-P.; Fedorov, A.Y. Three-step one-pot organobismuth-mediated synthesis of benzo[b]pyran compounds. Tetrahedron Lett. 2002, 43, 8245–8248. [Google Scholar]
- Boymond, L.; Rottländer, M.; Cahiez, G.; Knochel, P. Preparation of highly functionalized grignard reagents by an iodine-magnesium exchange reaction and its application in solid-phase synthesis. Angew. Chem. Int. Ed. 1998, 37, 1701–1703. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Finet, J.P. Bismuth(V) reagents in organic-synthesis. Pure Appl. Chem. 1987, 59, 937–946. [Google Scholar] [CrossRef]
- Naumann, D.; Tyrra, W.; Lewe, T. Synthesis and characterization of new pentafluorophenylbismuth(V) derivatives. J. Fluorine Chem. 1997, 84, 69–73. [Google Scholar] [CrossRef]
- Ooi, T.; Goto, R.; Maruoka, K. Fluorotetraphenylbismuth: A new reagent for efficient regioselective α-phenylation of carbonyl compounds. J. Am. Chem. Soc. 2003, 125, 10494–10495. [Google Scholar] [CrossRef]
- Jensen, K.A.; Buchardt, O. Derivatives of P-chlorobenzenesulfonic acid. Acta Chem. Scand. 1961, 15, 447–448. [Google Scholar] [CrossRef]
- Barton, D.H.R.; Bhatnagar, N.Y.; Blazejewski, J.C.; Charpiot, B.; Finet, J.-P.; Lester, D.J.; Motherwell, W.B.; Papoula, M.T.B.; Stanforth, S.P. Pentavalent organobismuth reagents. 2. The phenylation of phenols. J. Chem. Soc. Perkin Trans. 1 1985, 2657–2665. [Google Scholar]
- Rasmussen, L.K.; Begtrup, M.; Ruhland, T. Resin-bound triaryl bismuthanes and bismuth diacetates: Novel multidirectional linkers and novel resin-bound arylation reagents. J. Org. Chem. 2004, 69, 6890–6893. [Google Scholar] [CrossRef]
- Rasmussen, L.K.; Begtrup, M.; Ruhland, T. Solid-phase synthesis with resin-bound triarylbismuthanes: Traceless and multidirectional cleavage of unsymmetrical biphenyls. J. Org. Chem. 2006, 71, 1230–1232. [Google Scholar] [CrossRef]
- Gagnon, A.; St-Onge, M.; Little, K.; Duplessis, M.; Barabé, F. Direct N-cyclopropylation of cyclic amides and azoles employing a cyclopropylbismuth reagent. J. Am. Chem. Soc. 2007, 129, 44–45. [Google Scholar] [CrossRef]
- Shimada, S.; Yamazaki, O.; Tanaka, T.; Suzuki, Y.; Tanaka, M. Synthesis and structure of 5,6,7,12-tetrahydrodibenz[c,f][1,5]azabismocines. J. Organometal. Chem. 2004, 689, 3012–3023. [Google Scholar]
- Shimada, S.; Maruyama, J.; Choe, Y.-K. Yamashita, T. A unique Bi-Bi bond forming reaction using organobismuth oxides and phosphorus compounds bearing a P(=O)H group. Chem. Commun. 2009, 6168–6170. [Google Scholar]
- Caires, C.C.; Guccione, S. Synthesis, structure, and reactivity of borate ester coordinated organobismuth compounds. Organometallics 2008, 27, 747–752. [Google Scholar]
- Silvestru, C.; Breunig, H.J.; Althaus, H. Structural chemistry of bismuth compounds. I. Organobismuth derivatives. Chem. Rev. 1999, 99, 3277–3327. [Google Scholar]
- Wolf, R.; Fischer, J.; Fischer, R.C.; Fettinger, J.C.; Power, P.P. Reactions of terphenylbismuth dihalides with KSi(SiMe3)3, K2Si2(SiMe3)4 and Na2[Fe(CO)]4: Reduction vs. metathesis. Eur. J. Inorg. Chem. 2008, 2515–2521. [Google Scholar]
- Qiu, R.H.; Yin, S.F.; Zhang, X.W.; Xia, J.; Xu, X.H.; Luo, S.L. Synthesis and structure of an air-stable cationic organobismuth complex and its use as a highly efficient catalyst for the direct diastereoselective Mannich reaction in water. Chem. Commun. 2009, 4759–4761. [Google Scholar]
- Qiu, R.H.; Qiu, Y.M.; Yin, S.F.; Xu, X.H.; Luo, S.L.; Au, C.T.; Wong, W.Y.; Shimada, S. Highly efficient and selective synthesis of (E)-α,β-unsaturated ketones by crossed condensation of ketones and aldehydes catalyzed by an air-stable cationic organobismuth perfluorooctanesulfonate. Adv. Synth. Catal. 2010, 352, 153–162. [Google Scholar] [CrossRef]
- Zhang, X.W.; Qiu, R.H.; Tan, N.Y.; Yin, S.F.; Xia, J.; Luo, S.L.; Au, C.T. Air-stable hypervalent organobismuth(III) tetrafluoroborate as effective and reusable catalyst for the allylation of aldehyde with tetraallyltin. Tetrahedron Lett. 2010, 51, 153–156. [Google Scholar]
- Thomas, F.; Schulz, S.; Nieger, M. Synthesis and X-ray crystal structure of [Me2GaBi(SiMe3)2]3. Organometallics 2002, 21, 2793–2795. [Google Scholar]
- Monakhov, K.Yu.; Zessin, T.; Linti, G. Reduction vs. metathesis in the reactions of bismuth tribromide with a bulky lithium silanide—An experimental and theoretical study. Eur. J. Inorg. Chem. 2010, 322–332. [Google Scholar]
- Matano, Y.; Begum, S.A.; Miyamatsu, T.; Suzuki, H. Synthesis and stereochemical behavior of unsymmetrical tetraarylbismuthonium salts. Organometallics 1999, 18, 5668–5681. [Google Scholar] [CrossRef]
- Matano, Y.; Kurata, H.; Murafuji, T.; Azuma, N.; Suzuki, H. Synthesis and properties of a series of phenylene-bridged Bin-bismuthanes. Organometallics 1998, 17, 4049–4059. [Google Scholar] [CrossRef]
- Bao, M.; Hayashi, T.; Shimada, S. Synthesis and structural characterisation of a cationic trinuclear organobismuth complex with an unprecedented coordination mode of hydrotris(2-mercapto-imidazolyl)borate ligands. Dalton Trans. 2004, 2055–2056. [Google Scholar]
- Breunig, H.J.; Nema, M.G.; Silvestru, C.; Soran, A.; Varga, R.A. [2-{E(CH2CH2)2NCH2}C6H4]nBiX3–n (E = O, NMe; X = Cl, Br, I; n = 1–3) and [2-(Me2NCH2)C6H4]BiBr2—New hypervalent organobismuth(III) compounds. Z. Anorg. Allg. Chem. 2010, 636, 2378–2386. [Google Scholar]
- Hassan, A.; Wang, S.N. Organobismuth(V) complexes containing bifunctional ligands: Hydrogen-bonded extended structures and stereoselectivity. J. Chem. Soc. Dalton Trans. 1997, 2009–2017. [Google Scholar] [CrossRef]
- Yin, H.D.; Wang, C.H.; Wang, D.Q.; Xing, Q.J. Synthesis and crystal structure of the bismuth derivative of N, N-diethyldithiocarbamate, (Et2NCS2)2(NO3)Bi(NO3)Bi(S2CNEt2)2(HOCH3). Chinese J. Org. Chem. 2004, 24, 658–662. [Google Scholar]
- Sakurai, N.; Mukaiyama, T. New preparative method of aryl tosylates by using organobismuth reagents. Chem. Lett. 2007, 36, 928–929. [Google Scholar] [CrossRef]
- Chan, D.M.T. Promotion of reaction of N–H bonds with triarylbismuth and cupric acetate. Tetrahedron Lett. 1996, 37, 9013–9016. [Google Scholar] [CrossRef]
- Arnauld, T.; Barton, D.H.R.; Doris, E. The chemistry of pentavalent organobismuth reagents. New preparative methods for aryl bismuth (V) carboxylates and suffonates. Tetrahedron Lett. 1997, 38, 365–366. [Google Scholar] [CrossRef]
- Lermontov, S.A.; Rakov, I.M.; Zefirov, N.S. A novel method of C–C bond formation via phenylation of terminal acetylenes by triphenylbismuth difluoride. Tetrahedron Lett. 1996, 37, 4051–4054. [Google Scholar] [CrossRef]
- David, S.; Thieffry, A. Selective phenylation in mild conditions of one hydroxy group in glycols with triphenylbismuth diacetate—A new specific glycol reaction. Tetrahedron Lett. 1981, 22, 5063–5066. [Google Scholar] [CrossRef]
- Koech, P.K.; Krische, M.J. Phosphine catalyzed α-arylation of enones and enals using hypervalent bismuth reagents: Regiospecific enolate arylation via nucleophilic catalysis. J. Am. Chem. Soc. 2004, 126, 5350–5351. [Google Scholar] [CrossRef]
- Boyer, G.; Galy, J.P.; Barbe, J. Synthesis of substituted pyrazolo[3,4-B]phenothiazine and pyrazolo[4,3-C]phenothiazine derivatives. Heterocycles 1995, 41, 487–496. [Google Scholar] [CrossRef]
- Coles, S.J.; Costello, J.F.; Hursthouse, M.B.; Smith, S. A structural and mechanistic investigation of the mono-O-phenylation of diols with BiPh3(OAc)2. J. Organometal. Chem. 2002, 662, 98–104. [Google Scholar]
- Sakurai, N.; Ikegai, K.; Mukaiyama, T. Copper(II)-catalyzed O-phenylation of alcohols with organobismuth(V) reagents. Arkivoc 2007, 254–264. [Google Scholar]
- Tšubrik, O.; Mäeorg, U.; Ragnarsson, U. Highly selective arylation of disubstituted hydrazines by pentavalent organobismuth reagents. Tetrahedron Lett. 2002, 43, 6213–6215. [Google Scholar] [CrossRef]
- Tšubrik, O.; Mäeorg, U.; Sillard, R.; Ragnarsson, U. Arylation of diversely substituted hydrazines by tri- and pentavalent organobismuth reagents. Tetrahedron 2004, 60, 8363–8373. [Google Scholar] [CrossRef]
- Sorenson, R.J. Selective N-arylation of aminobenzanilides under mild conditions using triarylbismuthanes. J. Org. Chem. 2000, 65, 7747–7749. [Google Scholar]
- Rao, M.L.N.; Shimada, S.; Yamazaki, O.; Tanaka, M. Cross-coupling reaction of organobismuth dialkoxides with aryl bromides and iodides catalyzed by Pd(PPh3)4. J. Organometal. Chem. 2002, 659, 117–120. [Google Scholar]
- Zhou, W.J.; Wang, K.H.; Wang, J.X.; Huang, D.F. Reusable, polystyrene-resin-supported, palladium-catalyzed, atom-efficient cross-coupling reaction of aryl halides with triarylbismuths. Eur. J. Org. Chem. 2010, 416–419. [Google Scholar]
- Rao, M.L.N.; Jadhav, D.N.; Venkatesh, V. Atom-efficient vinylic arylations with triarylbismuths as substoichiometric multicoupling reagents under palladium catalysis. Eur. J. Org. Chem. 2009, 4300–4306. [Google Scholar]
- Rao, M.L.N.; Shimada, S.; Tanaka, M. Palladium complex-catalyzed cross-coupling reaction of organobismuth dialkoxides with triflates. Org. Lett. 1999, 1, 1271–1273. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Banerjee, D.; Giri, S. Palladium-catalyzed cross-couplings of allylic carbonates with triarylbismuths as multi-coupling atom-efficient organometallic nucleophiles. J. Organometal. Chem. 2010, 695, 1518–1525. [Google Scholar] [CrossRef]
- Rao, M.L.N.; Jadhav, D.N.; Dasgupta, P. Pd-catalyzed domino synthesis of internal alkynes using triarylbismuths as multicoupling organometallic nucleophiles. Org. Lett. 2010, 12, 2048–2051. [Google Scholar]
- Rao, M.L.N.; Banerjee, D.; Dhanorkar, R.J. Pd(0)-catalyzed couplings using bromide and chloride derivatives of baylis-hillman adducts with triarylbismuths as atom-efficient multi-coupling nucleophiles. Tetrahedron 2010, 66, 3623–3632. [Google Scholar]
- Rao, M.L.N.; Banerjee, D.; Dhanorkar, R.J. Pd-catalyzed coupling of aryl iodides with triarylbismuths as atom-economic multi-coupling organometallic nucleophiles under mild conditions. Tetrahedron Lett. 2010, 51, 6101–6104. [Google Scholar]
- Sato, I.; Toyota, Y.; Asakura, N. A highly enantioselective and catalytic aryl transfer reaction using mixed triarylbismuthane and dialkylzinc reagents. Eur. J. Org. Chem. 2007, 2608–2610. [Google Scholar]
- Nishikata, T.; Yamamoto, Y.; Miyaura, N. Asymmetric 1,4-addition of triarylbismuths to enones catalyzed by dicationic palladium(II) complexes. Chem. Commun. 2004, 1822–1823. [Google Scholar]
- Leonard, N.M.; Wieland, L.C.; Mohan, R.S. Applications of bismuth(III) compounds in organic synthesis. Tetrahedron 2002, 58, 8373–8397. [Google Scholar] [CrossRef]
- Miyake, Y.; Iwata, T.; Chung, K.-G.; Nishibayashi, Y.; Uemura, S. Kinetic resolution of secondary alcohols via chiral Pd(II)-complex-catalysed enantioselective benzoylation using CO and organobismuth(V) compound. Chem. Commun. 2001, 2584–2585. [Google Scholar]
- Morisaki, Y.; Ohashi, K.; Na, H.-S.; Chujo, Y. First Synthesis of the bismole-containing conjugated polymer. J. Polym. Sci. Part A: Polym. Chem. 2006, 44, 4857–4863. [Google Scholar]
- Pu, H.T.; Cai, X.Y.; Wan, D.C.; Yang, G.J. Living radical polymerization of N-vinylamides. Prog. Chem. 2008, 20, 1573–1577. [Google Scholar]
- Krawczuk, P.J.; Schöne, N.; Baran, P.S. A synthesis of the carbon skeleton of maoecrystal V. Org. Lett. 2009, 11, 4774–4776. [Google Scholar]
- Ikegai, K.; Fukumoto, K.; Mukaiyama, T. Copper(II)-catalyzed O-phenylation of tertiary alcohols with organobismuth(V) reagents. Chem. Lett. 2006, 35, 612–613. [Google Scholar] [CrossRef]
- Imachi, S.; Mukaiyama, T. Oxidative coupling of carbonyl compounds by using pentavalent biphenyl-2,2’-ylenebismuth reagents. Chem. Lett. 2007, 36, 718–719. [Google Scholar] [CrossRef]
- Sakurai, N.; Mukaiyama, T. Direct α-oxytosylation of ketones by using pentavalent organobismuth reagents. Chem. Lett. 2008, 37, 388–389. [Google Scholar] [CrossRef]
- Gaspard-Iloughmane, H.; Roux, C.L. Bismuth(III) triflate in organic synthesis. Eur. J. Org. Chem. 2004, 2517–2532. [Google Scholar] [CrossRef]
- Ollevier, T.; Li, Z.Y. Bismuth triflate-catalyzed addition of allylsilanes to N-alkoxycarbonylamino sulfones: Convenient access to 3-Cbz-protected cyclohexenylamines. Adv. Synth. Catal. 2009, 351, 3251–3259. [Google Scholar]
- Narsaiah, A.V.; Reddy, B.V.S.; Premalatha, K.; Reddy, S.S.; Yadav, J.S. Bismuth(III)-catalyzed hydrolysis of epoxides and aziridines: An efficient synthesis of vic-diols and β-amino alcohols. Catal. Lett. 2009, 131, 480–484. [Google Scholar]
- Desmurs, J.R. Surprising catalytic activity of bismuth(III) triflate in the friedel-crafts acylation reaction. Tetrahedron Lett. 1997, 38, 8871–8874. [Google Scholar]
- Kobayashi, S.; Ogino, T.; Shimizu, H.; Ishikawa, S.; Hamada, T.; Manabe, K. Bismuth triflate-chiral bipyridine complexes as water-compatible chiral lewis acids. Org. Lett. 2005, 7, 4729–4731. [Google Scholar] [CrossRef]
- Zeimentz, P.M.; Arndt, S.; Elvidge, B.R.; Okuda, J. Cationic organometallic complexes of scandium, yttrium, and the lanthanoids. Chem. Rev. 2006, 106, 2404–2433. [Google Scholar] [CrossRef]
- Qiu, R.H.; Qiu, Y.M.; Yin, S.F.; Song, X.X.; Meng, Z.G.; Xu, X.H.; Zhang, X.W.; Luo, S.L.; Au, C.-T.; Wong, W.-Y. Facile separation catalyst system: Direct diastereoselective synthesis of (E)-α,β-unsaturated ketones catalyzed by an air-stable Lewis acidic/basic bifunctional organobismuth complex in ionic liquids. Green Chem. 2010, 12, 1767–1771. [Google Scholar]
- Darensbourg, D.J.; Holtcamp, M.W. Catalysts for the reactions of epoxides and carbon dioxide. Coord. Chem. Rev. 1996, 153, 155–174. [Google Scholar] [CrossRef]
- Yin, S.F.; Maruyama, J.; Yamashita, T.; Shimada, S. Efficient fixation of carbon dioxide by hypervalent organobismuth oxide, hydroxide, and alkoxide. Angew. Chem. Int. Ed. 2008, 47, 6590–6593. [Google Scholar] [CrossRef]
- Yin, S.F.; Shimada, S. Synthesis and structure of bismuth compounds bearing a sulfur-bridged bis(phenolato) ligand and their catalytic application to the solvent-free synthesis of propylene carbonate from CO2 and propylene oxide. Chem. Commun. 2009, 1136–1138. [Google Scholar]
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Luan, J.; Zhang, L.; Hu, Z. Synthesis, Properties Characterization and Applications of Various Organobismuth Compounds. Molecules 2011, 16, 4191-4230. https://doi.org/10.3390/molecules16054191
Luan J, Zhang L, Hu Z. Synthesis, Properties Characterization and Applications of Various Organobismuth Compounds. Molecules. 2011; 16(5):4191-4230. https://doi.org/10.3390/molecules16054191
Chicago/Turabian StyleLuan, Jingfei, Lingyan Zhang, and Zhitian Hu. 2011. "Synthesis, Properties Characterization and Applications of Various Organobismuth Compounds" Molecules 16, no. 5: 4191-4230. https://doi.org/10.3390/molecules16054191
APA StyleLuan, J., Zhang, L., & Hu, Z. (2011). Synthesis, Properties Characterization and Applications of Various Organobismuth Compounds. Molecules, 16(5), 4191-4230. https://doi.org/10.3390/molecules16054191






