Antioxidant and Antimicrobial Attributes and Phenolics of Different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]
Abstract
:1. Introduction
2. Results and Discussion
2.1. Extraction Yields

2.2. Total Phenolics and Flavonoids Contents
2.3. HPLC Analysis of Phenolic Compounds
| Parameters | Solvent extracts | ||||||
|---|---|---|---|---|---|---|---|
| Leaves | Absolute | 80% | Absolute | 80% | Absolute | 80% | Deionized |
| Etanol | ethanol | methanol | methanol | acetone | acetone | water | |
| TPC (g/100 g DW) | 1.48 ± 0.06 bc | 2.31 ± 0.08 b | 2.40 ± 0.07 b | 3.63 ± 0.12 a | 1.01 ± 0.02 c | 1.21 ± 0.05 c | 0.89 ± 0.04 d |
| TFC (g/100 g DW) | 0.52 ± 0.03 bc | 0.78 ± 0.02 b | 0.99 ± 0.05 ab | 1.19 ± 0.07 a | 0.27 ± 0.02 d | 0.42 ± 0.01 c | 0.17 ± 0.03 cd |
| DPPH, IC50 (μg/mL) | 18.78 ± 0.5 b | 16.53 ± 0.7 bc | 13.26 ± 0.4 bc | 8.89 ± 0.46 c | 24.11 ± 0.81 ab | 22.74 ± 0.72 b | 34.93 ± 0.92 a |
| Inhibition of linoleic acid peroxidation (%) | 68.08 ± 2.8 b | 74.02 ± 3.5 ab | 79.58 ± 3.1 b | 85.54 ± 4.1 a | 48.14 ± 2.3 c | 52.56 ± 2.1 b | 32.26 ± 1.6 ab |
| Flowers | |||||||
| TPC (g/100 g DW) | 1.28 ± 0.04 cd | 1.36 ± 0.06 bc | 1.68 ± 0.08 b | 2.24 ± 0.11 a | 0.36 ± 0.02 d | 0.42 ± 0.05 cd | 1.02 ± 0.02 c |
| TFC (g/100 g DW) | 0.41 ± 0.02 bc | 0.47 ± 0.02 b | 0.48 ± 0.02 b | 0.81 ± 0.03 a | 0.11 ± 0.01 d | 0.27 ± 0.03 c | 0.31 ± 0.04 bc |
| DPPH, IC50 (μg/mL) | 26.61 ± 1.1 ab | 22.68 ± 1.02 b | 16.66 ± 0.39 c | 14.80 ± 0.48 c | 44.58 ± 2.3 a | 38.02 ± 0.9 ab | 31.74 ± 0.8 b |
| Inhibition of linoleic acid peroxidation (%) | 57.62 ± 2.3 bc | 61.15 ± 2.8 ab | 73.43 ± 3.2 b | 79.69 ± 3.7 a | 40.01 ± 1.8 d | 42.48 ± 2.3 c | 45.78 ± 1.8 a |
| Bark | |||||||
| TPC (g/100 g DW) | 0.42 ± 0.02 c | 0.49 ± 0.01 b | 0.58 ± 0.01 bc | 0.69 ± 0.02 a | 0.10 ± 0.01 d | 0.16 ± 0.01 cd | 0.09 ± 0.01 c |
| TFC (g/100 g DW) | 0.16 ± 0.01 b | 0.18 ± 0.01 c | 0.21 ± 0.01 ab | 0.28 ± 0.02 a | 0.09 ± 0.00 d | 0.18 ± 0.01 cd | 0.07 ± 0.01 c |
| DPPH, IC50 (μg/mL) | 41.45 ± 1.8 b | 36.16 ± 1.3 c | 34.14 ± 1.8 c | 29.86 ± 1.2 d | 44.64 ± 1.8 b | 49.98 ± 1.9 ab | 58.84 ± 1.8 a |
| Inhibition of linoleic acid peroxidation (%) | 32.23 ± 1.2 c | 38.35 ± 2.3 b | 42.1 ± 2.6 bc | 52.3 ± 2.3 a | 22.88 ± 1.9 d | 25.12 ± 1.2 c | 19.87 ± 1.2 d |
| Compounds | Leaves | Flowers | Bark |
|---|---|---|---|
| Sorbic acid | 1.02 ± 0.06 a | 0.14 ± 0.02 b | ND |
| Sinapic acid | 1.21 ± 0.03 a | 0.80 ± 0.05 b | 0.95 ± 0.03 b |
| p-Coumaric acid | 1.24 ± 0.07 a | 1.26 ± 0.05 a | 0.15 ± 0.01 b |
| Protocatechuic acid | 5.81 ± 0.32 a | 4.24 ± 0.26 a | ND |
| m-Coumaric acid | 0.57 ± 0.02 a | 0.16 ± 0.01 b | ND |
| trans-Cinnamic acid | ND | 3.95 ± 0.16 a | 0.46 ± 0.02 b |
| Ferulic acid | 1.12 ± 0.05 a | 0.47 ± 0.03 b | ND |
| Caffeic acid | 1.29 ± 0.06 a | 0.10 ± 0.01 b | ND |
| Salicylic acid | 5.43 ± 0.28 a | 4.01 ± 0.16 a | ND |
| Gentisic acid | ND | 1.45 ± 0.04 a | 1.20 ± 0.05 b |
| Chlorogenic acid | 1.53 ± 0.06 a | 2.13 ± 0.03 b | ND |
| Gallic acid | 8.15 ± 0.46 a | 6.16 ± 0.41 b | 1.99 ± 0.06 c |
| 3-Hydroxybenzoic acid | 0.76 ± 0.03 b | 0.98 ± 0.04 a | ND |
| 4-Hydroxycinnamic acid | ND | 0.18 ± 0.01 | ND |
| 4-Hydroxybenzoic acid | ND | 1.09 ± 0.05 a | 0.91 ± 0.01 b |
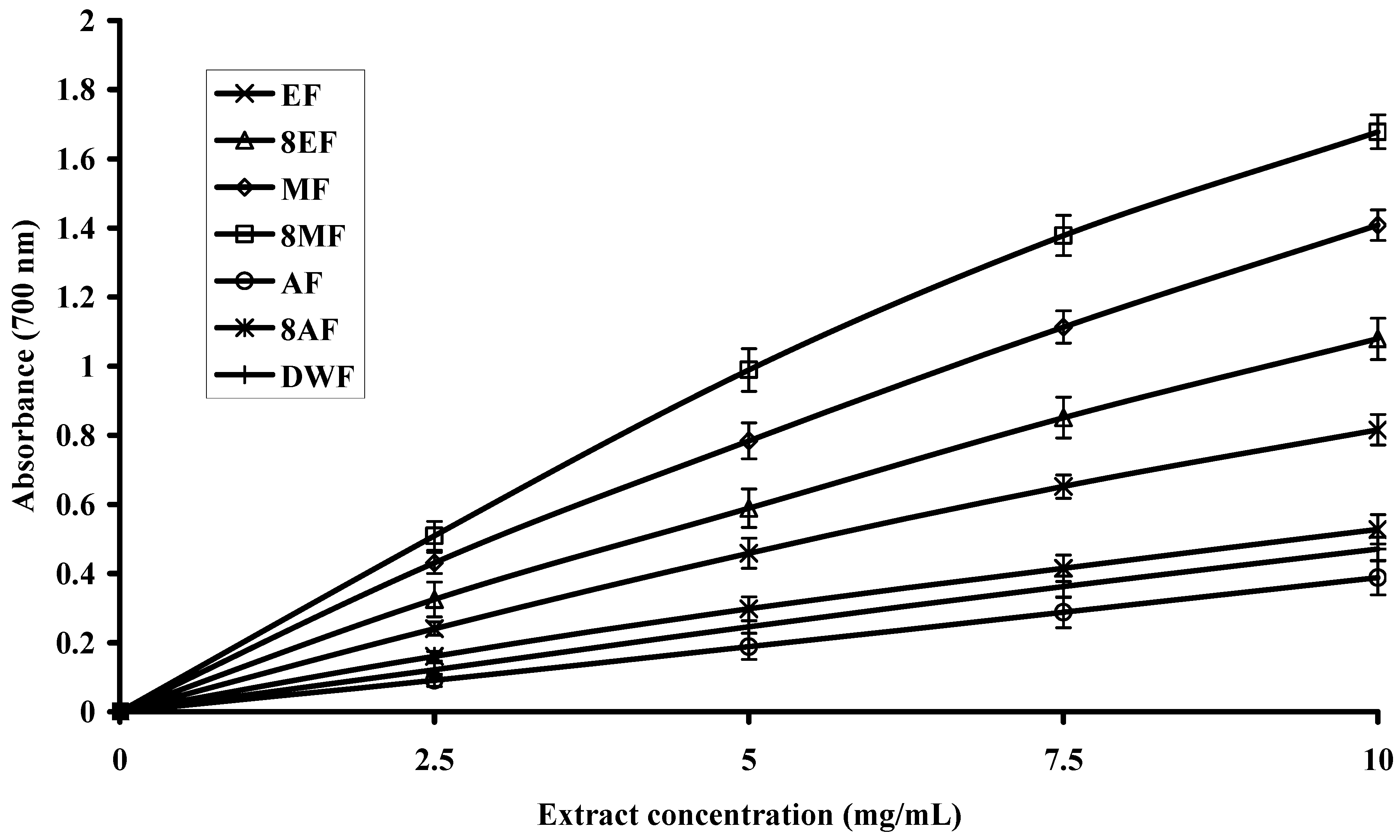
2.4. Reducing Power of Extracts


2.5. Antioxidant Activity of Extracts in Linoleic Acid Peroxidation System
2.6. DPPH Radical Scavenging Assay
2.7. Comparison between Different Antioxidant Assays
| Variable | TPC | TFC | DPPH | % Inhibition | RP |
|---|---|---|---|---|---|
| TPC | - | 0.988 | −0.959 | 0.883 | 0.933 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | ||
| TFC | 0.988 | - | −0.985 | 0.937 | 0.975 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | ||
| DPPH | −0.959 | −0.985 | - | −0.977 | −0.984 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | ||
| % Inhibition | 0.883 | 0.937 | −0.977 | - | 0.980 |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 | ||
| RP | 0.933 | 0.975 | −0.984 | 0.980 | - |
| P = 0.000 | P = 0.000 | P = 0.000 | P = 0.000 |
2.8. Antimicrobial Activity
| Organism | Leaves extracts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute | 80% | Absolute | 80% | Absolute | 80% | Deionized | Amoxicillin | Flumequine | |
| Etanol | ethanol | methanol | methanol | acetone | acetone | water | |||
| Minimum inhibitory concentration (mg/mL) | |||||||||
| Pseudomonas stutzeri | 25 ± 1.3 c | 22 ± 1.2 c | 21 ± 1.3 c | 20 ± 0.8 c | 65 ± 2.6 a | 50 ± 2.7 b | 70 ± 2.0 a | 21 ± 2.1 c | - |
| Pseudomonas aeruginosa | 35 ± 1.3 c | 30 ± 1.3 c | 26 ± 1.3 c | 23 ± 1.3 c | 75 ± 3.5 ab | 70 ± 3.3 b | 85 ± 1.8 a | 26 ± 2.4 c | - |
| Escherichia coli | 41 ± 2.1 c | 35 ± 2.4 c | 30 ± 2.7 c | 31 ± 1.4 c | 80 ± 3.2 b | 80 ± 3.1 b | 95 ± 2.3 a | 32 ± 1.8 c | - |
| Aspergilus orazae | 39 ± 2.1 b | 34 ± 2.3 bc | 32 ± 1.9 bc | 21 ± 1.9 c | 62 ± 3.2 a | 60 ± 4.1 a | 65 ± 3.4 a | - | 20 ± 1.1 c |
| Aspergilus niger | 45 ± 2.5 b | 40 ± 2.5 b | 35 ± 1.8 bc | 27 ± 1.1 c | 70 ± 4.2 a | 65 ± 4.4 a | 75 ± 3.3 a | - | 27 ± 1.2 c |
| Fusarium solani | 52 ± 3.3 b | 45 ± 3.1 bc | 42 ± 2.3 bc | 34 ± 1.2 c | 75 ± 4.3 a | 81 ± 2.8 a | 84 ± 2.9 a | - | 35 ± 1.3 c |
| Organism | Flowers extracts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute | 80% | Absolute | 80% | Absolute | 80% | Deionized | Amoxicillin | Flumequine | |
| ethanol | ethanol | methanol | methanol | acetone | acetone | water | |||
| Minimum inhibitory concentration (mg/mL) | |||||||||
| Pseudomonas stutzeri | 35 ± 1.8 c | 28 ± 0.9 c | 24 ± 1.1 d | 23 ± 1.3 d | 71 ± 3.3 a | 67 ± 2.6 ab | 65 ± 1.3 b | 21 ± 2.1 d | - |
| Pseudomonas aeruginosa | 45 ± 1.9 c | 41 ± 1.3 c | 35 ± 1.3 cd | 26 ± 1.1 d | 75 ± 3.4 a | 72 ± 3.3 a | 60 ± 1.7 b | 26 ± 2.4 d | - |
| Escherichia coli | 52 ± 2.0 bc | 50 ± 2.5 bc | 45 ± 2.3 c | 39 ± 1.1 cd | 88 ± 3.5 a | 83 ± 3.7 a | 66 ± 2.9 b | 32 ± 1.8 d | - |
| Aspergilus orazae | 56 ± 2.5 ab | 51 ± 1.3 b | 45 ± 1.2 c | 24 ± 1.1 cd | 70 ± 3.8 a | 68 ± 3.3 a | 68 ± 2.3 a | - | 20 ± 1.1 d |
| Aspergilus niger | 65 ± 2.8 ab | 57 ± 1.5 b | 49 ± 1.3 c | 30 ± 1.4 cd | 80 ± 2.9 a | 75 ± 2.3 a | 69 ± 2.7 ab | - | 27 ± 1.2 d |
| Fusarium solani | 70 ± 3.1 b | 65 ± 3.1 ab | 55 ± 2.8 c | 38 ± 1.8 d | 89 ± 3.4 a | 85 ± 2.8 a | 77 ± 2.9 ab | - | 35 ± 1.3 d |
| Organism | Bark extracts | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Absolute | 80% | Absolute | 80% | Absolute | 80% | Deionized | Amoxicillin | Flumequine | |
| ethanol | ethanol | methanol | methanol | acetone | acetone | water | |||
| Minimum inhibitory concentration (mg/mL) | |||||||||
| Pseudomonas stutzeri | 60 ± 2.4 a | 55 ± 2.3 ab | 50 ± 2.3 ab | 45 ± 1.3 b | - | - | - | 21 ± 2.1 c | - |
| Pseudomonas aeruginosa | 65 ± 2.6 a | 60 ± 2.8 a | 55 ± 1.6 ab | 50 ± 1.3 b | - | - | - | 26 ± 2.4 c | - |
| Escherichia coli | 70 ± 3.8 a | 70 ± 2.9 a | 60 ± 3.5 b | 58 ± 1.8 b | - | - | - | 32 ± 1.8 c | - |
| Aspergilus orazae | 65 ± 3.1 a | 65 ± 2.1 a | 70 ± 3.1 a | 55 ± 2.4 b | - | - | - | - | 20 ± 1.1 c |
| Aspergilus niger | 75 ± 3.2 a | 70 ± 2.7 a | 65 ± 2.9 b | 60 ± 2.6 b | - | - | - | - | 27 ± 1.2 c |
| Fusarium solani | 80 ± 3.2 a | 75 ± 2.9 ab | 70 ± 3.3 ab | 65 ± 2.3 b | - | - | - | - | 35 ± 1.3 c |
3. Experimental
3.1. Sample Collection and Preparation of Extracts
3.2. High Performance Liquid Chromatography (HPLC) Analysis
3.3. Determination of Total Phenolic Contents (TPC)
3.4. Determination of Total Flavonoid Contents (TFC)
3.5. Antioxidant Activity Determination in Linoleic Acid System
3.6. Determination of Reducing Power (RP)
3.7. DPPH Radical Scavenging Assay
3.8. Antimicrobial Activity
3.9. Statistical Analysis
4. Conclusions
References and Notes
- Audipudi, A.V.; Chakicherla, B.V.S. Antioxidative and antimicrobial activity of methanol and chloroform extracts of Gmelina arborea Roxb. Int. J. Biotechnol. Biochem. 2010, 6, 139–144. [Google Scholar]
- Jyothi, M.V.; Mandayan, S.N.; Kotamballi, N.C.; Bhagyalakshmi, N. Antioxidative efficacies of floral petal extracts of Delonix regia Raffin. Int. J. Biomed. Pharmaceutic. Sci. 2007, 1, 73–82. [Google Scholar]
- Adje, F.; Lozano, Y.F.; Meudec, E.; Lozano, P.; Adima, A.; Nzi, G.A.; Gaydou, E.M. Anthocyanin characterization of pilot plant water extracts of Delonix regia flowers. Molecules 2008, 13, 1238–1245. [Google Scholar] [CrossRef]
- Lawal, O.; Uzokwe, N.E.; Igboanugo, A.B.I.; Adio, A.F.; Awosan, E.A.; Nwogwugwu, J.O.; Faloye, B.; Olatunji, B.P.; Adesoga, A.A. Ethno medicinal information on collation and identification of some medicinal plants in Research Institutes of South-west Nigeria. Afr. J. Pharm. Pharmacol. 2010, 4, 1–7. [Google Scholar]
- Shanmukha, I.; Patel, H.; Patel, J.; Riyazunnisa. Quantification of total phenol and flavonoid content of Delonix regia flowers. Int. J. Chem. Tech. Res. 2011, 3, 280–283. [Google Scholar]
- Wang, S.Y.; Chang, H.N.; Lin, K.T.; Lo, C.P.; Yang, N.S.; Shyur, L.F. Antioxidant properties and phytochemical characteristics of extracts from Lactuca indica. J. Agric. Food Chem. 2003, 51, 1506–1512. [Google Scholar] [CrossRef]
- Sikwese, F.E.; Duodu, K.G. Antioxidant effects of crude phenolic extracts from sorghum bran in sunflower oil in the presence of ferric ions. Food Chem. 2007, 104, 324–331. [Google Scholar] [CrossRef]
- Behera, B.C.; Verma, N.; Sonone, A.; Makhija, U. Determination of antioxidative potential of lichen Usnea ghattensis in vitro. LWT- Food Sci. Technol. 2006, 39, 80–85. [Google Scholar] [CrossRef]
- Huang, S.C.; Yen, G.C.; Chang, L.W.; Yen, W.J.; Duh, P.D. Identification of an antioxidant, ethyl protocatechuate, in peanut seed testa. J. Agric. Food Chem. 2003, 51, 2380–2383. [Google Scholar]
- Liu, Q.; Yao, H. Antioxidant activities of barley seeds extracts. Food Chem. 2007, 102, 732–737. [Google Scholar] [CrossRef]
- Rawat, S.; Jugran, A.; Giri, L.; Bhatt, I.D.; Rawal, R.S. Assessment of antioxidant properties in fruits of Myrica esculenta: A popular wild edible species in Indian Himalayan region. Evid. Based Complement. Alternat. Med. 2011. [Google Scholar] [CrossRef]
- Sarikurkcu, C. Antioxidant activities of solvent extracts from endemic Cyclamen mirabile Hildebr. Tubers and leaves. Afirican J. Biotecnol. 2011, 10, 831–839. [Google Scholar]
- Aneja, K.R.; Sharma, C.; Joshi, R. In vitro efficacy of amaltas (Cassia fistula L.) against the pathogens causing otis externa. J. Microbiol. 2011, 4, 175–183. [Google Scholar]
- Bozin, B.; Mimica-Dukic, N.; Simin, N.; Anackov, G. Characterization of the volatile composition of essential oil of some lamiaceae species and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem. 2006, 54, 1822–1828. [Google Scholar] [CrossRef]
- Celiktasa, Y.O.; Nartop, P.; Gurel, A.; Bedir, E.; Vardar-Sukan, F. Determination of phenolic content and antioxidant activity of extracts obtained from Rosmarinus officinalis’ calli. J. Plant Physiol. 2007, 164, 1536–1542. [Google Scholar] [CrossRef]
- Wojdylo, A.; Oszmianski, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Ara, N.; Nur, H. In Vitro antioxidant activity of methanolic leaves and flowers extracts of Lippia alba. Res. J. Med. Sci. 2009, 4, 107–110. [Google Scholar]
- Tibiri, A.T.; Sawadogo, R.W.; Ouedraogo, N.J.T.; Banzouzi, J.; Guissou, I.P.; Nacoulma, G.O. Evaluation of antioxidant activity, total phenolics and flavonoid contents of Entada africana Guill. et. Perr. (Mimosaceae) organ extracts. Res. J. Med. Sci. 2010, 4, 81–87. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Antioxidant properties of various extracts of total phenolic constituents from three different agroclimatic origins of drumstick tree (Moringa oleifera Lam.) leaves. J. Agric. Food Chem. 2003, 51, 2144–2155. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef]
- Aqil, F.; Ahmad, I. Broad-spectrum antibacterial and antifungal properties of certain traditionally used Indian medicinal plants. World J. Microbiol. Biotechnol. 2003, 19, 653–657. [Google Scholar] [CrossRef]
- Siddiq, A.; Anwar, F.; Manzoor, M.; Fatima, M. Antioxidant activity of different solvent extracts of Moringa oleifera leaves under accelerated storage conditions of sunflower oil. Asian J. Plant Sci. 2005, 4, 630–635. [Google Scholar] [CrossRef]
- Choudhary, R.K.; Saroha, A.E.; Swarnkar, P.L. Radical scavenging activity of phenolics and flavonoids in some medicinal plants of India. J. Pharm. Res. 2011, 4, 712–713. [Google Scholar]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Jaffery, E.H.; Brown, A.F.; Kurilich, A.C.; Keek, A.S.; Matusheski, N.; Klein, B.P. Variation in content of bioactive components in broccoli. J. Food Compos. Anal. 2003, 16, 323–330. [Google Scholar] [CrossRef]
- Rafat, A.; Philip, K.; Muniandy, S. Antioxidant potential and content of phenolic compounds in ethanolic extracts of selected parts of Andrographis paniculata. J. Med. Plants Res. 2010, 4, 197–202. [Google Scholar] [CrossRef]
- Macheix, J.J.; Fleuriet, A.; Billot, J. Fruit Phenolics; CRC Press: Boca Raton, FL, USA, 1990; pp. 106–107. [Google Scholar]
- Randhir, Y.; Lin, T.; Shetty, K. Phenolics, their antioxidant and antimicrobial activity in dark germinated fenugreek sprouts in response to peptide and phytochemical elicitors. Asia Pac. J. Clin. Nutr. 2004, 13, 295–307. [Google Scholar]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts. Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Merkl, R.; Hrádková, I.; Filip, V.; Šmidrkal, J. Antimicrobial and antioxidant properties of phenolic acids alkyl esters. Czech J. Food Sci. 2010, 28, 275–279. [Google Scholar]
- Tomas-Barberan, F.; Clifford, M.N. Dietary hydroxybenzoic acid derivatives–nature, occurrence and dietary burden. J. Sci. Food Agric. 2000, 80, 1024–1032. [Google Scholar] [CrossRef]
- Ferguson, L.R.; Shuo-Tun, Z.; Harris, P.J. Antioxidant and antigenotoxic effects of plant cell wall hydroxycinnamic acids in cultured HT-29. Mol. Nutr. Food Res. 2005, 49, 585–693. [Google Scholar] [CrossRef]
- Jayaprakasha, G.K.; Singh, R.P.; Sakariah, K.K. Antioxidant activity of grape seed (Vitis vinifera) extracts on peroxidation models in vitro. Food Chem. 2001, 73, 285–290. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Mohan, P.S.; Becker, K. Studies on the antioxidant activity of Indian Laburnum (Cassia fistula L.): A preliminary assessment of crude extracts from stem bark, leaves, flower and fruit pulp. Food Chem. 2002, 79, 61–67. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef]
- Yen, G.C.; Duh, P.D.; Chuang, D.Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000, 70, 437–441. [Google Scholar] [CrossRef]
- Zainol, M.K.; Abd-Hamid, A.; Yusof, S.; Muse, R. Antioxidative activity and total phenolic compounds of leaf, root and petiole of four accessions of Centella asiatica (L.) Urban. Food Chem. 2003, 81, 575–581. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Przybylski, R. Antioxidant activity of phenolic components present in barks of Azadirachta indica, Terminalia arjuna, Acacia nilotica, and Eugenia jambolana Lam. Trees. Food Chem. 2007, 104, 1106–1114. [Google Scholar] [CrossRef]
- Porto, C.D.; Calligaris, S.; Cellotti, E.; Nicoli, M.C. Antiradical properties of commercial cognacs assessed by the DPPH• test. J. Agric. Food Chem. 2000, 48, 4241–4245. [Google Scholar] [CrossRef]
- Paixao, N.; Perestrelo, R.; Marques, J.C.; Camara, J.S. Relationship between antioxidant capacity and total phenolic content of red, rose and white wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Asi, M.R.; Chatha, S.A.S. Antioxidant potential of extracts from different agro wastes: Stabilization of corn oil. Grasas Aceites 2008, 59, 205–217. [Google Scholar]
- Jayaprakasha, G.K.; Girennavar, B.; Patil, B.S. Radical scavenging activities of Rio Red grapefruits and Sour orange fruit extracts in different in vitro model systems. Bioresour. Technol. 2008, 99, 4484–4494. [Google Scholar] [CrossRef]
- Li, H.; Qiu, N.; Ding, H.; Yao, R. Polyphenols contents and antioxidant capcitiy of 68 Chines herbals suitable for medical or food uses. Food Res. Int. 2008, 41, 363–370. [Google Scholar] [CrossRef]
- Li, H.; Wang, X.; Li, Y.; Li, P.; Wang, H. Polyphenolic compounds and antioxidant properties of selected China wines. Food Chem. 2009, 112, 454–460. [Google Scholar] [CrossRef]
- Anwar, F.; Qayyum, H.M.A.; Hussain, A.I.; Iqbal, S. Antioxidant activity of 100% and 80% methanol extracts from barley seeds (Hordeum vulgare L.): Stabilization of sunflower oil. Grasas Aceites 2010, 61, 237–243. [Google Scholar] [CrossRef]
- Silva, E.M.; Souza, J.N.S.; Rogez, H.; Rees, J.F.; Larondella, Y. Antioxidant activities and polyphenolic contents of fifteen selected plant species from the Amazonian region. Food Chem. 2006, 101, 1012–1018. [Google Scholar]
- Frankel, E.N.; Meyer, A.S. The problems of using one dimensional methods to evaluate multifunctional food and biological antioxidants. J. Sci. Food Agric. 2000, 80, 1925–1941. [Google Scholar] [CrossRef]
- Aqil, F.; Khan, M.S.A.; Owais, M.; Ahmad, I. Effect of certain bioactive plant extracts on clinical isolates of β-lactamase producing methicillin resistant Staphylococcus aureus. J. Basic Microbiol. 2005, 45, 106–114. [Google Scholar] [CrossRef]
- Parekh, J.; Chanda, S.V. In vitro antimicrobial activity and phytochemical analysis of some indian medicinal plants. Turk. J. Biol. 2007, 31, 53–58. [Google Scholar]
- Satish, S.; Raghavendra, M.P.; Raveesha, K.A. Evaluation of the antibacterial potential of some plants against human pathogenic bacteria. Adv. Biol. Res. 2008, 2, 44–48. [Google Scholar]
- Kasim, L.S.; Ferro, V.A.; Odukoya, O.A.; Ukpo, G.E.; Seidel, V.; Gray, A. I.; Waigh, R. Evaluation of cytotoxic and antimicrobial activities of Struchium sparganophora (Linn.) Kuntze Asteraceae. J. Med. Plants Res. 2011, 5, 862–867. [Google Scholar]
- Chapuis-Lardy, L.; Contour-Ansel, D.; Bernhard-Reversat, F. High-performance liquid chromatography of water-soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci. 2002, 163, 217–222. [Google Scholar] [CrossRef]
- Chaovanalikit, A.; Wrolstad, R.E. Total anthocyanins and total phenolics of fresh and processed cherries and their antioxidant properties. J. Food Sci. 2004, 69, 67–72. [Google Scholar]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Osawa, T.; Namiki, M. A novel type of antioxidant isolated from leaf wax of eucalyptus leaves. Agric. Biol. Chem. 1981, 45, 735–739. [Google Scholar] [CrossRef]
- Ayoola, G.A.; Coker, H.A.B.; Adesegun, S.A.; Adepoju-Bello, A.; Obaweya1, K.; Ezennia1, E.C.; Atangbayila, T.O. Phytochemical screening and antioxidant activities of some selected medicinal plants used for malaria therapy in southwestern Nigeria. Trop. J. Pharm. Res. 2008, 7, 1019–1024. [Google Scholar]
- NCCLS (National Committee for Clinical Laboratory Standards), Performance Standards for Antimicrobial Susceptibility Test, 9th ed; Wayne, PA, USA, 1999; International Supplement, M100-S9.
- Sample Availability: Samples of the plant materials are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shabir, G.; Anwar, F.; Sultana, B.; Khalid, Z.M.; Afzal, M.; Khan, Q.M.; Ashrafuzzaman, M. Antioxidant and Antimicrobial Attributes and Phenolics of Different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]. Molecules 2011, 16, 7302-7319. https://doi.org/10.3390/molecules16097302
Shabir G, Anwar F, Sultana B, Khalid ZM, Afzal M, Khan QM, Ashrafuzzaman M. Antioxidant and Antimicrobial Attributes and Phenolics of Different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]. Molecules. 2011; 16(9):7302-7319. https://doi.org/10.3390/molecules16097302
Chicago/Turabian StyleShabir, Ghulam, Farooq Anwar, Bushra Sultana, Zafar M. Khalid, Muhammad Afzal, Qaiser M. Khan, and M. Ashrafuzzaman. 2011. "Antioxidant and Antimicrobial Attributes and Phenolics of Different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]" Molecules 16, no. 9: 7302-7319. https://doi.org/10.3390/molecules16097302
APA StyleShabir, G., Anwar, F., Sultana, B., Khalid, Z. M., Afzal, M., Khan, Q. M., & Ashrafuzzaman, M. (2011). Antioxidant and Antimicrobial Attributes and Phenolics of Different Solvent Extracts from Leaves, Flowers and Bark of Gold Mohar [Delonix regia (Bojer ex Hook.) Raf.]. Molecules, 16(9), 7302-7319. https://doi.org/10.3390/molecules16097302





