Chemical Profile of the Organic Residue from Ancient Amphora Found in the Adriatic Sea Determined by Direct GC and GC-MS Analysis
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Headspace Chemical Composition of the Residue (HS-SPME)
2.2. The Residue CH2Cl2 Solution Chemical Composition
3. Experimental
3.1. The Samples of the Ancient Organic Residue and Pine Resin
3.2. Headspace Solid-Phase Microextraction (HS-SPME)
3.3. Dissolution of the Samples in CH2Cl2
3.4. Gas Chromatography and Mass Spectrometry (GC, GC-MS)
3.5. Data Analysis and Data Evaluation
4. Conclusions
Acknowledgments
References
- Petrić, M. Amphorae of the Adriatic; Logos: Split, Croatia, 1989. [Google Scholar]
- Font, J.; Salvadó, N.; Butí, S.; Enrich, J. Fourier transform infrared spectroscopy as a suitable technique in the study of the materials used in waterproofing of archaeological amphorae. Anal. Chim. Acta 2007, 598, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Connan, J.; Maurin, B.; Long, L.; Sebire, H. Identification of pitch and conifer resin in archaeological samples from the Sanguinet lake: Export of pitch on the Atlantic Ocean during the Gallo-Roman period. Rev. Archéométrie 2002, 26, 177–196. [Google Scholar] [CrossRef]
- Pollard, A.M.; Heron, C. Archaeological Chemistry; Royal Society of Chemistry: Cambridge, UK, 1996; pp. 239–260. [Google Scholar]
- Colombini, M.P.; Giachi, G.; Modugno, F.; Ribechini, E. Characterisation of organic residues in pottery vessels of the Roman age of Antione (Egypt). Microchem. J. 2005, 79, 83–90. [Google Scholar] [CrossRef]
- Langenheim, J.H. Plant Resins: Chemistry, Evolution, Ecology, and Ethnobotany; Timber Press: Cambridge, UK, 2003. [Google Scholar]
- Wiyono, B.; Tachibana, S.; Tinambunan, D. Chemical composition of pine resin, rosin and turpentine oil from west Java. J. Forest. Res. 2006, 3, 7–17. [Google Scholar] [CrossRef]
- Regent, M.; Rolando, C. Identification of archaeological adhesives using direct inlet electron ionization mass spectrometry. Anal. Chem. 2002, 74, 965–975. [Google Scholar] [CrossRef]
- Evershed, R.P.; Heron, C.; Goad, J. Analysis of organic residues of archaeological origin by high-temperature gas chromatography and gas chromatography-mass spectrometry. Analyst 1990, 115, 1339–1342. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Falk, M.J. Fourier Transform Raman spectroscopic study of ancient resins: A feasibility study of application to archaeological artefacts. J. Raman Spectrosc. 1997, 28, 211–218. [Google Scholar] [CrossRef]
- Kimpe, K.; Jacobs, P.A.; Waelkens, M. Mass spectrometric methods prove the use of beeswax and ruminant fat in late Roman cooking pots. J. Chromatogr. A 2002, 968, 151–160. [Google Scholar] [CrossRef]
- Petit-Dominguez, M.D.; Martinez-Maganto, J. MCF fast derivatization procedure for the identification of resinous deposit components from the inner walls of roman age amphorae by GC-MS. Talanta 2000, 51, 727–734. [Google Scholar] [CrossRef]
- Ebeler, S.E. Analytical chemistry: Unlocking the secrets of wine flavor. Food Rev. Int. 2001, 17, 45–64. [Google Scholar] [CrossRef]
- Howard, K.L.; Mike, J.H.; Riesen, R. Validation of a solid-phase microextraction method for headspace analysis of wine aroma components. Am. J. Enol. Vitic. 2005, 56, 37–45. [Google Scholar]
- Antalick, G.; Perello, M.-C.; de Revel, G. Development, validation and application of a specific method for the quantitative determination of wine esters by headspace-solid-phase microextraction-gas chromatography-mass spectrometry. Food Chem. 2010, 121, 1236–1245. [Google Scholar] [CrossRef]
- Guasch-Jané, M.R.; Andrés-Lacueva, C.; Jáuregui, O.; Lamuela-Raventós, R.M. First evidence of white wine in ancient Egypt from Tutankhamun’s tomb. J. Archaeol. Sci. 2006, 33, 1075–1080. [Google Scholar] [CrossRef]
- McGovern, P.E.; Mirzoian, A.; Hall, G.R. Ancient Egyptian herbal wines. Anthropology 2009, 106, 7361–7366. [Google Scholar] [CrossRef] [PubMed]
- Guasch-Jané, M.R.; Ibern-Gómez, M.; Andrés-Lacueva, C.; Jáuregui, O.; Lamuela-Raventós, R.M. Liquid chromatography with mass spectrometry in tandem mode applied for the identification of wine markers in residues from ancient Egyptian vessels. Anal. Chem. 2004, 76, 1672–1676. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.J.; Bakker, J. Wine Flavour Chemistry; Blackwell: Oxford, UK, 2004. [Google Scholar]
- Marchand-Geneste, N.; Carpy, A. Theoretical study of the thermal degradation pathways of abietane skeleton diterpenoids: Aromatization to retene. J. Mol. Struc. Theochem. 2003, 635, 55–82. [Google Scholar] [CrossRef]
- Hjulström, B.; Isaksson, S.; Hennius, A. Organic geochemical evidence for pine tar production in Middle Eastern Sweden during the Roman Iron Age. J. Archaeol. Sci. 2006, 33, 283–294. [Google Scholar] [CrossRef]
- Lamboglia, N. Sulla cronologia delle amfore romane di etá repubblicana. Riv. St. Lig. 1955, 18, 247–250. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 25 July 2011).
Sample Availability: Contact the authors. |

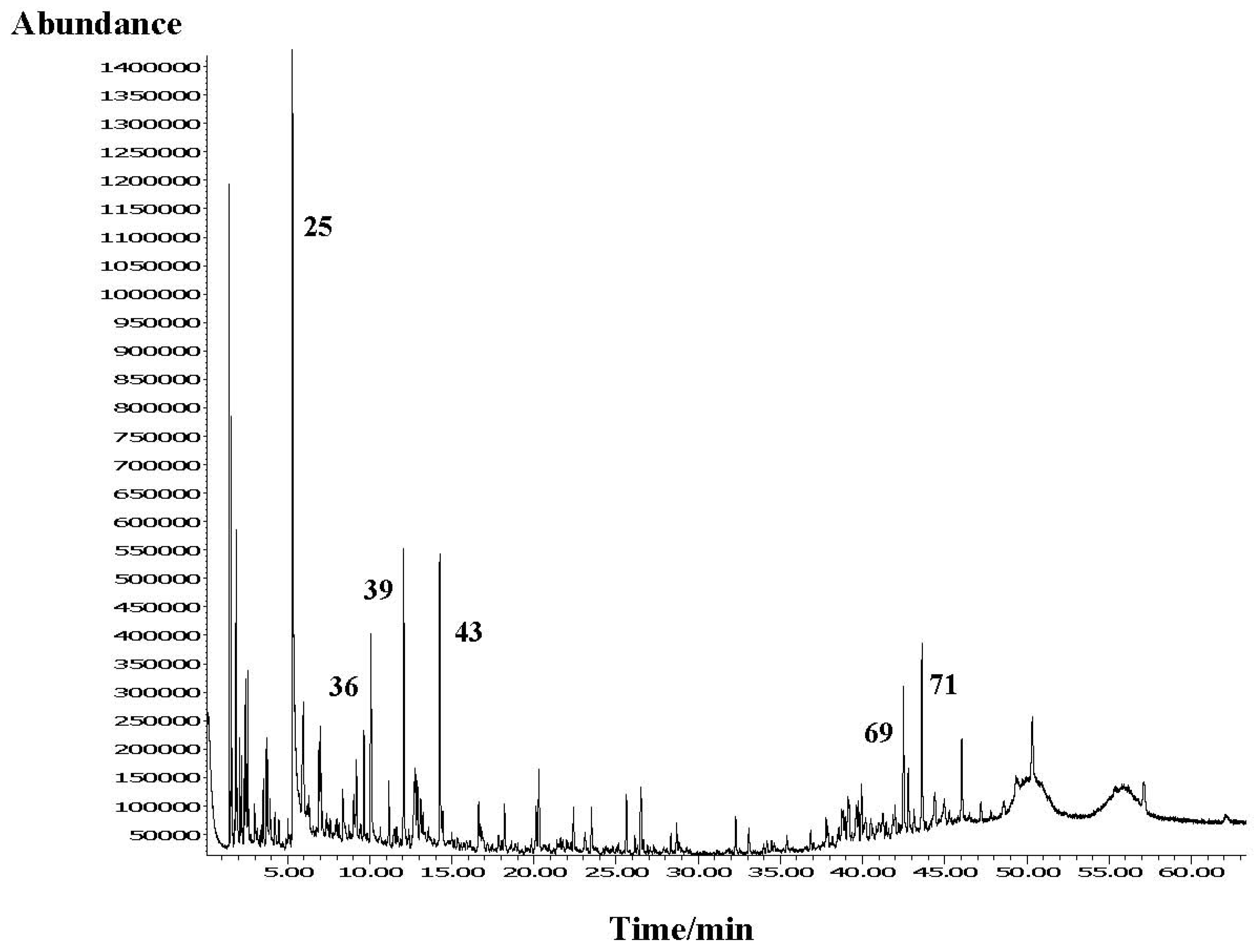


| No. | Compound | RI | A | B | C |
|---|---|---|---|---|---|
| 1. | Acetone | < 900 | 0.3 | 2.8 | - |
| 2. | Acetic acid | < 900 | 0.1 | 2.0 | - |
| 3. | Butan-2-one | < 900 | - | 0.6 | - |
| 4. | Ethyl acetate | < 900 | - | 0.4 | - |
| 5. | Benzene | < 900 | - | 0.2 | - |
| 6. | Propionic acid | < 900 | 0.1 | 2.1 | - |
| 7. | Heptane | < 900 | - | 0.7 | - |
| 8. | Ethyl propionate | < 900 | - | 0.4 | - |
| 9. | Isoamyl alcohol | < 900 | 0.3 | 0.9 | - |
| 10. | Toluene | < 900 | - | 0.5 | - |
| 11. | Butanoic acid | < 900 | - | 1.6 | - |
| 12. | Octane | < 900 | - | 1.6 | - |
| 13. | Furfural | < 900 | - | 0.5 | - |
| 14. | Isovaleric acid | < 900 | - | 0.4 | - |
| 15. | Ethylbenzene | < 900 | - | 0.4 | - |
| 16. | Isoamyl acetate | < 900 | 0.1 | 0.8 | - |
| 17. | p-Xylene | < 900 | - | 0.8 | - |
| 18. | Valeric acid | < 900 | - | 1.2 | - |
| 19. | Heptan-2-one | < 900 | - | 1.0 | - |
| 20. | Nonane | 900 | - | 0.6 | - |
| 21. | Heptanal | 905 | - | 0.4 | - |
| 22. | Isopropylbenzene (cumene) | 932 | - | 0.4 | - |
| 23. | α-Pinene | 941 | 0.1 | 0.2 | - |
| 24. | Camphene | 958 | 0.2 | 0.4 | - |
| 25. | Benzaldehyde | 965 | 0.6 | 20.4 | - |
| 26. | Hexanoic acid (caproic acid) | 994 | 0.1 | 4.6 | - |
| 27. | Octanal | 1007 | - | 0.7 | - |
| 28. | p-Cymene | 1032 | 0.1 | 2.7 | - |
| 29. | Limonene | 1036 | - | 0.5 | - |
| 30. | 2-Hydroxybenzaldehyde (salicylaldehyde) | 1052 | - | 0.2 | - |
| 31. | 1-Phenylethanone (acetophenone) | 1074 | - | 0.7 | - |
| 32. | Heptanoic acid | 1093 | - | 0.8 | - |
| 33. | Fenchone | 1094 | 0.2 | ||
| 34. | Nonan-2-one | 1097 | - | 1.3 | - |
| 35. | 1-Ethyl-4-(1-methylethyl)-benzene | 1110 | - | 1.6 | - |
| 36. | 2-Phenylethanol | 1116 | 1.2 | 3.3 | - |
| 37. | Camphor | 1153 | - | 0.9 | - |
| 38. | Pentylbenzene | 1165 | - | 0.4 | - |
| 39. | Borneol | 1175 | 1.1 | 4.5 | 0.2 |
| 40. | Naphthalene | 1190 | - | 1.2 | - |
| 41. | Octanoic acid | 1191 | 0.5 | 1.4 | - |
| 42. | 2-Methoxy-4-methylphenol (p-cresol) | 1198 | 0.3 | 0.9 | - |
| 43. | 3-Isopropylbenzaldehyde | 1229 | 0.4 | 4.7 | - |
| 44. | 4-Ethyl-2-methoxyphenol (4-ethylguaiacol) | 1286 | 0.4 | 0.9 | - |
| 45. | Nonanoic acid | 1288 | - | 0.5 | - |
| 46. | Bornyl acetate | 1291 | - | 0.6 | - |
| 47. | 1-Methylnaphthalene | 1316 | - | 0.4 | - |
| 48. | p-Acetylacetophenone | 1325 | - | 0.7 | - |
| 49. | 2-Methoxy-4-propylphenol | 1375 | 1.2 | 1.5 | - |
| 50. | 4-Hydroxy-3-methoxy-benzaldehyde (vanillin) | 1406 | 0.3 | - | - |
| 51. | 1,7-Dimethylnaphthalene ** | 1427 | 0.4 | 0.7 | - |
| 52. | 3,5-Dimethoxybenzaldehyde (syringaldehyde) | 1445 | 0.3 | 0.5 | - |
| 53. | 4-Hydroxy-3-methoxy-acetophenone (acetovanillone) | 1494 | 0.3 | - | - |
| 54. | α-Muurolene | 1507 | 0.6 | 0.9 | - |
| 55. | Calamenene | 1531 | 1.0 | 1.3 | - |
| 56. | γ-Cadinene | 1535 | 0.1 | - | - |
| 57. | α-Calacorene | 1551 | 0.1 | - | - |
| 58. | 2,3-Dimethylbenzofuran | 1578 | 0.3 | - | - |
| 59. | 1-Methyl-7-(1-methylethyl)-naphthalene (eudalin) | 1587 | 0.6 | 0.5 | - |
| 60. | 1,6,7-Trimethylnaphthalene * | 1590 | 0.3 | - | - |
| 61. | 1,6-Dimethyl-4-(1-methylethyl)-naphthalene (cadalin) | 1684 | 1.1 | 0.7 | - |
| 62. | 3,4-Diethyl-1,1′-biphenyl | 1751 | 0.4 | - | - |
| 63. | Anthracene | 1780 | 0.1 | - | - |
| 64. | 4-(1-Methyl-1-phenyl)-phenol (4-cumylphenol) | 1814 | 0.8 | - | - |
| 65. | Cembrene ** | 1912 | 2.0 | 0.7 | - |
| 66. | 2-Methylanthracene ** | 1925 | 1.8 | 0.5 | 0.3 |
| 67. | 1-Methoxy-8-methylnaphthalene * | 1942 | 2.2 | - | 0.3 |
| 68. | Hexadecanoic acid | 1963 | - | - | 0.9 |
| 69. | 7-(1-Methylethyl)-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-naphthalene | 1988 | 9.3 | 2.7 | 2.8 |
| 70. | 7-(1-Methylethyl)-1,4a-dimethyl-2,3,4,4a,9,10-hexahydro-phenanthrene | 1996 | 7.7 | 1.0 | 1.9 |
| 71. | 7-(1-Methylethyl)-1,4a-dimethyl-1,2,3,4,4a,9,10,10a-octahydro-phenanthrene | 2023 | 12.2 | 3.4 | 3.6 |
| 72. | 3,6-Dimethylphenanthrene ** | 2049 | 6.6 | 1.0 | 2.9 |
| 73. | 1,4-Dimethoxyphenathrene * | 2103 | 10.0 | 1.7 | 6.8 |
| 74. | 2,3,5-Trimethylphenanthrene | 2167 | 1.2 | - | 3.2 |
| 75. | 7-Ethenyl-1,2,3,4,4a,4b,5,6,7, 9,10,10a-dodecahydro-1,4a,7-trimethyl-1-phenanthrene-carboxaldehyde (pimaral) | 2187 | 0.6 | - | 0.8 |
| 76. | Methyl 8,15-pimaradien-18-oate | 2227 | 0.6 | - | 0.8 |
| 77. | 1-Methyl-7-(1-methylethyl)-phenanthrene (retene) | 2242 | 9.9 | 1.6 | 24.1 |
| 78. | Methyl 8,15-isopimaradien-18-oate | 2256 | 0.5 | - | 0.6 |
| 79. | 4-(2-Ethyl-5-phenyl-1H-pyrrol-3-yl)-pyridine * | 2382 | 0.7 | - | 4.2 |
| 80. | Methyl 6,8,11,13-abietatetraen-18-oate * | 2451 | - | - | 1.7 |
| 81. | Methyl dehydroabietate | 2477 | 6.0 | 1.0 | 33.6 |
| Total identified (%) | 85.1 | 94.7 | 88.7 | ||
| No. | Compound | RI | A | B | C |
|---|---|---|---|---|---|
| 1. | Toluene | < 900 | 0.3 | 3.2 | - |
| 2. | α-Pinene | 941 | 73.4 | 66.2 | 52.0 |
| 3. | Camphene | 958 | 2.1 | 3.8 | 4.2 |
| 4. | Verbenene | 961 | 0.6 | 0.7 | 3.5 |
| 5. | Benzaldehyde | 965 | - | 0.2 | - |
| 6. | β-Pinene | 983 | 1.0 | 0.9 | 0.3 |
| 7. | Myrcene | 994 | 0.4 | 0.2 | - |
| 8. | Δ-3-Carene | 1016 | 0.3 | 0.2 | - |
| 9. | p-Cymene | 1032 | 0.5 | 1.4 | 0.9 |
| 10. | Limonene | 1035 | 2.9 | 4.4 | 1.5 |
| 11. | α-Terpinolene | 1093 | 0.3 | 0.3 | - |
| 12. | p-Cymenyl | 1095 | 0.4 | 2.3 | 1.5 |
| 13. | Fenchol | 1123 | 0.1 | 0.2 | 0.7 |
| 14. | α-Campholene aldehyde | 1133 | 0.4 | 0.4 | 0.9 |
| 15. | trans-Pinocarveol | 1148 | 0.3 | 0.4 | 1.0 |
| 16. | Camphor | 1153 | 0.2 | 0.1 | 0.3 |
| 17. | Borneol | 1175 | 0.2 | 0.3 | 3.8 |
| 18. | Terpinen-4-ol | 1185 | 0.1 | 0.1 | - |
| 19. | p-Cymen-8-ol | 1194 | 0.2 | 0.4 | 0.6 |
| 20. | α-Terpineol | 1198 | 0.7 | 1.1 | 2.3 |
| 21. | Estragole | 1204 | 0.8 | 0.9 | - |
| 22. | Verbenone | 1217 | 0.4 | 0.6 | 2.4 |
| 23. | β-Caryophyllene | 1425 | 5.7 | 2.0 | 3.0 |
| 24. | β-Selinene | 1460 | 1.1 | 0.4 | - |
| 25. | α-Muurolene | 1506 | 2.3 | 1.6 | 2.0 |
| 26. | Caryophyllene oxide | 1589 | 0.8 | 0.2 | - |
| 27. | Methyl dehydroabietate | 2477 | - | - | 2.7 |
| Total identified (%) | 95.5 | 92.5 | 83.6 | ||
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jerković, I.; Marijanović, Z.; Gugić, M.; Roje, M. Chemical Profile of the Organic Residue from Ancient Amphora Found in the Adriatic Sea Determined by Direct GC and GC-MS Analysis. Molecules 2011, 16, 7936-7948. https://doi.org/10.3390/molecules16097936
Jerković I, Marijanović Z, Gugić M, Roje M. Chemical Profile of the Organic Residue from Ancient Amphora Found in the Adriatic Sea Determined by Direct GC and GC-MS Analysis. Molecules. 2011; 16(9):7936-7948. https://doi.org/10.3390/molecules16097936
Chicago/Turabian StyleJerković, Igor, Zvonimir Marijanović, Mirko Gugić, and Marin Roje. 2011. "Chemical Profile of the Organic Residue from Ancient Amphora Found in the Adriatic Sea Determined by Direct GC and GC-MS Analysis" Molecules 16, no. 9: 7936-7948. https://doi.org/10.3390/molecules16097936
APA StyleJerković, I., Marijanović, Z., Gugić, M., & Roje, M. (2011). Chemical Profile of the Organic Residue from Ancient Amphora Found in the Adriatic Sea Determined by Direct GC and GC-MS Analysis. Molecules, 16(9), 7936-7948. https://doi.org/10.3390/molecules16097936






