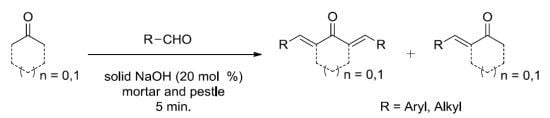
A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones
Abstract
Share and Cite
Rahman, A.F.M.M.; Ali, R.; Jahng, Y.; Kadi, A.A. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules 2012, 17, 571-583. https://doi.org/10.3390/molecules17010571
Rahman AFMM, Ali R, Jahng Y, Kadi AA. A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules. 2012; 17(1):571-583. https://doi.org/10.3390/molecules17010571
Chicago/Turabian StyleRahman, A. F. M. Motiur, Roushown Ali, Yurngdong Jahng, and Adnan A. Kadi. 2012. "A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones" Molecules 17, no. 1: 571-583. https://doi.org/10.3390/molecules17010571
APA StyleRahman, A. F. M. M., Ali, R., Jahng, Y., & Kadi, A. A. (2012). A Facile Solvent Free Claisen-Schmidt Reaction: Synthesis of α,α′-bis-(Substituted-benzylidene)cycloalkanones and α,α′-bis-(Substituted-alkylidene)cycloalkanones. Molecules, 17(1), 571-583. https://doi.org/10.3390/molecules17010571