Extract of Lillium candidum L. Can Modulate the Genotoxicity of the Antibiotic Zeocin
Abstract
:1. Introduction
2. Results and Discussion
2.1. LE and Zeo Cytotoxicity

2.2. Zeocin Genotoxicity and Effects of Lilium candidum Extract on P. sativum L.
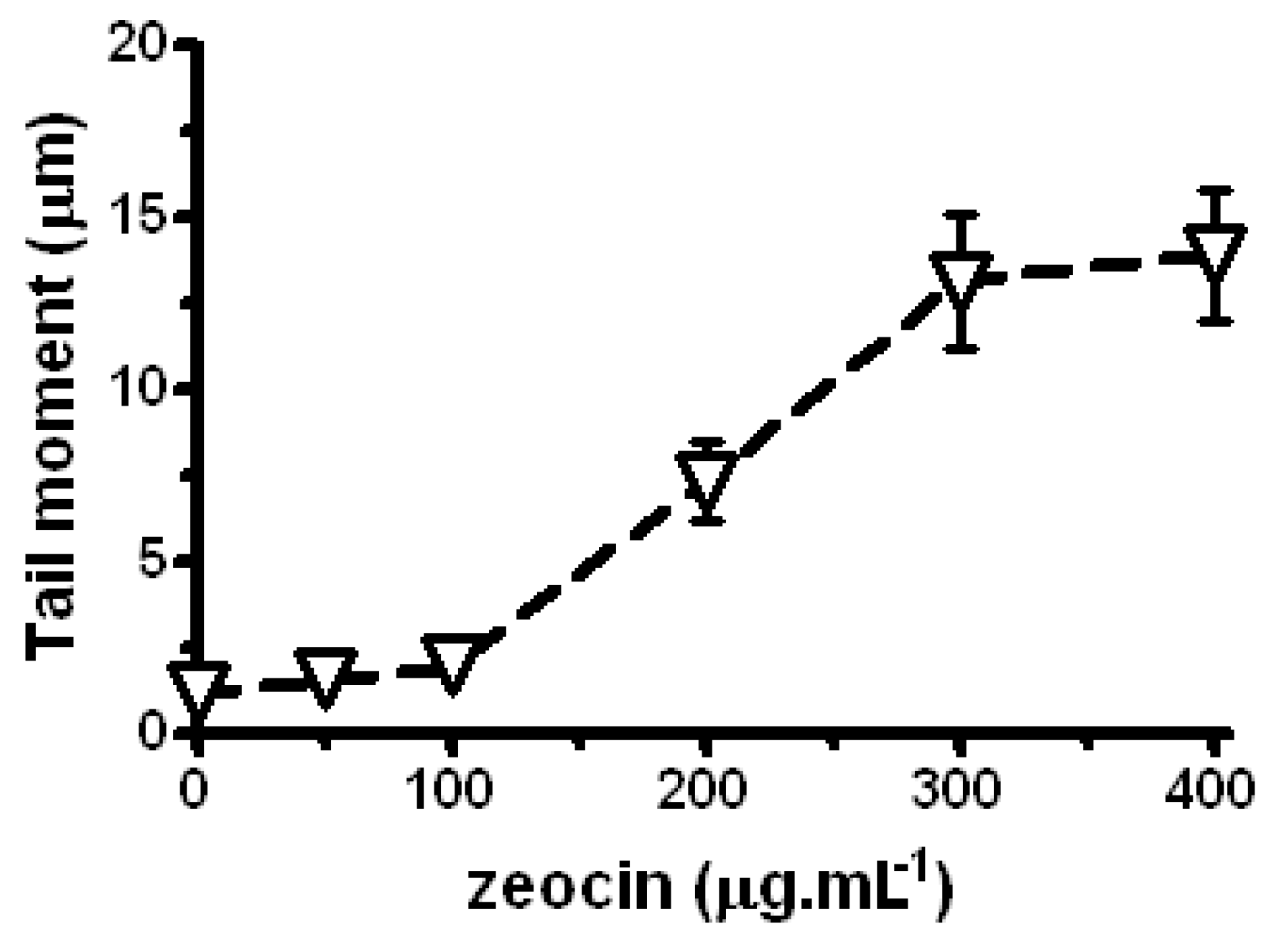
2.3. LE and Zeo Clastogenicity on H. vulgare L.


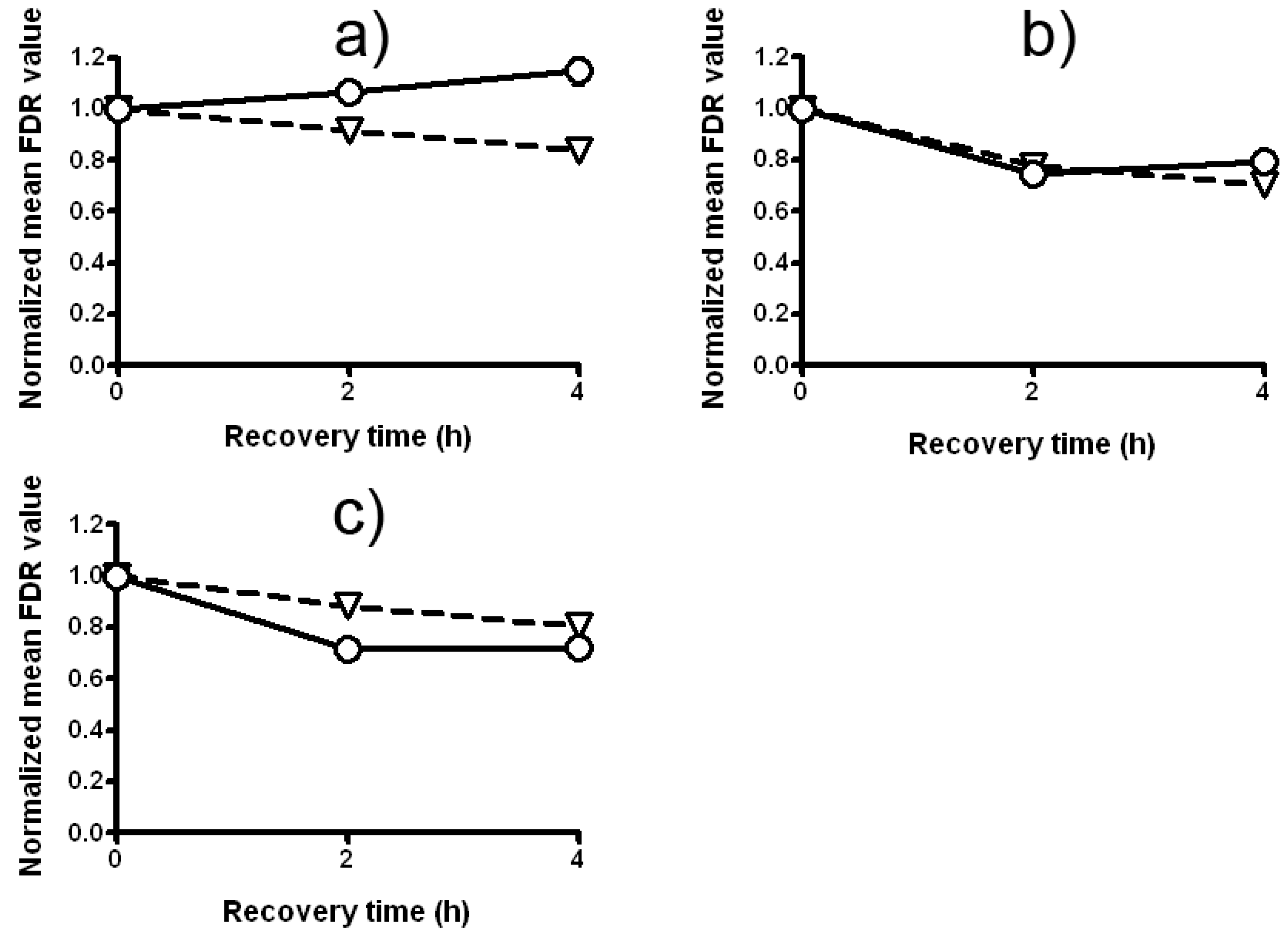
2.4. The Antigenotoxic Effect of Lilium candidum Extract in C. reinhardtii and P. sativum L.

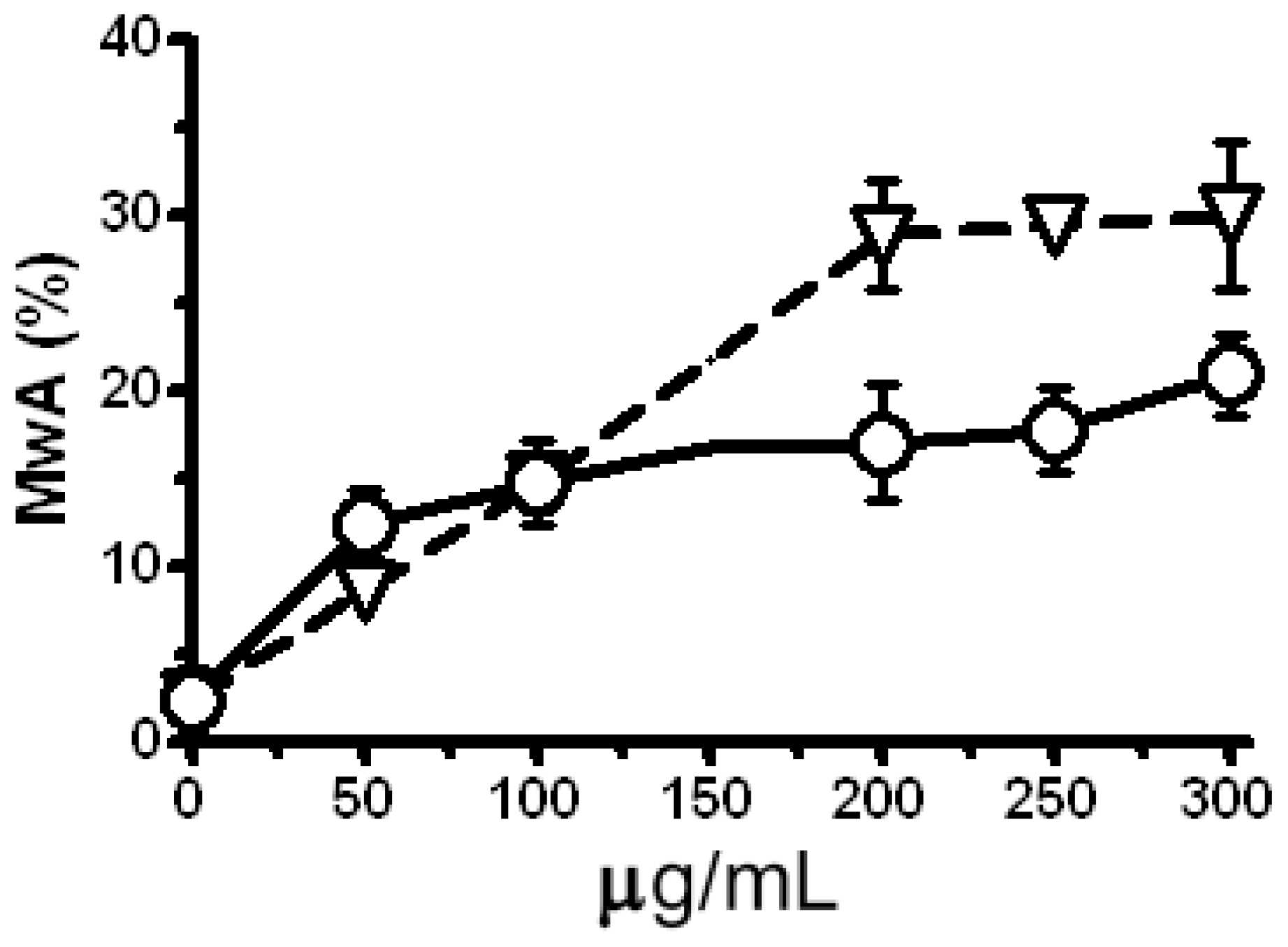
2.5. Modulation of the Clastogenic Effect of Zeocin in H. vulgare L.



3. Experimental
3.1. Extraction Procedure
3.2. Test Systems and Treatments

3.3. Mitotic Index (MI)
3.4. Comet Assay (CA)
3.5. Constant Field Gel Electrophoresis (CFGE)
3.6. Micronuclei (MN) and Metaphases with Chromatid Aberrations (MwA)
3.7. Experimental Designs
3.8. Statistics
4. Conclusions
Acknowledgements
References and Notes
- Miadoková, E.; Naďová, S.; Trebatická, M.; Grolmus, J.; Kopásková, M.; Rauko, P.; Mučaji, P.; Grančai, D. Research on biomodulatory effect of natural compounds. Neuro. Endocrinol. Lett. 2006, 27, 53–56. [Google Scholar]
- Miadoková, E.; Naďová, S.; Vlčková, V.; Dúhová, V.; Kopásková, M.; Čipák, L.; Rauko, P.; Mučaji, P.; Grančai, D. Antigenotoxic effect of extract from Cynara cardunculus L. Phytother. Res. 2008, 22, 77–81. [Google Scholar] [CrossRef]
- Afoulous, S.; Ferhout, H.; Raoelison, E.G.; Valentin, A.; Moukarzel, B.; Couderc, F.; Bouajila, J. Helichrysum gymnocephalum Essential oil: Chemical composition and cytotoxic, antimalarial and antioxidant activities, attribution of the activity origin by correlations. Molecules 2011, 16, 8273–8291. [Google Scholar] [CrossRef]
- Santos, M.L.; Toyama, D.O.; Oliveira, S.C.B.; Cotrim, C.A.; Diz-Filho, E.B.S.; Fagundes, F.H.R.; Soares, V.C.G.; Aparicio, R.; Toyama, M.H. Modulation of the pharmacological activities of secretory phospholipase A2 from Crotalus durissus cascavella induced by naringin. Molecules 2011, 16, 738–761. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, J.Y.; Chen, S.Y.; Shi, L.L.; Liu, Y.J.; Ma, C. Antioxidant potential of polyphenols and tannins from burs of Castanea mollissima blume. Molecules 2011, 16, 8590–8600. [Google Scholar] [CrossRef]
- Zhou, K.; Wang, H.; Mei, W.; Li, X.; Luo, Y.; Dai, H. Antioxidant activity of papaya seed extracts. Molecules 2011, 16, 6179–6192. [Google Scholar] [CrossRef]
- Nikolić, B.; Mitić-Ćulafić, D.; Vuković-Gačić, B.; Knežević-Vukčević, J. Modulation of genotoxicity and DNA repair by plant monoterpenes camphor, eucalyptol and thujone in E. coli and mammalian cells. Food Chem. Toxicol. 2011, 49, 2035–2045. [Google Scholar] [CrossRef]
- Shaughnessy, D.T.; Schaaper, R.M.; Umbach, D.M.; DeMarini, D.M. Inhibition of spontaneous mutagenesis by vanillin and cinnamaldehyde in Escherichia coli: Dependence on recombinational repair. Mutat. Res. 2006, 602, 54–64. [Google Scholar] [CrossRef]
- Mimaki, Y.; Satou, T.; Kuroda, M.; Sashida, Y.; Hatakeyama, Y. Steroidal saponins from the bulbs of Lilium candidum. Phytochemistry 1999, 51, 567–573. [Google Scholar] [CrossRef]
- Eisenreichová, E.; Haladová, M.; Mučaji, P.; Grančai, D. The study of constituents of Lilium candidum L. Acta Facult. Pharm. Univ. Comen. 2004, 51, 27–37. [Google Scholar]
- Dimova, E.G.; Bryant, P.E.; Chankova, S.G. Adaptive response”—Some underlying mechanisms and open question. Gen. Mol. Biol. 2008, 31, 396–408. [Google Scholar]
- Achary, V.M.M.; Panda, B.B. Aluminium-induced DNA damage and adaptive response to genotoxic stress in plant cells are mediated through reactive oxygen intermediates. Mutagenesis 2010, 25, 201–209. [Google Scholar] [CrossRef]
- Chankova, S.G.; Dimova, E.; Dimitrova, M.; Bryant, P.E. Induction of DNA double-strand breaks by zeocin in Chlamydomonas reinhardtii and the role of increased DNA double-strand breaks rejoining in the formation of an adaptive response. Radiat. Environ. Biophys. 2007, 46, 409–416. [Google Scholar] [CrossRef]
- Dimova, E.; Dimitrova, M.; Miteva, D.; Mitrovska, Z.; Yurina, N.P.; Bryant, P.E.; Chankova, S. Does single-dose cell resistance to the radio-mimetic zeocin correlate with a zeocin-induced adaptive response in Chlamydomonas reinhardtii strains? Radiat. Environ. Biophys. 2009, 48, 77–84. [Google Scholar] [CrossRef]
- Yarmolinsky, L.; Zaccai, M.; Ben-Shabat, S.; Mills, D.; Huleihel, M. Antiviral activity of ethanol extracts of Ficus binjamina and Lilium candidum in vitro. New Biotechnol. 2009, 26, 307–313. [Google Scholar]
- Jaloszynski, P.; Kujawski, M.; Czub-Swierczek, M.; Markowska, J.; Szyfter, K. Bleomycin-induced DNA damage and its removal in lymphocytes of breast cancer patients studied by comet assay. Mutat. Res. 1997, 385, 223–233. [Google Scholar] [CrossRef]
- Menke, M.; Chen, I.P.; Angelis, K.J.; Schubert, I. DNA damage and repair in Arabidopsis thaliana as measured by the comet assay after treatment with different classes of genotoxins. Mutat. Res. 2001, 493, 87–93. [Google Scholar] [CrossRef]
- Pfeifer, T.A.; Hegedus, D.D.; Grigliatti, T.A.; Theilman, D.A. Baculovirus immediate-early promoter-mediated expression of the Zeocin resistant gene for use as a dominant selectable marker in Dipteran and Lepidopteran insect cell lines. Gene 1997, 188, 183–190. [Google Scholar] [CrossRef]
- Cenkci, B.; Petersen, J.L.; Small, G.D. REX1, a novel gene required for DNA repair. J. Biol. Chem. 2003, 278, 22574–22577. [Google Scholar]
- Mason, R.W.; Bergman, C.A.; Lu, G.; Holbrook, J.F.; Sol-Church, K. Expression and characterization of cathepsin P. Biochem. J. 2004, 378, 657–663. [Google Scholar] [CrossRef]
- Moore, C.W.; McKoy, J.; Dardalhon, M.; Davermann, D.; Martinez, M.; Averbeck, D. DNA damage-inducible and RAD52-independent repair of DNA double-strand breaks in Saccharomyces cerevisiae. Genetics 2000, 154, 1085–1099. [Google Scholar]
- Zaka, R.; Chenal, C.; Misset, M.T. Study of external low irradiation dose effects on induction of chromosome aberrations in Pisum sativum root tip meristem. Mutat. Res. 2002, 517, 87–99. [Google Scholar] [CrossRef]
- Noël, G.; Giocanti, N.; Fernet, M.; Mégnin-Chanet, F.; Favaudon, V. Poly(ADP-ribose) polymerase (PARP-1) is not involved in DNA double-strand break recovery. BMC Cell Biol. 2003, 4, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Jovtchev, G.; Stergois, M.; Schubert, I. A comparison of N-methyl-N-nitrosourea-induced chromatid aberrations and micronuclei in barley meristems using FISH techniques. Mutat. Res. 2002, 517, 47–51. [Google Scholar] [CrossRef]
- Yi, H.; Meng, Z. Genotoxicity of hydrated sulfur dioxide on root tips of Allium sativum and Vicia faba. Mutat. Res. 2003, 537, 109–114. [Google Scholar] [CrossRef]
- Esnault, M.A.; Legue, F.; Chenal, C.H. Ionizing radiation: Advances in plant response. Environ. Exp. Bot. 2010, 68, 231–237. [Google Scholar] [CrossRef]
- Chankova, S.G.; Bryant, P.E. Acceleration of DNA-double strand rejoining during the adaptive response of Chlamydomonas reinhardtii. Radiats. Biol. Radioecol. 2002, 42, 600–603. [Google Scholar]
- Araujo, M.C.P.; Dias, F.L.; Takahashi, C.S. Potentiation by turmeric and curcumin of γ-radiation-induced chromosome aberrations in Chinese hamster ovary cells. Teratog. Carcinog. Mutagen. 1999, 19, 9–18. [Google Scholar] [CrossRef]
- Leung, H.W.; Lin, C.J.; Hour, M.J.; Yang, W.H.; Wang, M.Y.; Lee, H.Z. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem. Toxicol. 2007, 45, 2005–2013. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Tsang, S.F.; Lin, K.W.; Yang, S.C.; Lin, C.N. Cell death induced by flavonoid glycosides with and without copper. Food Chem. Toxicol. 2008, 46, 2394–2401. [Google Scholar] [CrossRef]
- Naďová, S.; Miadoková, E.; Čipák, L. Flavonoids potentiate the efficacy of cytarabine through modulation of drug-induced apoptosis. Neoplasma 2007, 54, 202–206. [Google Scholar]
- Yoshida, M.; Shimizu, N.; Suzuki, M.; Watanabe, C.; Satoh, M.; Mori, K.; Yasutake, A. Emergence of delayed methylmercury toxicity after perinatal exposur in metallothionein-null and wild-type C57BL mice. Environ. Health Perspect. 2008, 116, 746–751. [Google Scholar] [CrossRef]
- Rakba, N.; Loyer, P.; Gilot, D.; Delcros, J.G.; Glaise, D.; Baret, P.; Pierre, J.L.; Brissot, P.; Lescoat, G. Antiproliferative and apoptotic effects of O-Trensox, a new synthetic iron chelator, on differentiated human hepatoma cell lines. Carcinogenesis 2000, 21, 943–951. [Google Scholar] [CrossRef]
- Francis, G.; Kerem, Z.; Makkar, J.P.S.; Becker, K. The biological action of saponins in animal systems:a review. Br. J. Nutr. 2002, 88, 587–605. [Google Scholar] [CrossRef]
- Rieger, R.; Michaelis, A.; Jovtchev, G.; Nicolova, T. Copper-sulfate and lead-nitrate pretreatments trigger adaptive responses to the induction of chromatid aberrations by maleic hydrazide (MH) and or TEM in Vicia faba, Hordeum vulgare, and human peripheral blood lymphocytes. Biol. Zentralbl. 1993, 112, 18–27. [Google Scholar]
- Mahat, M.Y.; Kulkarni, N.M.; Vishwakarma, S.L.; Khan, F.R.; Thippeswamy, B.S.; Hebballi, V.; Adhyapak, A.A.; Benade, V.S.; Ashfaque, S.M.; Tubachi, S.; et al. Modulation of the cyclooxygenase pathway via inhibition of nitric oxide production contributes to the anti-inflammatory activity of kaempferol. Eur. J. Pharmacol. 2010, 642, 169–176. [Google Scholar] [CrossRef]
- El-Shehawi, A.M.; Eldakak, M.A.; Seehy, M.A. Assessment of anticlastogenic activity of cinnamic acid: Anticlastogenic index (ACI) and model simulation. Afr. J. Biotechnol. 2011, 10, 6863–6873. [Google Scholar]
- Niering, P.; Michels, G.; Wätjen, W.; Ohler, S.; Steffen, B.; Chovolou, Y.; Kampkötter, A.; Proksch, P.; Kahl, R. Protective and detrimental effects of kaempferol in rat H4IIE cells: Implication of oxidative stress and apoptosis. Toxicol. Appl. Pharm. 2005, 209, 114–122. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormesis is central to toxicology, pharmacology and risk assessment. Hum. Exp. Toxicol. 2010, 29, 249–261. [Google Scholar] [CrossRef]
- Mučaji, P.; Haladová, M.; Eisenreichová, E.; Šeršeň, F.; Ubik, K.; Grančai, D. Constituents in Lilium candidum L. and their antioxidant activity. Ceska. Slov. Farm. 2007, 56, 27–29, In Slovak. [Google Scholar]
- Slovak Pharmacopoeia [in Slovak]; Herba: Bratislava, Czech Republic, 2001; prima ed; Part IV.
- Fathkiev, F.; Samikov, K.; Shakirov, R. Dynamics of the alkaloid content of Lilium martagon. Chem. Nat. Compd. 1990, 26, 476–477. [Google Scholar]
- Künzel, G.; Nicoloff, H. Further results on karyotype reconstruction in barley. Biol. Zentralbl. 1979, 98, 587–592. [Google Scholar]
- Nicoloff, H. Mutations in rDNA. 1. Dependence of chromosome mutation induction on positions and activity of nucleolus organizer regions. Theor. Appl. Genet. 1981, 60, 383–393. [Google Scholar]
- Nicoloff, H.; Rieger, R.; Russev, G. Mutations in rDNA. 5. Is translocation involvement of barley nucleolus organizer regions influenced by chromatin architecture? Mutat. Res. 1987, 181, 147–155. [Google Scholar] [CrossRef]
- Jovtchev, G.; Gateva, S.; Stergios, M.; Kulekova, S. Cytotoxic and genotoxic effects of paraquat in Hordeum vulgare and human lymphocytes in vitro. Environ. Toxicol. 2010, 25, 294–303. [Google Scholar]
- Gichner, T.; Znidar, I.; Wagner, E.D.; Plewa, M.J. The use of higher plants in the comet assay. In Issues of Toxicology №5, The Comet Assay in Toxicology; Dhawan, A., Anderson, D., Eds.; Royal Society of Chemistry: Cambridge, UK, 2009; pp. 98–119. [Google Scholar]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods; Iowa State University Press: Ames, IA, USA, 1967. [Google Scholar]
- Bryant, P.E. Change in sensitivity of cells after split dose recovery a further test of the repair hypothesis. Int. J. Radiat. Biol. 1974, 26, 499–504. [Google Scholar] [CrossRef]
- Chankova, S.G.; Matos, J.A.; Simoes, F.; Bryant, P.E. Adaptive response of a new radioresistant strain of Chlamydomonas reinhardtii and correlation with increased DNA double-strand break rejoining. Int. J. Radiat. Biol. 2005, 81, 509–514. [Google Scholar] [CrossRef]
- Rieger, R.; Michaelis, A.; Schubert, I.; Döbel, P.; Jank, H.W. Non-random intrachromosomal distribution of chromatid aberrations induced by X-rays, alkylating agents and ethanol in Vicia faba. Mutat. Res. 1975, 27, 69–79. [Google Scholar] [CrossRef]
- Sample Availability: Sample of the LE is available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kopaskova, M.; Hadjo, L.; Yankulova, B.; Jovtchev, G.; Galova, E.; Sevcovicova, A.; Mucaji, P.; Miadokova, E.; Bryant, P.; Chankova, S. Extract of Lillium candidum L. Can Modulate the Genotoxicity of the Antibiotic Zeocin. Molecules 2012, 17, 80-97. https://doi.org/10.3390/molecules17010080
Kopaskova M, Hadjo L, Yankulova B, Jovtchev G, Galova E, Sevcovicova A, Mucaji P, Miadokova E, Bryant P, Chankova S. Extract of Lillium candidum L. Can Modulate the Genotoxicity of the Antibiotic Zeocin. Molecules. 2012; 17(1):80-97. https://doi.org/10.3390/molecules17010080
Chicago/Turabian StyleKopaskova, Marcela, Lina Hadjo, Bisera Yankulova, Gabriele Jovtchev, Eliska Galova, Andrea Sevcovicova, Pavel Mucaji, Eva Miadokova, Peter Bryant, and Stephka Chankova. 2012. "Extract of Lillium candidum L. Can Modulate the Genotoxicity of the Antibiotic Zeocin" Molecules 17, no. 1: 80-97. https://doi.org/10.3390/molecules17010080
APA StyleKopaskova, M., Hadjo, L., Yankulova, B., Jovtchev, G., Galova, E., Sevcovicova, A., Mucaji, P., Miadokova, E., Bryant, P., & Chankova, S. (2012). Extract of Lillium candidum L. Can Modulate the Genotoxicity of the Antibiotic Zeocin. Molecules, 17(1), 80-97. https://doi.org/10.3390/molecules17010080



