Abstract
A number of interesting heterocycles were prepared through interaction of the intermediate 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno-[2,3-d]pyrimidine (1) and reagents such as hydrazonyl halides 2 to furnish triazine derivatives 4a–l. Reaction of 1 with phenacyl bromide afforded compound 5. Moreover, the title compound 1 was subjected to condensation with active methylene compounds (ethyl acetoacetate and ethyl benzoylacetate) to give triazipinones 8a,b. The condensation with aromatic aldehydes afforded either the triazole derivatives 10a–d or Schiff base 11. In addition, the behaviour of compound 1 towards activated unsaturated compounds namely dimethyl acetylene dicarboxylate and ethoxymethylenemalonitrile was studied and it was found to furnish the triazine 13 and triazepine derivative 15, respectively. Combination of title compound 1 with chlorinated active methylene compounds delivered the triazine derivatives 18a–c. Reaction of 1 with chloroacetonitrile furnished compound 20. The structures of the products were elucidated based on their microanalyses and spectroscopic data. Finally, the antitumor activity of the new compounds 4a and 8a against human breast cell MCF-7 line and liver carcinoma cell line HepG2 were recorded.
1. Introduction
The word tumor is commonly used as a synonym for a neoplasm [a solid or fluid-filled (cystic) lesion that may or may not be formed by an abnormal growth of neoplastic cells] that appears enlarged in size [1]. In modern medicine, the term tumor means a neoplasm that has formed a lump. While cancer is by definition malignant, a tumor can be benign, pre-malignant, or malignant, or can represent a lesion without any cancerous potential whatsoever. Development of novel drugs, and in particular new antitumour agents is a constantly growing need that concerns researchers throughout the World, consequently, as cancers continue to be an emerging problem. Numerous antitumor chemical drugs have been widely synthesized, including the chromenopyrimidines, which present interesting biological activities. The authors, who have contributed in the past to the exploration of this research topic, were interested in expanding their work by developing a facile synthesis of new derivatives and then test their antimicrobial, cytotoxicity activities [1,2,3], and in vitro antitubercular activity [4], in addition to antitumour activity. It was reported that pyrimidotriazines themselves posses biological activities with a wide range of applications [5,6,7,8,9]. The research done in this article could be regarded as an extension to our previous work [10] for constructing fused chromenopyrimidines heterocycles through reactions of the key compound 1 with a variety of reagents, especially hydrazonyl halides [11,12,13,14], which lead to interesting azoheterocyclic compounds.
2. Results and Discussion
2.1. Chemistry
The title compound 1 was prepared according to the procedure reported in literature [10], and it was proved to be highly reactive towards various reagents, resulting in the formation of a wide range of annulated chromenopyrimidine systems. With compound 1 in hand, a number of valuable heterocycles could be prepared. Firstly, the interaction between the aminopyrimidine 1 and hydrazonyl halides 2 in refluxing ethanol delivered the azotriazine derivatives 4a–l in good yields (Scheme 1). Structure assessment was based on their spectroscopic data. The IR spectra showed absorption bands at 3,470–3,410 (OH), 3,350–3,310 (NH) and at 1,593–1,573 cm−1 (C=N), while the mass spectra revealed molecular ion peaks consistent with the proposed structures. The 1H-NMR spectra, for example for compound 4a, showed enrichment of the aromatic signals due to the additional aryl group, while two signals at 9.30 and 9.66 ppm for two D2O exchangeable protons (NH, OH) also appeared. The spectral data presented here indicate collectively that such compounds 4a–l exist predominantly in the hydrazone tautomeric form 4A rather than 4B [15,16,17,18,19,20].
In order to prepare an authentic sample of compound 4a through an alternative route, the aminopyrimidine 1 was condensed with phenacyl bromide 6 in ethanol to afford the triazine derivative 5, whose structure was elucidated from its spectroscopic data. The mass spectrum showed a peak of m/z = 420 corresponding to the M.F. C26H20N4O2, while the 1H-NMR displayed a signal at 4.23 ppm attributable to a CH2 group. When compound 5 was coupled to phenyldiazonium chloride, unfortunately, it failed to yield the desired compound 4a because the diazonium salt coupled preferentially to the more reactive phenolic ring to give compound 6 (Scheme 1). The structure of compound 6 was established by its spectroscopic data compatible with the proposed structure (see Experimental).
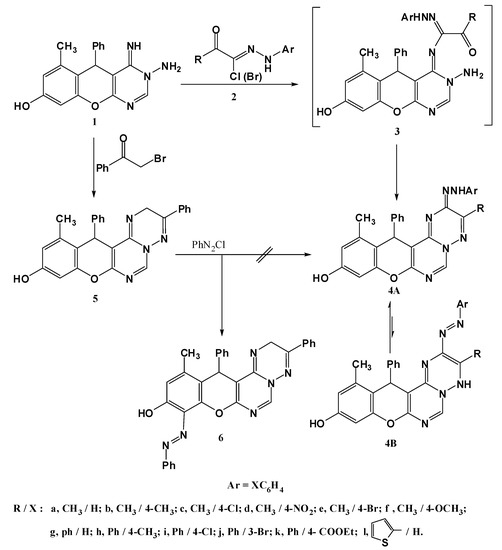
Scheme 1.
Synthesis of 5-arylazotriazine derivatives 4a–l.
Scheme 1.
Synthesis of 5-arylazotriazine derivatives 4a–l.
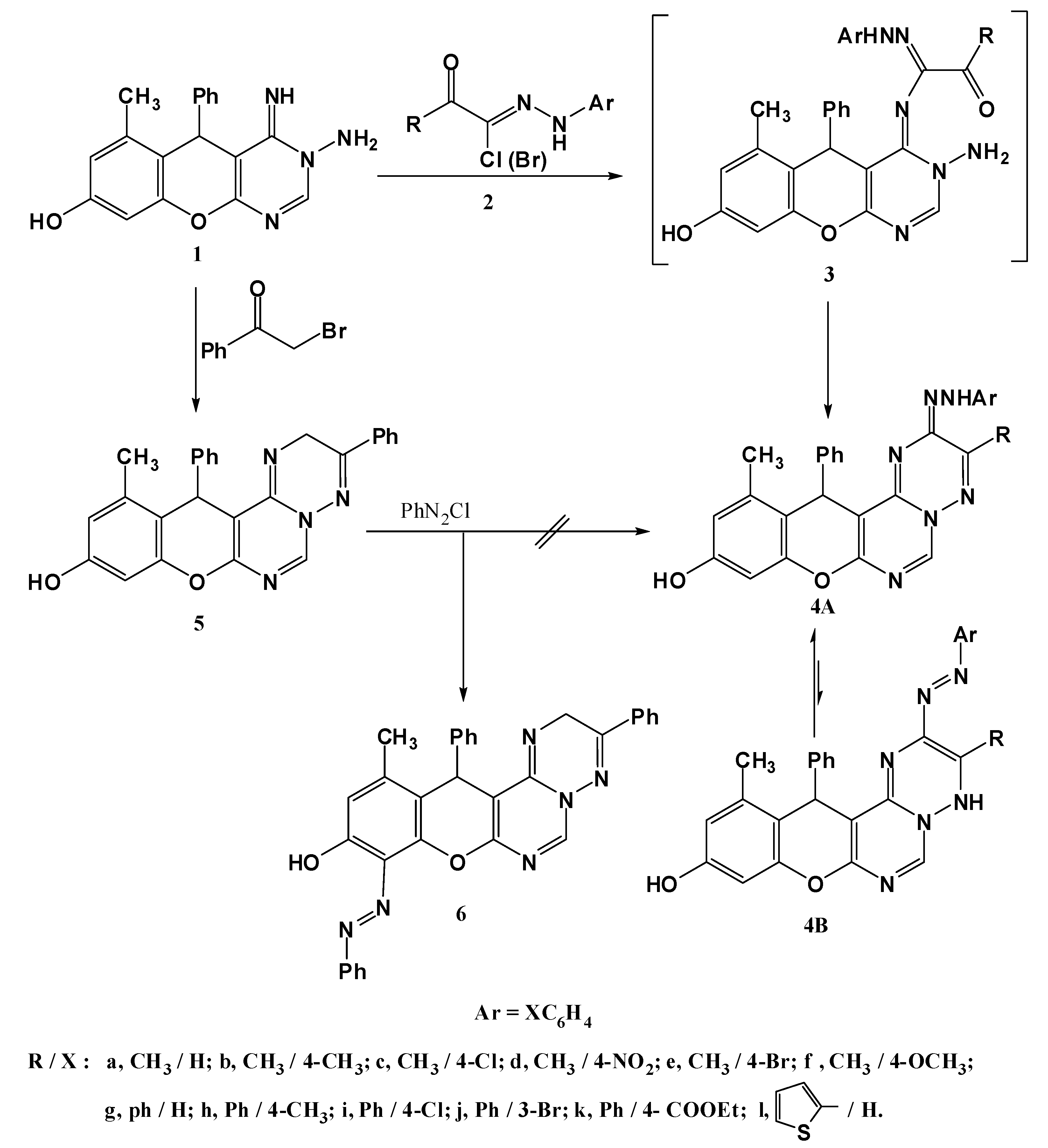
An even more convenient access for constructing triazepines based on the aminopyrimidine compound 1 was established using readily available active methylene reagents such as ethyl acetoacetate (7a) and ethyl benzoylacetate (7b to convert the pyrimidin-8-ol 1 into the triazepinone derivatives 8a,b instead of 9a,b [18,19,20,21,22,23,24,25] (Scheme 2). The mass spectra of these compounds revealed peaks at characteristic m/z values corresponding to their molecular weights. In the 1H-NMR spectra, for example R= CH3, a signal at 4.06 ppm integrating for two protons (CH2), and only one downfield characteristic signal (D2O exchangeable) corresponding to OH proton at 9.72 ppm, excluded the structures 9a,b as reaction products since they lack a CH2 group and contain an NH function (no characteristic signal in 1H-NMR).
Scheme 2.
Synthesis of triazepine derivatives 8a,b.
Scheme 2.
Synthesis of triazepine derivatives 8a,b.
In continuation to our previous work on the title compound 1 and studying its behaviour towards aromatic aldehydes via condensation in basic medium (piperidine), the aromatic aldehydes were reacted in a different manner, all of which afforded the triazole derivatives 10, except salicyaldehyde that just gives the ordinary Schiff base 11 (Scheme 3).
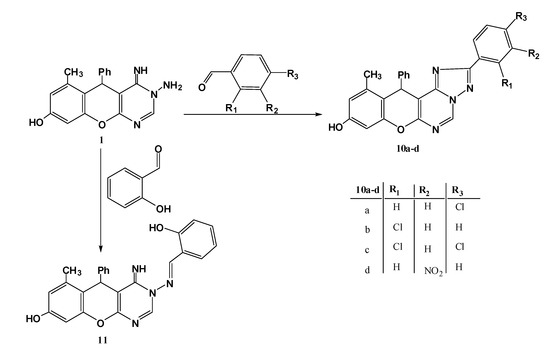
Scheme 3.
Reaction of title compound 1 with aromatic aldehydes.
Scheme 3.
Reaction of title compound 1 with aromatic aldehydes.
It worth mention that when an EWG (Cl, NO2) is in o-, m- or p-positions with respect to the aldehydic function, a condensation followed by cyclization occurred to give the triazoles 10, otherwise, the Schiff bases were produced, which is no doubt due to the + and –M effect of these substituents. The mass and 1H-NMR spectra were sufficient to indicate the correct structures, for example the mass spectrum for the reaction product obtained from reaction with p-chlorobenzaldehyde showed a peak at m/z = 440 consistent with structure 10a (the Schiff base should give m/z = 442). The 1H-NMR spectrum revealed one downfield CH=N-signal at 9.67 ppm (the Schiff base should show two downfield signals for CH=N- protons). On the other hand, reaction of 1 with salicylaldehyde afforded the Schiff base 11, based on its spectroscopic data; the mass spectrum showed a molecular ion peak at 424 (triazole 10 should give 422). Also, the 1H-NMR displayed two downfield signals at 8.34, 8.36 ppm corresponding to CH=N and 2-H protons, in addition to two D2O exchangeable signals (NH, OH) at 5.86 and 8.62 ppm.
Consequently, we aimed to investigate further the behaviour of the aminopyrimidine 1 towards activated unsaturated compounds such as dimethyl acetylenedicarboxylate and ethoxymethylene malonitrile. The reactions were performed without catalyst in ethanol. It was found that this reaction proceeds in a simple manner through addition to acetylenic function or the olefinic double bond followed by loss of methanol or ethanol to furnish the expected triazine derivative 13 or the triazepine derivative 15 respectively (Scheme 4).
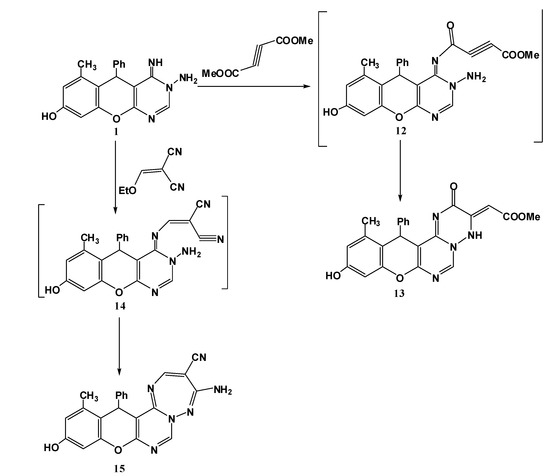
Scheme 4.
Reaction of title compound 1 with activated unsaturated compounds.
Scheme 4.
Reaction of title compound 1 with activated unsaturated compounds.
Confirmatory evidence for the structure assignment for compound 13 was provided by spectroscopic data. The IR spectrum revealed absorption bands at 1665, 1712 cm−1 characteristic for C=O, and COOMe; in the 1H-NMR spectrum, two signals at 2.17, 3.48 ppm assignable to two CH3 groups (CH3, OCH3) a more characteristic signal at 5.60 ppm integrating for one proton (=CHCOOMe).
The spectroscopic data for compound 15 were in a good agreement with this proposed structure, IR should show no great difference, while the electron ionization mass spectrum was consistent with the expected molecular mass for the proposed structure (m/z = 326). Furthermore, the 1H-NMR spectrum displayed a new signal at 8.57 ppm attributable to H5 in the trizepine ring.
Finally, an additional pathway for synthesis of substituted triazine derivatives 18a–c was achieved through reaction of the title compound 1 with α-halo compounds (namely ethyl α-chloroacetoacetate, α-chloroacetylacetone and α-chloroacetoacetanilide) in refluxing ethanol containing triethylamine (Scheme 5).
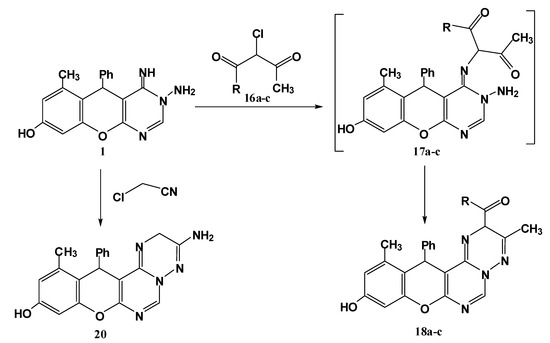
Scheme 5.
Synthesis of triazine derivatives 18a–cand 20.
Scheme 5.
Synthesis of triazine derivatives 18a–cand 20.
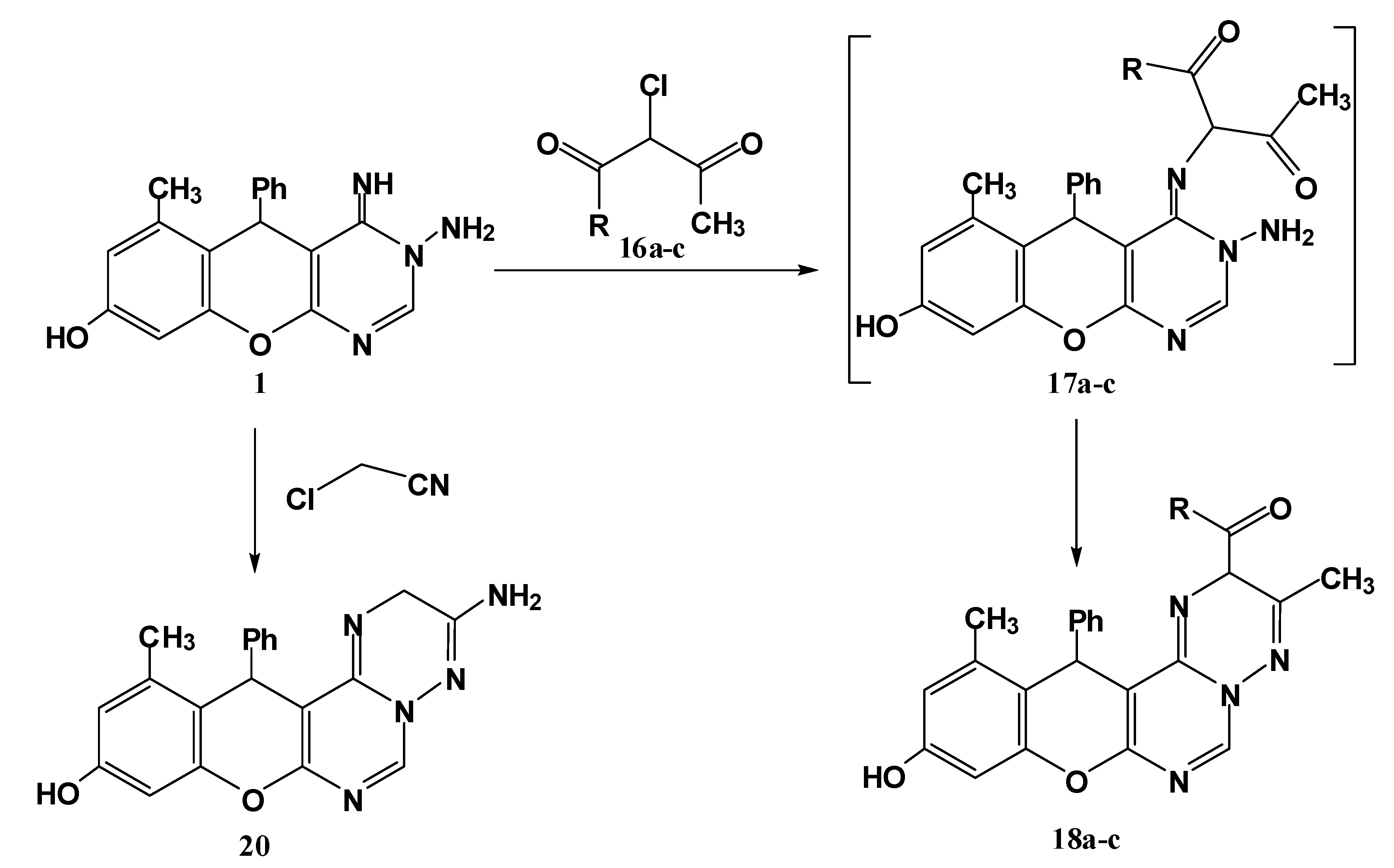
The reaction proceeds through nucleophilic substitution followed by cyclocondensation. The structural assignment of these compounds was based on spectral evidence and microanalyses. The mass spectra of these products 18a–c showed the molecular ion peaks at the expected m/z values. In their IR spectra, the appearance of absorption bands in the range 1712–1660 cm−1 confirmed the presence of a C=O group. The 1H-NMR spectrum, for example for compound 18a, revealed two signals at 2.21, 2.23 ppm each integrating for three protons (CH3-phenolic ring, CH3-triazine ring) in addition to the characteristic ethoxy triplet-quartet pattern; a new characteristic signal at 5.50 ppm assignable for H5 in triazine ring. In a similar manner, alkylation of the imino function of compound 1 with chloroacetonitrile followed by in situ cyclization through the addition of the amino group to the cyano function delivered the aminotriazine derivative 20 (Scheme 5).
2.2. Antitumor Screening Test
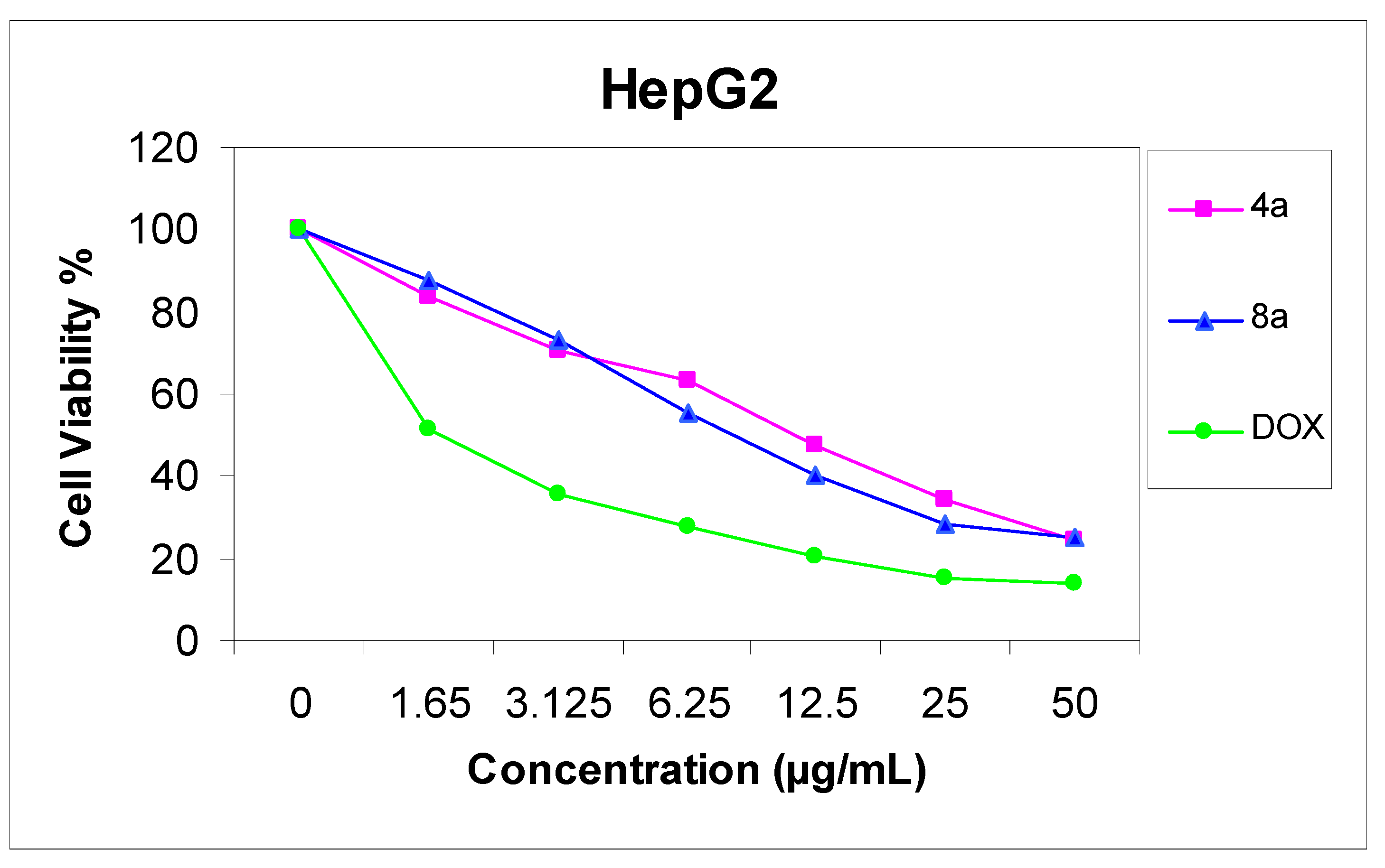
The cytotoxicity of compounds 4a and 8a was evaluated against two cell lines representing two common forms of human cancer i.e. human hepatocellular carcinoma cell line (HepG2) and human breast adenocarcinoma cell line (MCF-7). For comparison purposes, the cytotoxicity of doxorubicin, a standard antitumor drug, was evaluated under the same conditions (IC50 value of doxorubicin = 0.59 ± 0.04 and 0.72 ± 0.08 µg/mL, respectively). The analysis of the data obtained indicated that the IC50 values (dose of the compound which causes a 50% reduction of survival values) for such compounds against human breast cell MCF-7 line are 5.36 ± 0.12 and 6.71 ± 0.09 µg/mL, respectively (Figure 1), but against liver carcinoma cell line HepG2 they are 9.94 ± 0.15 and 6.93 ± 0.08 µg/mL, respectively (Figure 2). All values were calculated from dose-response curve done in triplicate for each compound. Values were given ± standard deviation. The value of IC50 indicated that:
- (1) Generally, both the tested compounds tended to be more active cytotoxic agents against human breast cell MCF-7 line, than HepG2 cell line;
- (2) Compound 4a is a more active cytotoxic agent against human breast cell MCF-7 line;
- (3) Compound 8a is a more active cytotoxic agent against human hepatocellular carcinoma cell line HepG2.
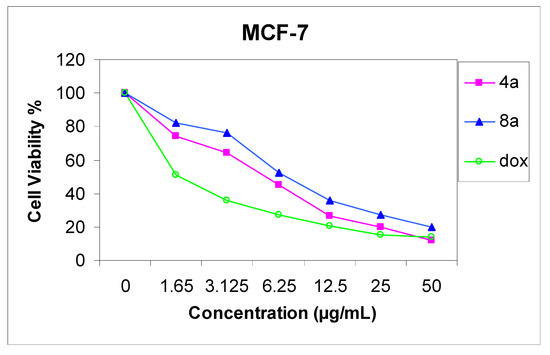
Figure 1.
Effect of concentration of compound 4a and 8a on MCF-7 cell.
Figure 1.
Effect of concentration of compound 4a and 8a on MCF-7 cell.
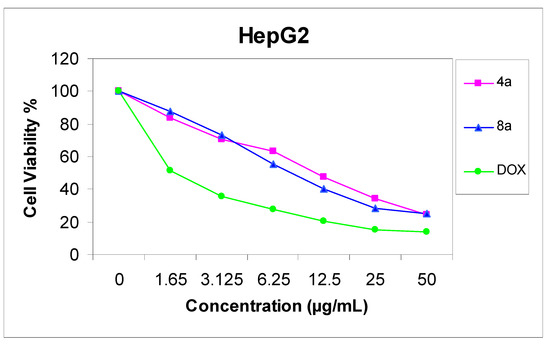
Figure 2.
Effect of concentration of compound 4a and 8a on HepG2 cell.
Figure 2.
Effect of concentration of compound 4a and 8a on HepG2 cell.
3. Experimental
3.1. Chemistry
3.1.1. General
Melting points were determined on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded in a Pye-Unicam SP300 instrument in potassium bromide discs. 1H-NMR spectra were recorded in a Varian Mercury VXR-300 spectrometer at 300 MHz in DMSO-d6 and the chemical shifts were related to TMS as standard solvent. Mass spectra were recorded in a GCMS-QP 1000 EX Shimadzu spectrometer, the ionizing voltage was 70 eV. Elemental analyses were carried out at the Microanalytical Laboratory of Cairo University, Giza, Egypt. Antitumor activity was evaluated by the Regional Center for Mycology and Biotechnology, Al-Azhar University, Cairo, Egypt. 3-Amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d]pyrimidine (1) [10] and hydrazonoyl halides 2 [26,27] were prepared as reported in the literature.
3.1.2. Synthesis of 10-Hydroxy-3-substituted-12-methyl-13-phenyl-2-(2-substituted phenyl-hydrazono)-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazines 4a–l
General procedure: A mixture of 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d] pyrimidine (1, 0.32 g, 1 mmol) and the appropriate hydrazonoyl halide 2 (1 mmol) in ethanol (20 mL) was refluxed for 2 h (monitored by TLC), then allowed to cool and the solid formed was filtered off, washed with ethanol, dried and recrystallized from DMF to give 4a–l.
10-Hydroxy-3,12-dimethyl-13-phenyl-2-(2-phenylhydrazono)-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4a). Yield 71%; reddish-brown solid; mp. 334 °C; IR (KBr): v 1630 (C=N), 3427 (br, OH, NH) cm−1; 1H-NMR (DMSO-d6): δH 2.14 (3H, s, 12-CH3), 2.16 (3H, s, 3-CH3), 5.40 (1H, s, 13-H), 6.45 (1H, s, 9-H), 6.52 (1H, s, 11-H), 6.75–7.45 (10H, m, Ar-H), 8.39 (1H, s, 6-H), 9.30 (1H, br s, NH), 9.66 (1H, s, OH); MS m/z (%): 463 (M+ + 1, 38), 462 (M+, 100), 385 (23), 192 (24), 77 (33). Anal. Calcd for C27H22N6O2 (462.18): C, 70.12; H, 4.79; N, 18.17. Found C, 70.10; H, 4.65; N, 18.03%.
10-Hydroxy-3,12-dimethyl-13-phenyl-2-[2-(p-tolyl)hydrazono]-2H,13H-chromeno[2,3-d]-pyrimido[1,6-b][1,2,4] triazine (4b). Yield 74%; reddish-brown solid; mp. 345 °C; IR (KBr): v 1639 (C=N), 3421 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.18 (3H, s, 12-CH3), 2.19 (3H, s, p-toly-CH3), 2.20 (3H, s, 3-CH3), 5.44 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.56 (1H, s, 11-H), 6.82–7.55 (9H, m, Ar-H), 8.42 (1H, s, 6-H), 9.37 (1H, br s, NH), 9.72 (1H, s, OH); MS m/z (%): 477 (M+ + 1, 34), 476 (M+, 100), 399 (14), 253 (18), 200 (22), 77 (28). Anal. Calcd for C28H24N6O2 (476.20): C, 70.57; H, 5.08; N, 17.64. Found C, 70.51; H, 5.11; N, 17.34%.
2-[2-(4-Chlorophenyl)hydrazono]-10-Hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4c). Yield 76%; reddish-brown solid; mp. 352 °C; IR (KBr): v 1639 (C=N), 3448 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.19 (3H, s, 12-CH3), 2.21 (3H, s, 3-CH3), 5.42 (1H, s, 13-H), 6.49 (1H, s, 9-H), 6.58 (1H, s, 11-H), 6.88–7.63 (9H, m, Ar-H), 8.40 (1H, s, 6-H), 9.39 (1H, br s, NH), 9.75 (1H, s, OH); MS m/z (%): 498 (M+ + 2, 36), 497 (M+ + 1, 23), 496 (M+, 100), 428 (27), 253 (18), 209 (46), 77 (60), 55 (73). Anal. Calcd for C27H21ClN6O2 (496.14): C, 65.26; H, 4.26; N, 16.91. Found C, 65.10; H, 4.34; N, 16.69%.
10-Hydroxy-3,12-dimethyl-2-[2-(4-nitrophenyl)hydrazono]-13-phenyl--2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4d). Yield 76%; dark red solid; mp. 358 °C; IR (KBr): v 1639 (C=N), 3440 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.20 (3H, s, 12-CH3), 2.21 (3H, s, 3-CH3), 5.48 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.60 (1H, s, 11-H), 6.89–7.77 (9H, m, Ar-H), 8.43 (1H, s, 6-H), 9.42 (1H, br s, NH), 9.77 (1H, s, OH); MS m/z (%): 508 (M+ + 1, 17), 507 (M+, 46), 429 (20), 294 (37), 253 (50), 77 (44), 55 (100). Anal. Calcd for C27H21N7O4 (507.17): C, 63.90; H, 4.17; N, 19.32. Found C, 63.76; H, 4.02; N, 19.12%.
2-[2-(4-Bromophenyl)hydrazono]-10-Hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4e). Yield 71%; dark reddish-brown solid; mp. 358 °C; IR (KBr): v 1633 (C=N), 3452 (br, OH, NH) cm−1; 1H-NMR (DMSO-d6): δH 2.18 (3H, s, 12-CH3), 2.20 (3H, s, 3-CH3), 5.43 (1H, s, 13-H), 6.51 (1H, s, 9-H), 6.60 (1H, s, 11-H), 6.90–7.69 (9H, m, Ar-H), 8.41 (1H, s, 6-H), 9.38 (1H, br s, NH), 9.69 (1H, s, OH); MS m/z (%): 542 (M+ + 2, 93), 541 (M+ + 1, 71), 540 (M+, 100),463 (13), 253 (25), 90 (40), 77 (9). Anal. Calcd for C27H21BrN6O2 (540.09): C, 59.90; H, 3.91; N, 15.52. Found C, 59.70; H, 3.86; N, 15.37.%.
10-Hydroxy-2-[2-(4-methoxyphenyl)hydrazono]-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4f). Yield 74%; dark red solid; mp. 320 °C; IR (KBr): v 1638 (C=N), 3412 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.12 (3H, s, 12-CH3), 2.15 (3H, s, 3-CH3), 3.71 (3H, s, OCH3), 5.37 (1H, s, 13-H), 6.45 (1H, s, 9-H), 6.51 (1H, s, 11-H), 6.86–7.44 (9H, m, Ar-H), 8.35 (1H, s, 6-H), 9.14 (1H, br s, NH), 9.65 (1H, s, OH); MS m/z (%): 493 (M+ + 1, 33), 492 (M+, 100), 415 (10), 253 (15), 122 (31), 77 (22). Anal. Calcd for C28H24N6O3 (492.19): C, 68.28; H, 4.91; N, 17.06. Found C, 68.11; H, 4.78; N, 16.89%.
10-Hydroxy-12-methyl-3,13-diphenyl-2-(2-phenylhydrazono)-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4g). Yield 70%; orange solid; mp. 316 °C; IR (KBr): v 1637 (C=N), 3438 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.19 (3H, s, 12-CH3), 5.47 (1H, s, 13-H), 6.51 (1H, s, 9-H), 6.59 (1H, s, 11-H), 6.73–7.85 (15H, m, Ar-H), 8.47 (1H, s, 6-H), 9.48 (1H, br s, NH), 9.74 (1H, s, OH); MS m/z (%): 524 (M+, 16), 384 (12), 228 (100), 77 (20). Anal. Calcd for C32H24N6O2(524.20): C, 73.27; H, 4.61; N, 16.02. Found C, 73.12; H, 4.45; N, 15.22%.
10-Hydroxy-12-methyl-3,13-diphenyl-2-[2(p-tolyl)hydrazono]-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4h). Yield 74%; dark brown solid; mp. 312 °C; IR (KBr): v 1628 (C=N), 3427 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.18 (3H, s, 12-CH3), 2.50 (3H, s, p-tolyl-CH3), 5.44 (1H, s, 13-H), 6.47 (1H, s, 9-H), 6.54 (1H, s, 11-H), 6.76–7.87 (14H, m, Ar-H), 8.42 (1H, s, 6-H), 9.44 (1H, br s, NH), 9.64 (1H, s, OH); MS m/z (%): 540 (M+ + 2, 9), 539 (M+ + 1, 42), 538 (M+, 100), 316 (10), 238 (17), 77 (27). Anal. Calcd for C33H26N6O2 (538.21): C, 73.59; H, 4.87; N, 15.60. Found C, 73.59; H, 4.87; N, 15.60%.
2-[2-(4-Chlorophenyl)hydrazono]-10-Hydroxy-12-methyl-3,13-diphenyl2H,13H-chromeno-[2,3-d]pyrimido[1,6-b][1,2,4]triazine (4i). Yield 72%; dark brown solid; mp. 330 °C; IR (KBr): v 1643 (C=N), 3444 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.15 (3H, s, 12-CH3), 5.43 (1H, s, 13-H), 6.40 (1H, s, 9-H), 6.51 (1H, s, 11-H), 6.72–7.98 (14H, m, Ar-H), 8.43 (1H, s, 6-H), 9.45 (1H, br s, NH), 9.70 (1H, s, OH); MS m/z (%): 559 (M+ + 1, 9), 558 (M+, 21), 391 (12), 201 (21), 105 (47), 77 (47), 55 (100). Anal. Calcd for C32H23ClN6O2 (558.16): C, 68.75; H, 4.15; N, 15.03. Found C, 68.73; H, 4.10; N, 15.00%.
2-[2-(3-Bromophenyl)hydrazono]-10-Hydroxy-12-methyl-3,13-diphenyl-2H,13H-chromeno-[2,3-d]pyrimido[1,6-b][1,2,4] triazine (4j). Yield 70%; dark brown solid; mp. 180 °C; IR (KBr): v 1643 (C=N), 3413 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.17 (3H, s, 12-CH3), 5.46 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.56 (1H, s, 11-H), 6.69–7.90 (14H, m, Ar-H), 8.47 (1H, s, 6-H), 9.50 (1H, br s, NH), 9.74 (1H, s, OH); MS m/z (%): 604 (M+ + 2, 3), 603 (M+ + 1, 4), 602 (M+, 3), 503 (4), 471 (5), 305 (15), 228 (88), 105 (100), 77 (79). Anal. Calcd for C32H23BrN6O2 (602.11): C, 63.69; H, 3.84; N, 13.93. Found C, 63.57; H, 3.75; N, 13.68%.
Ethyl4-[10-hydroxy-12-methyl-3,13-diphenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazin-2-ylidene)hydrazinyl]benzoate (4k). Yield 72%; dark brown solid; mp. 352 °C; IR (KBr): v 1639 (C=N), 1715 (C=O), 3420 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 1.34 (3H, t, CH3), 2.21 (3H, s, 12-CH3), 4.51 (2H, q, CH2), 5.51 (1H, s, 13-H), 6.52 (1H, s, 9-H), 6.58 (1H, s, 11-H), 6.70–7.92 (14H, m, Ar-H), 8.40 (1H, s, 6-H), 9.51 (1H, br s, NH), 9.70 (1H, s, OH); MS m/z (%): 597 (M+ + 1, 13), 596 (M+, 24), 567 (100), 524 (30), 432 (18), 228 (49), 103 (78), 77 (66). Anal. Calcd for C35H28N6O4 (596.22): C, 70.46; H, 4.73; N, 14.09. Found C, 70.34; H, 4.45; N, 13.97%.
10-Hydroxy-12-methyl-13-phenyl-3-2-(2-phenylhydrazono)-(thiophen-2-yl)-2H,13H-chromeno2,3-d]pyrimido[1,6-b][1,2,4]triazine (4l). Yield 76%; dark red solid; mp. 228 °C; IR (KBr): v 1647 (C=N), 3387 (br, OH,NH) cm−1; 1H-NMR (DMSO-d6): δH 2.19 (3H, s, 12-CH3), 5.46 (1H, s, 13-H), 6.48 (1H, s, 9-H), 6.56 (1H, s, 11-H), 6.68–7.96 (13H, m, Ar-H), 8.49 (1H, s, 6-H), 9.54 (1H, br s, NH), 9.71 (1H, s, OH); MS m/z (%): 531 (M+ + 1, 2), 530 (M+ , 7), 385 (9), 306 (39), 228 (100), 77 (29). Anal. Calcd for C30H22N6O2S (530.15): C, 67.91; H, 4.18; N, 15.84. Found C, 67.91; H, 4.18; N, 15.84%.
3.1.3. Synthesis of 10-hydroxy-12-methyl-9-phenylazo-2,13-diphenyl-13H-chromeno[2,3-d]pyrimido [1,6-b][1,2,4] triazine 6
Synthesis of 10-hydroxy-12-methyl-2,13-diphenyl-13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (5). A mixture of 1 (0.320 g, 1 mmol) and phenacyl bromide (0.198 g, 1 mmol) in absolute ethanol (30 mL) was refluxed for 2 h (monitored by TLC). The product started to separate out during the course of reaction. The crystalline solid was filtered, washed with water, dried and recrystallized from dioxane to give compound 5 in 76% yield as yellow solid; mp. 230 °C; IR (KBr): v 1632 (C=N), 3425 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.14 (3H, s, 12-CH3), 4.23 (2H, s, CH2), 5.55 (1H, s, 13-H), 6.52 (1H, s, 9-H), 6.54 (1H, s, 11-H), 7.18–7.38 (10H, m, Ar-H),), 8.63 (1H, s, 6-H), 9.82 (1H, s, OH); MS m/z (%): 421 (M+ +1, 1), 420 (M+, 2), 329 (14), 305 (16), 253 (21), 228 (100), 201 (23), 105 (15), 77 (26); Anal. Calcd for C26H20N4O2 (420.47): C, 74.27; H, 4.79; N, 13.32. Found C, 74.14; H, 4.87; N, 13.35%.
Coupling of 5 with benzenediazonium chloride. To a solution of 5 (0.421g, 1 mmol) in ethanol (20 mL) was added sodium acetate trihydrate (0.138 g, 1 mmol), and the mixture was cooled to 0–5 °Cin an ice bath. To the resulting cold solution was added portionwise a cold solution of benzenediazonium chloride (1 mmol) [prepared by diazotizing aniline] dissolved in hydrochloric acid (6 M, 1 mL) with a solution of sodium nitrite (0.07 g, 1 mmol) in water (2 mL). After complete addition of the diazonium salt, the reaction mixture was stirred for a further 30 min in an ice bath. The solid that separated was filtered off, washed with water and finally recrystallized from ethanol to give product 6. Yield 78%; orange solid; mp. 298 °C; IR (KBr): v 1634 (C=N), 3466 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.16 (3H, s, 12-CH3), 4.24 (2H, s, CH2), 5.56 (1H, s, 13-H), 6.54 (1H, s, 11-H), 7.03–7.41 (15H, m, Ar-H), 8.63 (1H, s, 6-H), 9.88 (1H, s, OH); MS m/z (%): 524 (M+, 23), 305 (100), 228 (87), 77 (64). Anal. Calcd for C32H24N6O2(524.20): C, 73.27; H, 4.61; N, 16.02. Found C, 73.10; H, 4.65; N, 15.12%.
3.1.4. Synthesis of 4-substituted-11-hydroxy-13-methyl-14-phenyl-3H,14H-chromeno[2,3-d]pyrimido [1,6-b][1,2,4] triazepin-2(3H)-ones 8a,b.
A mixture of compound 1 (0.32 g, 1 mmol) and ethyl acetoacetate or ethyl benzoylacetate (1.5 mmol) was heated under reflux for 2 h. After cooling, the solid precipitated was collected and crystallized from dioxane to give 8a,b, respectively.
11-Hydroxy-4,13-dimethyl-14-phenyl-3H,14H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazepin-2(3H)-one (8a). Yield 74%; yellow solid; mp. 189 °C; IR (KBr): v 1635 (C=N), 1720 (CO), 3251 (NH), 3406 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.07 (3H, s, 13-CH3), 2.17 (3H, s, 4-CH3), 4.06 (2H, s, CH2), 5.57 (1H, s, 14-H), 6.50 (1H, s, 10-H), 6.60 (1H, s, 12-H), 7.14–7.29 (5H, m, Ar-H), 9.50 (1H, s, 7-H), 9.72 (1H, s, OH); MS m/z (%): 388 (M+ + 2, 1), 387 (M+ + 1, 5), 386 (M+, 16), 309 (100), 266 (19) 77 (11), 55. Anal. Calcd for C22H18N4O3 (386.14): C, 68.38; H, 4.70; N, 14.50. Found C, 68.32; H, 4.65; N, 14.36%.
11-Hydroxy-13-methyl-4,14-diphenyl-3H,14H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazepin-2(3H)-one (8b). Yield 74%; yellow solid; mp. 214 °C; IR (KBr): v 1632 (C=N), 1702 (CO), 3416 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.10 (3H, s, 13-CH3), 4.15 (2H, s, CH2), 5.62 (1H, s, 14-H), 6.55 (1H, s, 10-H), 6.68 (1H, s, 12-H), 7.06–7.67 (10H, m, Ar-H), 9.52 (1H, s, 7-H), 9.82 (1H, s, OH); MS m/z (%): 449 (M++1, 8), 448 (M+, 20), 371 (100), 266 (24), 105 (76), 77 (75). Anal. Calcd for C27H20N4O3 (448.15): C, 72.31; H, 4.49; N, 12.49. Found C, 72.18; H, 4.34; N, 12.29%.
3.1.5. Reaction of N-aminopyrimidine 1 with Aromatic Aldehydes
General procedure: An appropriate aromatic aldehyde (1 mmol.) was added to a solution of the N-aminopyrimidine 1 (0.32 g, 1 mmol) in absolute ethanol (15 mL) containing a few drops of piperidine, the resulting mixture refluxed for 3 h. The solids formed after cooling were collected by filtration, washed with ether and crystallized from DMF.
9-Hydroxy-11-methyl-12-phenyl-2-(4-chlorophenyl)-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10a). Yield 76%; yellow solid; mp. 270 °C; IR (KBr): v 1639 (C=N), 3441 (br, OH) cm−1; 1H-NMR (DMSO-d6): δH 2.20 (3H, s, 11-CH3), 6.01 (1H, s, 12-CH), 5.86 (1H, s, NH), 6.47 (1H, s, 8-H), 6.55 (1H, s, 10-H), 7.08–8.40 (9H, m, Ar-H), 9.67 (1H, s, 5-H), 11.36 (1H, s, OH); MS m/z (%): 440 (M+, 2), 366 (12), 305 (100), 228 (69), 201 (33), 138(73), 77(52). Anal.Calcd for C25H17ClN4O2S (440.10): C, 68.11; H, 3.89; N, 12.71. Found C, 68.01; H, 3.67; N, 12.56%.
2-(2-Chlorophenyl)-9-hydroxy-11-methyl-12-phenyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10b). Yield 74%; yellow solid; mp. 252 °C; IR (KBr): v 1636 (C=N), 3438 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.21 (3H, s, 11-CH3), 6.02 (1H, s, 12-H), 6.48 (1H, s, 8-H), 6.52 (1H, s, 10-H), 7.05–8.33 (9H, m, Ar-H), 9.60 (1H, s, 5-H), 11.03 (1H, s, OH); MS m/z (%): 440 (M+, 12), 305 (66), 228 (100), 138(58), 77(47). Anal.Calcd for C25H17ClN4O2S (440.10): C, 68.11; H, 3.89; N, 12.71. Found C, 68.21; H, 3.71; N, 12.49%.
2-(2,4-Dichlorophenyl)-9-Hydroxy-11-methyl-12-phenyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10c). Yield 74%; yellow solid; mp. 298 °C; IR (KBr): v 1639 (C=N), 3444 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.25 (3H, s, 11-CH3), 6.12 (1H, s, 12-H), 5.88 (1H, s, NH), 6.49 (1H, s, 8-H), 6.61 (1H, s, 10-H), 7.08–8.46 (8H, m, Ar-H), 9.74 (1H, s, 5-H), 11.36 (1H, s, OH); MS m/z (%): 476 (M+ + 2, 7), 474 (M+, 6), 397 (26), 305 (24), 228 (75), 145 (100), 77 (38). Anal. Calcd for C25H16Cl2N4O2 (474.07): C, 63.17; H, 3.39; N, 11.79. Found C, 63.10; H, 3.21; N, 11.53%.
9-Hydroxy-11-methyl-2-(3-nitrophenyl)-12-phenyl-12H-chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine (10d). Yield 72%; yellow solid; mp. 240 °C; IR (KBr): v 1632 (C=N), 3412 (OH) cm−1; 1H-NMR (DMSO-d6): 2.09 (3H, s, CH3), 5.58 (1H, s, 11-H), 6.42 (1H, s, 8- H), 6.57 (1H, s, 10-H), 7.10–8.46 (9H, m, Ar-H), 9.50 (1H, s, 5-H), 9.72 (1H, br s, OH); MS m/z (%): 452 (M+ + 1, 6), 451 (M+, 17), 374 (100), 172 (13),77 (18). Anal.Calcd for C25H17N5O4 (451.13): C, 66.51; H, 3.80; N, 15.51. Found C, 66.34; H, 3.65; N, 15.41%.
3-(2-Hydroxybenzylideneamino)-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d]pyrimidin-8-ol (11). Yield 78%; cannary yellow solid; mp. 192 °C; IR (KBr): v 1616 (C=N), 3433 (very br, 2OH,NH) cm−1; 1H-NMR (DMSO-d6): 1H-NMR δH 2.19 (3H, s, 6-CH3), 5.68 (1H, s, 5-H), 6.45 (1H, s, 9-H), 6.55 (1H, s, 7-H), 6.89–7.56 (9H, m, Ar-H), 8.27 (1H, s, CH=N-N), 8.37 (1H, s, 2-H), 8.53 (1H, s, NH), 8.57 (1H, s, OH); MS m/z (%):424 (M+, 29), 304 (100), 228 (94), 173 (35), 105 (29), 77 (75); Anal.Calcd for C25H20N4O3 (424.15): C, 70.74; H, 4.75; N, 13.20. Found C, 70.31; H, 4.56; N, 13.02%.
3.1.6. Reaction of 1 with Activated Unsaturated Compounds
Synthesis of 10-hydroxy-12-methyl-2-oxo-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazin-3(4H)-ylidene)acetate (13). An equimolar mixture of 1 (0.32 g, 1 mmol) and dimethylacetylene dicarboxylate (0.142 g, 1 mmol) in methanol (20 mL) was refluxed for 2 h (monitored by TLC). The formed solid was collected by filtration and recrystallized from DMF to give compound 13. Yield 78%; canary yellow solid; mp. 184 °C; IR (KBr): v = 1633 (C=N), 1665, 1712 (2C=O), 3466 (br, OH and NH) cm−1; 1H-NMR (DMSO-d6): δH 2.17 (3H, s, 12-CH3), 3.48 (3H, s, OCH3), 5.42 (1H, s, 13-H), 6.60 (1H, s, CH=CO2Me), 6.52 (1H, s, 9-H), 6.62 (1H, s, 11-H), 7.11–7.34 (5H, m, Ar-H), 9.12 (1H, s, 6-H), 9.95 (1H, s, NH), 10.15 (1H, s, OH); MS m/z (%): 432 (M+ + 2, 2), 431 (M+ + 1, 2), 430 (M+, 5), 305 (22), 253 (25), 228 (100), 105 (69), 77 (62). Anal. Calcd for C23H18N4O5 (430.13): C, 64.18; H, 4.22; N, 13.02. Found C, 64.12; H, 4.13; N, 12.82%.
Synthesis of 4-Amino-11-hydroxy-13-methyl-14-phenyl-3H,14H-chromeno[2,3-d]pyrimido[1,6-b]1,2,4]triazepine-3-carbonitrile (15). A mixture of 1 (0.32 g, 1 mmol) and ethoxymethylene malononitrile (0.122 g, 1 mmol) in methanol (20 mL) was refluxed for 1 h (monitored by TLC). The reaction mixture was cooled and the resulting precipitate was filtered off and recrystallized from DMF/EtOH to give 15. Yield 76%; yellow solid; mp. 330 °C; IR (KBr): v 1628 (C=N), 2197 (CN), 3214, 3180 (NH2), 3466 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.07 (3H, s, 13-CH3), 5.61 (1H, s, 14-H), 6.50 (1H, s, 10-H), 6.60 (1H, s, 12-H), 7.12–7.33 (7H, m, Ar-H + NH2), 8.57 (1H, s, 2-H), 9.57 (1H, s, 7-H), 9.72 (1H, s, OH); MS m/z (%): 396 (M+, 3), 330 (13), 253 (100), 77 (11). Anal. Calcd for C22H16N6O2 (396.13): C, 66.66; H, 4.07; N, 21.20. Found C, 66.38; H, 4.01; N, 21.03%.
3.1.7. Reaction of 1 with Active Chloromethylene Compounds 16a–c and Chloroacetonitrile
General procedure: To a solution of 1 (0.32 g, 1 mmol) in ethanol was added triethylamine (0.7 mL) and the mixture was stirred for 10 min at room temperature. To the resulting clear solution was added active chloromethylene compounds 16a–c and chloroacetonitrile (1 mmol) dropwise while stirring the reaction mixture. After complete addition, the reaction mixture was refluxed for 2 h (monitored by TLC). The solid that precipitated was filtered off, washed with H2O, dried and finally crystallized from ethanol to give the respective 18a–c and 20.
2-Acetyl-10-hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (18a). Yield 74%; yellow solid; mp. 212 °C; IR (KBr): v 1639 (C=N), 1698 (C=O), 3410 (OH) cm−1; 1H-NMR (DMSO-d6): δH 2.17 (3H, s, 12-CH3), 2.19 (3H, s, 3-CH3), 2.25 (3H, s, CH3CO), 5.48 (1H, s, 2-H), 5.6 (1H, s, 13-H), 6.46 (1H, s, 9-H), 6.58 (1H, s, 11-H), 7.14–7.54 (5H, m, Ar-H), 8.20 (1H, s, 6-H), 9.69 (1H, br s, OH); MS m/z (%): 400 (M+, 8), 305 (12), 276 (100), 253 (11), 228 (92), 105 (18), 77 (15). Anal. Calcd for C23H20N4O3 (400.15): C, 68.99; H, 5.03; N, 13.99. Found C, 68.78; H, 4.83; N, 13.86%.
Ethyl-10-hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine-2-carboxylate (18b). Yield 74%; yellow solid; mp. 160 °C; IR (KBr): v 1634 (C=N), 1712 (C=O), 3422 (OH) cm−1; 1H-NMR (DMSO-d6): δH 1.30 (3H, t, CH3), 2.21 (3H, s, 12-CH3), 2.23 (3H, s, 3-CH3), 4.22 (2H, q, CH2), 5.50 (1H, s, 2-H), 5.58 (1H, s, 13-H), 6.40 (1H, s, 9-H), 6.49 (1H, s, 11-H), 7.11–7.36 (5H, m, Ar-H), 8.17 (1H, s, 6-H), 9.61 (1H, br s, OH); MS m/z (%): 430 (M+, 12), 354 (16), 305 (59), 268 (43), 228 (100), 105 (18), 76 (52). Anal. Calcd for C24H22N4O4 (430.16): C, 66.97; H, 5.15; N, 13.02. Found C, 66.76; H, 5.01; N, 12.92%.
10-Hydroxy-3,12-dimethyl-N,13-diphenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4] triazine-2-carboxamide (18c). Yield 76%; yellow solid; mp. 198 °C; IR (KBr): v 1636 (C=N), 1660 (C=O), 3433 (br, OH and NH) cm−1. 1H-NMR (DMSO-d6): δH 2.14 (3H, s, 12-CH3), 2.17 (3H, s, 3-CH3), 5.37 (1H, s, 2-H), 5.59 (1H, s, 13-H), 6.40 (1H, s, 9-H), 6.52 (1H, s, 11-H), 7.19–7.84 (10H, m, Ar-H), 8.18 (1H, s, 6-H), 9.45 (1H, s, NH), 9.79 (1H, br s, OH); MS m/z (%): 477 (M+, 16), 305 (100), 268 (43), 228 (94), 105 (46), 77 (68). Anal. Calcd for C28H23N5O3 (477.18): C, 70.43; H, 4.85; N, 14.67. Found C, 70.33; H, 4.74; N, 14.62%.
3-Amino-10-hydroxy-3,12-dimethyl-13-phenyl-2H,13H-chromeno[2,3-d]pyrimido[1,6-b][1,2,4]triazine (20). Yield 73%; yellow solid; mp. 165 °C; IR (KBr): v 1639 (C=N), 3356, 3198 (NH2), 3406 (OH) cm−1. 1H-NMR (DMSO-d6): δH 2.11 (3H, s, 12-CH3), 3.88 (2H, s, CH2), 5.57 (1H, s, 13-H), 6.35 (2H, br s, NH2), 6.52 (1H, s, 9-H), 6.50 (1H, s, 11-H), 7.11–7.42 (5H, m, Ar-H), 8.67 (1H, s, 6-H), 9.84 (1H, s, OH); MS m/z (%): 359 (M+, 3), 253 (25), 228 (100), 77 (23). Anal. Calcd for C20H17N5O2 (359.14): C, 66.84; H, 4.77; N, 19.49. Found C, 66.67; H, 4.54; N, 19.36%.
3.2. Cytotoxic Activity
Potential cytotoxicity of the compounds was tested using the method of Skehan et al. [28], using Sulfo-Rhodamine-B stain (SRB). Cells were plated in 96-multiwill plates (104 cells/well) for 24 h before treatment with the tested compound to allow attachment of cell to the wall of the plate. Different concentrations of the compound under test (0, 1.56, 3.125, 6.25, 12.5, 25, and 50 µg/mL) were added to the cell monolayer in triplicate wells individual dose, monolayer cells were incubated with the compounds for 48 h at 37 °C and in atmosphere of 5% CO2. After 48 h, cells were fixed, washed and stained with SRB stain, excess stain was washed with acetic acid and attached stain was recovered with tris-EDTA buffer, color intensity was measured in an ELISA reader. The relation between surviving fraction and drug concentration is plotted to get the survival curve of each tumor cell line after the specified compound and the IC50 was calculated (Figure 1 and Figure 2).
4. Conclusions
In this report, a simple method for the synthesis of new chromeno[2,3-d]]pyrimido[1,6-b][1,2,4]triazines, chromeno[2,3-d]]pyrimido[1,6-b][1,2,4] triazepinones and chromeno[3,2-e][1,2,4]triazolo[1,5-c]pyrimidines by the reactions of 3-amino-8-hydroxy-4-imino-6-methyl-5-phenyl-4,5-dihydro-3H-chromeno[2,3-d]pyrimidine and hydrazonoyl halides, ethyl acetoacetate, ethyl benzoylacetate, and aromatic aldehydes are demonstrated. The new compounds 4a and 8a have been evaluated for the antitumor activity against human breast cell MCF-7 line and liver carcinoma cell line HepG2.
- Sample Availability: Samples of the synthesized compounds are available from the authors.
References
- Sabry, N.M.; Mohamed, H.M.; Khattab, E.S.; Motlaq, S.S.; El-Agrody, A.M. Synthesis of 4H-chromene, coumarin, 12H-chromeno[2,3-d]pyrimidine derivatives and some of their antimicrobial and cytotoxicity activities. Eur. J. Med. Chem. 2011, 46, 765–772. [Google Scholar] [CrossRef]
- Rai, U.S.; Isloor, A.M.; Shetty, P.; Vijesh, A.M.; Prabhu, N.; Isloor, S.; Thiageeswaran, M.; Fun, H.K. Novel chromeno[2,3-b]-pyrimidine derivatives as potential anti-microbial agents. Eur. J. Med. Chem. 2010, 45, 2695–2699. [Google Scholar]
- Abd El-Wahab, H.F.; Mohamed, A.M.; El-Agrody, H.M.; El-Nassag, A.A.; Bedair, M.H. Synthesis and Reactions of Some New Benzylphthalazin-1-ylaminophenols, 2H-Chromene and 5H-Chromeno[2,3-d]pyrimidine Derivatives with Promising Antimicrobial Activities. Lett. Org. Chem. 2012, 9, 360–368. [Google Scholar] [CrossRef]
- Kamdar, N.R.; Haveliwala, D.D.; Mistry, P.T.; Patel, S.K. Synthesis and evaluation of in vitro antitubercular activity and antimicrobial activity of some novel 4H-chromeno[2,3-d]pyrimidine via 2-amino-4-phenyl-4H-chromene-3-carbonitriles. Med. Chem. Res. 2011, 20, 854–864. [Google Scholar] [CrossRef]
- Attaby, F.A.; Eldin, S.M. Synthesis of Pyrimidine, Thiazolopyrimidine, Pyrimidotriazine and Triazolopyrimidine Derivatives and their Biological Evaluation. Z. Naturforsch. 1999, 54b, 78–798. [Google Scholar]
- Aly, A.A.; Gad El-Karim, I.A. Facile synthesis of new pyrazolopyrimidine derivatives of potential biosignificant interest. J. Korean Chem. Soc. 2011, 55, 781–786. [Google Scholar] [CrossRef]
- El-Mahdy, K.M.; Abdel-Rahman, R.M.A. Convenient methods for synthetic isomeric structures of pyrimido-1,2,4-triazine derivatives as biocidal agents. Acta Chim. Slov. 2011, 58, 755–764. [Google Scholar]
- Guertin, K.R.; Setti, L.; Qi, L.; Dunsdon, R.M.; Dymock, B.W.; Jones, P.; Overton, S.H. Identification of a novel class of orally active pyrimido[5,4-e][1,2,4]triazine-5,7-diamine-based hypoglycemic agents with protein tyrosine phosphatase inhibitory activity. Bioorg. Med. Chem. Lett. 2003, 13, 2895–2898. [Google Scholar] [CrossRef]
- Sugimoto, T.; Matsuura, S. A new synthesis of pyrimido[4,5-e][1,2,4]triazines from 5,5-dibromopyrimidines. Bull. Chem. Soc. Jpn. 1975, 48, 1679–1680. [Google Scholar] [CrossRef]
- Mohammed, F.K.; Badrey, M.G. Synthesis of pyrimidines and heteroannulated pyrimidine ring systems. J. Korean Chem. Soc. 2011, 55, 218–229. [Google Scholar] [CrossRef]
- Gomha, S.M.; Abdel-Aziz, H.A. Synthesis of new heterocycles derived from 3-(3-methyl-1H-indol-2-yl)-3-oxopropanenitrile as potent antifungal agents. Bull. Korean Chem. Soc. 2012, in press.. [Google Scholar]
- Gomha, S.M.; Khalil, K.D. A Convenient ultrasound-promoted synthesis and cytotoxic activity of some new thiazole derivatives bearing a coumarin nucleus. Molecules 2012, 17, 9335–9347. [Google Scholar] [CrossRef]
- Gomha, S.M.; Riyadh, S.M. Synthesis of triazolo[4,3-b][1,2,4,5]tetrazines and triazolo[3,4-b] [1,3,4]thiadiazines using chitosan as ecofriendly catalyst under microwave irradiation. ARKIVOC 2009, XI, 58–68. [Google Scholar]
- Gomha, S.M.; Abdel-Aziz, H.A. Enaminones as building blocks in heterocyclic preparations: synthesis of novel pyrazoles, pyrazolo [3,4-d]pyridazines, pyrazolo[1,5-a]pyrimidines, pyrido[2,3-d] pyrimidines linked to imidazo[2,1-b]thiazole system. Heterocycles 2012, 85, 2291–2303. [Google Scholar] [CrossRef]
- Shawali, A.S.; Sherif, S.M.; Farghaly, T.A.; Shehata, M.R.; Darwish, M.A.A. Site selectivity synthesis and tautomerism of arylazo derivatives of pyrazolo[3,4-d]pyrimido[1,6-b][1,2,4]triazine. AFINIDAD 2008, LXV, 314–319. [Google Scholar]
- Shawali, A.S.; Farghaly, T.A. Synthesis and tautomeric structure of 2-[N-aryl-2-oxo-2-arylethanehydrazonoyl]-6-methyl-4(3H)-pyrimidinones. Tetrahedron 2004, 60, 3051–3057. [Google Scholar] [CrossRef]
- Mosselhi, M.A.; Pfleiderer, W. Purines. Part XVI: Syntheses, properties, and reactions of 8-aminoxanthines. Helv. Chim. Acta 2010, 93, 2115–2134. [Google Scholar]
- Shawali, A.S.; Mosselhi, M.A.; Altablawy, F.M.A.; Farghaly, T.A.; Tawfik, N.M. Synthesis and tautomeric structure of 3,7-bis(arylazo)-6-methyl-2-phenyl-1H-imidazo[1,2-b]pyrazoles in ground and excited states. Tetrahedron 2008, 64, 5524–5530. [Google Scholar]
- Shawali, A.S.; Mosselhi, M.A.; Farghaly, T.A.; Shehata, M.R.; Tawfik, N.M. Synthesis and tautomeric structure of 3,6-bis(arylazo)pyrazolo[1,5-a]pyrimidine-5,7(4H,6H)-diones. J. Chem. Res. 2008, 452–456. [Google Scholar]
- Farghaly, T.A.; Edrees, M.M.; Mosselhi, A.M. Synthesis, tautomeric structure and antimicrobial activity of 3-arylhydrazono-4-phenyl-[1,2,4]-triazepino[2,3-a]quinazoline-2,7(1H)-diones. Molecules 2012, 17, 8483–8493. [Google Scholar] [CrossRef]
- Shawali, A.S.; Farghaly, T.A. Synthesis and tautomeric structure of 6-arylhydrazono 1H-pyrazolo[3',4':4,5]-pyrimido[1,6-b][1,2,4]triazepines. Tetrahedron 2009, 65, 644–647. [Google Scholar] [CrossRef]
- Essassi, E.M.; Lavergne, J.P.; Viallefont, P.; Daunis, J. Recherches en série triazepine-1,2,4:1-détermination de la structure de la triazolotriazépinone obtenue par action de l'acétylacétate d'éthyle sur le diamino-3,4 triazole-1,2,4. J. Heterocycl. Chem. 1975, 12, 661–663. [Google Scholar] [CrossRef]
- Hassan, K.M.; Ahmed, R.A.; Abdel-Hafez, S.H.; Abdel-Azim, M.A. Condensed thioxocyclopentapyridine (isoquinoline)-1,2,4-azines. Phosphorus Sulfur Silicon Relat. Elem. 2006, 181, 481–496. [Google Scholar] [CrossRef]
- Romano, C.; de la Cuesta, E.; Avendano, C.; Florencio, F.; Sainz-Aparicio, J. Reactions of 1,2-diaminobenzimidazoles with β-dicarbonyl compounds. Tetrahedron 1988, 44, 7185–7192. [Google Scholar] [CrossRef]
- Shawali, A.S.; Sherif, S.M.; Farghaly, T.A.; Shehata, M.R.; Darwish, M.A.A. Synthesis and tautomeric structure of the azo-coupling products of 2-methyl-7-phenylpyrimido[1,2-b][1,2,4]triazepine-4,9(3H,5H)-dione. J. Chem. Res. 2007, 44–47. [Google Scholar]
- Eweiss, N.F.; Osman, A. Synthesis of heterocycles. Part II: New routes to acetylthiadiazolines and alkylazothiazoles. J. Heterocycl. Chem. 1980, 17, 1713–1717. [Google Scholar]
- Shawali, A.S.; Abdelhamid, A.O. Reaction of dimethylphenacylsulfonium bromide with N-nitrosoacetarylamides and reactions of the products with nucleophiles. Bull. Chem. Soc. Jpn. 1976, 49, 321–327. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.I.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Nat. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
