Solvent-Free Synthesis of Modified Pectin Compounds Promoted by Microwave Irradiation
Abstract
:1. Introduction
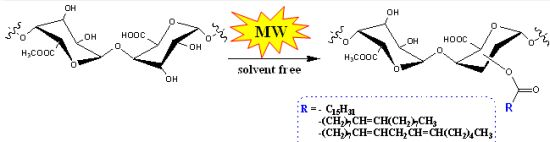
2. Results and Discussion

| Compound | Fatty acid | Time(min) |
|---|---|---|
| 1a | Linoleic acid | 3 |
| 1b | Linoleic acid | 6 |
| 2a | Oleic acid | 3 |
| 2b | Oleic acid | 6 |
| 3a | Palmitic acid | 3 |
| 3b | Palmitic acid | 6 |
2.1. Infrared Analysis



2.2. Thermal Analysis

3. Experimental
3.1. Materials
3.2. Methods
3.3. Synthesis of Modified Pectin Samples
4. Conclusions
Supplementary Materials
Acknowledgments
References
- Tanaka, K.; Toda, F. Solvent-free organic synthesis. Chem. Rev. 2000, 100, 1025–1074. [Google Scholar] [CrossRef]
- Lidstrom, P.; Tierney, J.; Wathey, B.; Westman, J. Microwave assisted organic synthesis—A review. Tetrahedron 2001, 57, 9225–9283. [Google Scholar] [CrossRef]
- Ravichandran, S.; Karthikeyan, E. Microwave synthesis—A potential tool for green chemistry. Int. J. ChemTech Res. 2011, 3, 466–470. [Google Scholar]
- Loupy, A. Solvent-free microwave organic synthesis as an efficient procedure for green chemistry. C. R. Chimie 2004, 7, 103–112. [Google Scholar] [CrossRef]
- Li, J.; Zhang, L.; Peng, F.; Bian, J.; Yuan, T.; Xu, F.; Sun, R. Microwave-assisted solvent-free acetylation of cellulose withacetic anhydride in the presence of iodine as a catalyst. Molecules 2009, 14, 3551–3566. [Google Scholar] [CrossRef]
- Varma, R.S. Solvent-free organic syntheses using supported reagents and microwave irradiation. Green Chem. 1999, 1, 43–55. [Google Scholar] [CrossRef]
- Kappe, C.O. Controlled microwave heating in modern organic synthesis. Angew. Chem. Int. Ed. 2004, 43, 6250–6284. [Google Scholar] [CrossRef]
- Monfregola, L.; Bugatti, V.; Amodeo, P.; De Luca, S.; Vittoria, V. Physical and water sorption properties of chemically modified pectin with an environmentally friendly process. Biomacromolecules 2011, 12, 2311–2318. [Google Scholar] [CrossRef]
- Monfregola, L.; Leone, M.; Vittoria, V.; Amodeo, P.; De Luca, S. Chemical modification of pectin: environmental friendly process for new potential material development. Polym. Chem. 2011, 2, 800–804. [Google Scholar]
- Gandini, A. Polymers from renewable resources: A challenge for the future of macromolecular materials. Macromolecules 2008, 41, 9491–9504. [Google Scholar]
- Williams, C.K.; Hillmyer, M.A. Polymers from renewable resources: A perspective for a special issue of polymer reviews. Polym. Rev. 2008, 48, 1–10. [Google Scholar] [CrossRef]
- Guilherme, M.R.; Moia, T.A.; Reis, A.V.; Paulino, A.T.; Rubira, A.F.; Mattoso, L.H.C.; Muniz, E.C.; Tambourgi, E.B. Synthesis and water absorption transport mechanism of a pH sensitive polymer network structured on vinyl-functionalized pectin. Biomacromolecules 2009, 10, 190–196. [Google Scholar] [CrossRef]
- Junistia, L.; Sugih, A.K.; Manurung, R.; Picchioni, F.; Janssen, L.P.B.M.; Heeres, H.J. Synthesis of higher fatty acid starch esters using vinyl laurate and stearate as reactants. Starch/Stärke 2008, 60, 667–675. [Google Scholar]
- Sashiwa, H.; Yajima, H.; Aiba, S. Synthesis of a chitosan−dendrimer hybrid and its biodegradation. Biomacromolecules 2003, 4, 1244–1249. [Google Scholar] [CrossRef]
- Coffin, D.R.; Fishman, M.L. Physical and mechanical properties of highly plasticized pectin/starch films. J. Appl. Polym. Sci. 1994, 54, 1311–1320. [Google Scholar] [CrossRef]
- Kaplan, D.L. Biopolymers from Renewable Resources; Springer-Verlag: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Thakur, B.R.; Singh, R.K.; Handa, A.K. Chemistry and uses of pectin—A review. Crit. Rev. Food Sci. Nutr. 1997, 37, 47–73. [Google Scholar] [CrossRef]
- Mitchel, J.R.; Hill, S.E.; Ledward, D.A. Functional Properties of Food Macromolecules, 2nd ed.; Aspen Publication: Gaithersburg, MA, USA, 1998. [Google Scholar]
- Zaleska, H.; Ring, S.G.; Tomasik, P. Apple pectin complexes with whey protein isolate. Food Hydrocoll. 2000, 14, 377–382. [Google Scholar] [CrossRef]
- Synytsya, A.; Copikova, J.; Matejka, P.; Machovic, V. Fourier transform Raman and IR spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar]
- Filippov, M.P. Practical infrared spectroscopy of pectic substances. Food Hydrocoll. 1992, 6, 115–142. [Google Scholar] [CrossRef]
- Kamnev, A.A.; Ptitchkina, N.M.; Ristic, M.; Shkodina, O.G.; Ignatov, V.V. Spectroscopy of Biological Molecules; Merlin, J.C., Turrell, S., Huvenne, J.P., Eds.; Kluwer: Dordrecht, The Netherlands, 1995; pp. 445–446. [Google Scholar]
- Zhbankov, R.G. Vibrational spectra and structure of mono- and polysaccharides. J. Mol. Struct. 1992, 275, 65–84. [Google Scholar]
- Matora, A.V.; Korshunova, V.E.; Shkodina, O.G.; Zhemerichkin, D.A.; Ptitchkina, N.M. Morris E.R. The application of bacterial enzymes for extraction of pectin from pumpkin and sugar beet. Food Hydrocoll. 1995, 9, 43–46. [Google Scholar] [CrossRef]
- Zhemerichkin, D.A.; Ptichkina, N.M. The composition and properties of pumpkin and sugar beet pectins. Food Hydrocoll. 1995, 9, 147–149. [Google Scholar] [CrossRef]
- Gnanasambandam, R.; Proctor, A. Determination of pectin degree of esterification by diffuse reflectance Fourier transform infrared spectroscopy. Food Chem. 2000, 68, 327–332. [Google Scholar] [CrossRef]
- Xin, J.Y.; Wang, Y.; Liu, T.; Lin, K.; Chang, L.; Xia, C.G. Biosynthesis of corn starch palmitate by lipase Novozym 435. Int. J. Mol. Sci. 2012, 13, 7226–7236. [Google Scholar] [CrossRef]
- Lu, X.; Luo, Z.; Yu, S.; Fu, X. Lipase-catalyzed synthesis of starch palmitate in mixed ionic liquids. J. Agric. Food Chem. 2012, 60, 9273–9279. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H. Thermoanalytical characterisation of processing-dependent structural changes and state transitions of citrus pectin. Food Hydrocoll. 2009, 23, 40–52. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H. The influence of the storage conditions heat and humidity on conformation, state transitions and degradation behaviour of dried pectins. Food Hydrocoll. 2009, 23, 856–866. [Google Scholar]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Calce, E.; Bugatti, V.; Vittoria, V.; De Luca, S. Solvent-Free Synthesis of Modified Pectin Compounds Promoted by Microwave Irradiation. Molecules 2012, 17, 12234-12242. https://doi.org/10.3390/molecules171012234
Calce E, Bugatti V, Vittoria V, De Luca S. Solvent-Free Synthesis of Modified Pectin Compounds Promoted by Microwave Irradiation. Molecules. 2012; 17(10):12234-12242. https://doi.org/10.3390/molecules171012234
Chicago/Turabian StyleCalce, Enrica, Valeria Bugatti, Vittoria Vittoria, and Stefania De Luca. 2012. "Solvent-Free Synthesis of Modified Pectin Compounds Promoted by Microwave Irradiation" Molecules 17, no. 10: 12234-12242. https://doi.org/10.3390/molecules171012234
APA StyleCalce, E., Bugatti, V., Vittoria, V., & De Luca, S. (2012). Solvent-Free Synthesis of Modified Pectin Compounds Promoted by Microwave Irradiation. Molecules, 17(10), 12234-12242. https://doi.org/10.3390/molecules171012234