Abstract
The one-pot synthesis of three dragmacidin derivatives is reported. Sarcosine anhydride (4) is brominated and immediately reacted with the corresponding indole to produce the products, namely 3,6-bis(5′-methoxy-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (1), 3,6-bis(7′-methyl-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (2) and 3,6-bis-(6′-chloro-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (3), which are characterized by 1H-NMR.
1. Introduction
During the past twenty years, a number of natural products containing the indole moiety that display a significant amount of biological activity have been isolated from marine organisms [1,2,3,4]. Among these is a rapidly expanding class of bisindole alkaloid compounds isolated from marine sponges sp. Dragmacidon, Spongosorites, and Hexadella. Classified as dragmacidins [5,6], these compounds contain substituted indole residues joined by a piperazine linker at the 3-and 6-positions [7]. They have received considerable amounts of attention in the scientific community due to their applications as antiviral, antibacterial, anti-inflammatory, and cytotoxic agents [8,9,10,11,12,13,14,15,16,17,18,19].
Evidence supporting the need for further investigation into the activity of the dragmacidin compounds has arisen from multiple arenas. In relation to the cytotoxic activity of the dragmacidin class, it has been shown that serine-threonine protein phosphatases were significantly inhibited by dragmacidin D [20]. Dragmacidin D has also been shown to act as a potent inhibitor in vivo of resiniferitoxin-induced inflammation in murine ear tissue, as well as a selective inhibitor of neural nitric oxide synthase [21,22]. Dragmacidin has been reported to inhibit the in vitro growth of P388 (murine leukemia), A-549 (human lung), HCT-8 (human colon), and MDAMB (human mammary) cancer cell lines [8]. The broad range of abilities displayed by the dragmacidin compounds could prove useful in the development of cancer therapeutics and anti-inflammatory drugs, as well as prospective treatments for Alzheimer’s, Parkinson’s, and Huntington’s diseases [23] and depression and anxiety [24]. It is evident from the current studies that an effective method for synthetically producing dragmacidin derivatives would be beneficial.
Current synthetic methods center on the sequential implementation of palladium(0)-catalyzed Suzuki and Stille cross-coupling reactions [25,26]. A transamidation-cyclization has also been proposed as a synthetic method for the hamacanthin alkaloids [6]. These methods have proved to be moderately successful, but are impractical to implement on a large scale due to the stringent conditions required during multiple steps of the reactions. A short step, one-pot synthetic method was developed in our lab in order to prepare a dragmacidin intermediate [19]. This method was utilized to prepare novel dragmacidin precursors.
2. Results and Discussion
We now describe the synthesis of three novel bisindolylpiperazine-2,5-diones using a previously developed method [18]. The synthesis of 3,6-bis(5′-methoxy-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (1) is shown in Scheme 1. Bromine is directly added to sarcisine anhydride (4) with heat and the illumination of a sun lamp. After one hour, the solution is cooled to provide the dibrominated product as an unstable precipitate. This precipitate is then reacted with the corresponding indole in DMF to produce 1, 2, or 3. Using a similar approach, 3,6-bis(6′-chloro-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (2), and 3,6-bis(7′-methyl-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (3) were prepared (Scheme 2). In conclusion, an important precursor to a dragmacidin derivative has been prepared by efficient means.
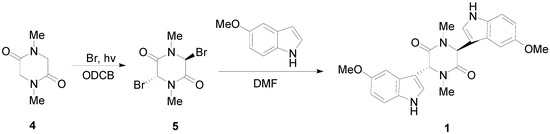
Scheme 1.
Synthesis of 3,6-bis(5′-methoxy-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (1).
Scheme 1.
Synthesis of 3,6-bis(5′-methoxy-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (1).
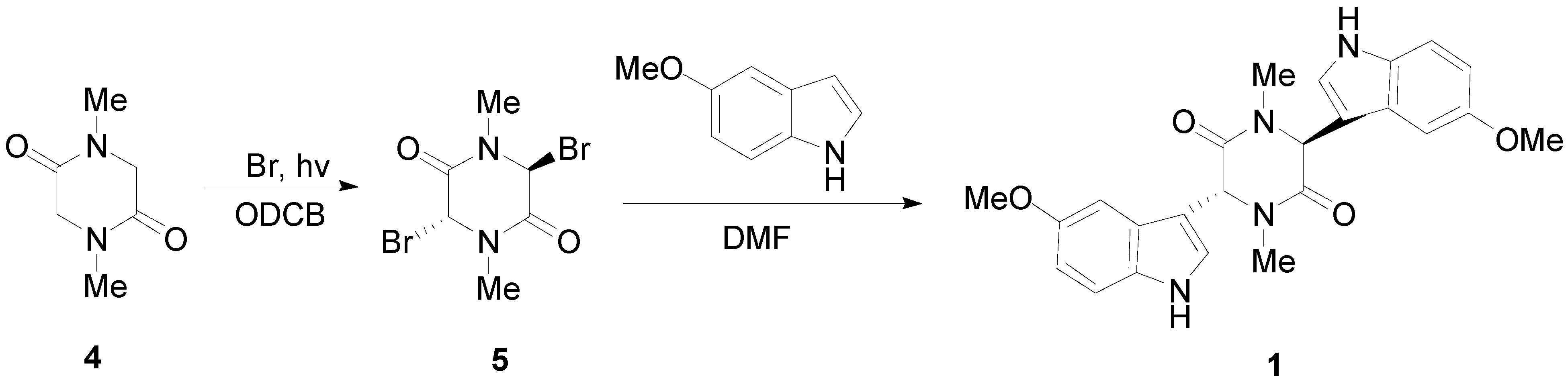
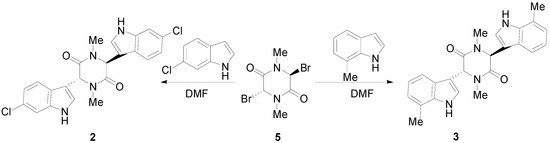
Scheme 2.
Syntheses of 3,6-bis(6′-chloro-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (2) and 3,6-bis(7′-methyl-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (3).
Scheme 2.
Syntheses of 3,6-bis(6′-chloro-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (2) and 3,6-bis(7′-methyl-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (3).
3. Experimental
General
Melting points are uncorrected. 1H-NMR spectra were obtained using a Bruker 250 MHz multi-nuclear spectrometer. Sarcosine anhydride (99.5%), 5-methoxyindole (99%), 6-chloroindole (99%), 7-methylindole (99%) were obtained from Acros Organics.
3,6-Bis(5′-methoxy-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (1). Step 1. Preparation of 3,6-dibromo-1,4-dimethylpiperazine-2,5-dione (5). Br2 (3.0 mL, 58.1 mmol) in o-dichlorobenzene (6 mL), was added dropwise under illumination of a sun lamp to a solution of sarcosine anhydride (4, 3.75 g, 26.4 mmol) in o-dichlorobenzene (15 mL), at 150 °C. The solution was heated for 1 h and then cooled to rt. A stream of N2 was bubbled through the reaction mixture for 10 min, and the solution was diluted with hexanes. The resulting beige crystals of 5 (an unstable precipitate) were filtered and rinsed with hexanes (7.06 g, 89.1%): mp 128–132 °C (lit. [27] 139–143 °C. 1H-NMR (CDCl3): 3.10 (s, 3H), 6.01 (s, 1H).
Step 2. Compound 5 (1.88 g, 6.27 mmol) was slowly added to a solution of 5-methoxyindole (1.00 g, 6.79 mmol) in DMF (15 mL), while the reaction temperature was maintained at room temperature with a water bath. The reaction mixture was stirred for 24 h, concentrated and diluted with methanol. The resulting solid was filtered to yield the product 1 as a white crystalline solid (0.33 g; 22.4%): mp > 250 °C. 1H-NMR (d6-DMSO): 2.66 (s, 3H), 3.27 (s, 3H), 5.58 (s, 1H), 6.82 (dd, 1H, J = 2.4, 8.8), 6.97 (d, 1H, J = 2.6), 7.33 (d, 1H, J = 28.8), 7.46 (d, 1H, J = 2.6), 11.09 (bs, 1H).
3,6-Bis(6′-chloro-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (2). To a solution of 6-chlorolindole (1.00 g, 6.60 mmol) in DMF (15 mL) was slowly added 5, while the reaction temperature was maintained at room temperature with a water bath. The reaction mixture was stirred for 24 h, concentrated and diluted with methanol. The resulting solid was filtered to yield the product 7 as a white crystalline solid (0.28 g; 19.4%): mp > 250 °C. 1H-NMR (d6-DMSO): 2.57 (s, 3H), 5.66 (s, 1H), 7.09 (d, 1H, J = 8.9), 7.22 (s, 1H), 7.51 (d, 1H, J = 6.7), 7.53 (d, 1H, J = 6.1), 11.23 (bs, 1H).
3,6-Bis(7′-methyl-3′-indolyl)-1,4-dimethylpiperazine-2,5-dione (3). To a solution of 7-methylindole (1.00 g, 7.62 mmol) in DMF (15 mL) was slowly added 5, while the reaction temperature was maintained at room temperature with a water bath. The reaction mixture was stirred for 24 h, concentrated and diluted with methanol. The resulting solid was filtered to yield the product 6 as a white crystalline solid (0.35 g; 23.1%): mp > 250 °C. 1H-NMR (d6-DMSO): 1H NMR (d6-DMSO): 2.49 (s, 3H), 3.36 (s, 3H), 5.63 (s, 1H), 6.95 (d, 2H, J = 2.9), 7.32 (d, 1H, J = 3.1), 7.49 (s, 1H), 11.20 (s, 1H).
4. Conclusions
We have developed a reproducible method for preparing bis-indolylpiperazine-2,5-diones. These symmetrical compounds can serve as precursors in the development of biologically active alkaloids related to the dragmacidon, spongosorites, and hexadella series.
Acknowledgments
This work was supported by the Allen E. Paulson College of Science and Technology Paulson Scholars Program at Georgia Southern University.
- Sample Availability: Not available.
References
- Aygün, A.; Pindur, U. Chemistry and Biology of New Marine Alkaloids from the Indole and Annelated Indole Series. Curr. Med. Chem. 2003, 10, 1113–1127. [Google Scholar] [CrossRef]
- Gupta, L.; Talwar, A.; Chauhan, P.M. Bis and Tris Indole Alkaloids from Marine Organisms: New Leads for Drug Discovery. Curr. Med. Chem. 2007, 14, 1789–1803. [Google Scholar] [CrossRef]
- Gul, W.; Hamann, M.T. Indole Alkaloid Marine Natural Products: An Established Source of Cancer Drug Leads with Considerable Promise for the Control of Parasitic, Neurological and Other Diseases. Life Sci. 2005, 78, 442–453. [Google Scholar] [CrossRef]
- Mollica, A.; Locatelli, M.; Stefanucci, A.; Pinnen, F. Synthesis and Bioactivity of Secondary Metabolites from Marine Sponges Containing Dibrominated Indolic Systems. Molecules 2012, 17, 6083–6099. [Google Scholar] [CrossRef]
- Kawasaki, T.; Ohno, K.; Enoki, H.; Umemoto, Y.; Sakamoto, M. Syntheses of Bis(indolyl)-piperazine Alkaloids, Dragmacidin B and C, and Dihydrohamacanthin A. Tetrahedron Lett. 2002, 43, 4245–4248. [Google Scholar] [CrossRef]
- Kawasaki, T.; Kouko, T.; Totsuka, H.; Hiramatsu, K. Synthesis of Marine Bisindole Alkaloids, Hamacanthins A and B through Intramolecular Transamidation-cyclization. Tetrahedron Lett. 2003, 44, 8849–8852. [Google Scholar] [CrossRef]
- Garg, N.K.; Sarpong, R.; Stolts, B.M. The First Total Synthesis of Dragmacidin D. J. Am. Chem. Soc. 2002, 124, 3179–3184. [Google Scholar]
- Kohomoto, S.; Kashman, Y.; McConnell, O.J.; Rinehart, K.L.; Wright, A.; Koehn, F. Dragmacidin, a New Cytotoxic Bis(indole) Alkaloid from a Deep Water Marine Sponge. J. Org. Chem. 1988, 53, 3116–3118. [Google Scholar] [CrossRef]
- Wright, A.E.; Pomponi, S.A.; Cross, S.S.; McCarthy, P. A new bis-(indole) alkaloid from a deep- water marine sponge of the genus Spongosorites. J. Org. Chem. 1992, 57, 4772–4775. [Google Scholar] [CrossRef]
- Capon, R.J.; Rooney, F.; Murray, L.M.; Collins, E.; Sim, A.T.R.; Rostas, J.A.P.; Butler, M.S.; Carroll, A.R. Dragmacidins: New protein phosphatase inhibitors from a southern Australian deep- water marine sponge, Spongosorites sp. J. Nat. Prod. 1998, 61, 660–662. [Google Scholar] [CrossRef]
- Capon, R.J.; Rooney, F.; Murray, L.M. 1,9-Dimethylhypoxanthine from a southern Australian marine sponge Spongosorites species. J. Nat. Prod. 2000, 63, 261–262. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Q.; Yao, X.; Hong, J.; Lee, C.O.; Cho, H.Y.; Jung, J.H. Bisindole alkaloids of the topsentin and hamacanthin classes from a marine sponge Spongosorites sp. J. Nat. Prod. 2007, 70, 2–8. [Google Scholar] [CrossRef]
- Morris, S.A.; Andersen, R.J. Brominated bis(indole) alkaloids from the marine sponge hexadella sp. Tetrahedron 1990, 46, 715–720. [Google Scholar] [CrossRef]
- Yang, C.-G.; Huang, H.; Jiang, B. Progress in studies of novel marine bis(indole) alkaloids. Curr. Org. Chem. 2004, 8, 1691–1720. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Q.; Xinsheng, Y.; Hong, J.; Lee, C.-O.; Sim, C.J.; Im, K.S.; Jung, J.H. Cytotoxic bisindole alkaloids from a marine sponge Spongosorites sp. J. Nat. Prod. 2005, 68, 711–715. [Google Scholar] [CrossRef]
- Sakemi, S.; Sun, H.H. Nortopsentins A, B, and C. Cytotoxic and antifungal imidazolediylbis[indoles] from the sponge Spongosorites ruetzleri. J. Org. Chem. 1991, 56, 4304–4307. [Google Scholar] [CrossRef]
- Gunasekera, S.P.; McCarthy, P.J.; Kelly-Borges, M. Hamacanthins A and B, new antifungal bis indole alkaloids from the deep-water marine sponge, Hamacantha sp. J. Nat. Prod. 1994, 57, 1437–1441. [Google Scholar] [CrossRef]
- Whitlock, C.R.; Cava, M.P. A total synthesis of dragmacidin B. Tetrahedron Lett. 1994, 35, 371–374. [Google Scholar] [CrossRef]
- Miles, D.; Whitlock, C. An improved, one-pot synthesis of 3,6-bis(3’-indolyl)-1,4- dimethylpiperazine-2,5-dione. Heterocycl. Commun. 2009, 15, 61–62. [Google Scholar]
- Capon, R.J.; Rooney, F.; Murray, L.M.; Collins, E. Dragmacidins: New Protein Phospatase Inhibitors from a Southern Australian Deep-Water Marine Sponge, Spongosorites Sp. J. Nat. Prod. 1998, 61, 660–662. [Google Scholar] [CrossRef]
- Jacobs, R.S.; Pomponi, S.; Gunasekera, S.; Wright, A. Anti-neurogenic inflammatory compounds and compositions and methods of use thereof. PCT Int. Appl WO 9818466, 7 May 1998. [Google Scholar]
- Longley, R.E.; Isbrucker, R.A.; Wright, A. Use of imidazole and indole compounds as inhibitors of nitric oxide synthase. U.S. Patent 6,087,363, 7 July 2000. [Google Scholar]
- Thorns, V.; Hansen, L.; Masliah, E. nNOS Expressing Neurons in the Entorhinal Cortex and Hippocampus Are Affected in Patients with Alzheimer’s Disease. Exp. Neurol. 1998, 150, 14–20. [Google Scholar] [CrossRef]
- Kochanowska-Karamyan, A.J.; Hamann, M.T. Indole alkaloids: Potential new drug leads for depression and anxiety. Chem. Rev. 2010, 110, 4489–4497. [Google Scholar] [CrossRef]
- Jiang, B.; Smallheer, J.M.; Amaral-Ly, C.; Wuonola, M.A. Total Synthesis of (+/−)-Dragmacidin: A Cytotoxic Bis(indole)alkaloid of Marine Origin. J. Org. Chem. 1994, 59, 6823–6827. [Google Scholar] [CrossRef]
- Yang, C.-G.; Liu, G.; Jiang, B. Preparing Functional Bis(indole) Pyrazine by Stepwise Cross-coupling Reactions: An Efficient Method to Construct the Skeleton of Dragmacidin D. J. Org. Chem. 2002, 67, 9392–9396. [Google Scholar] [CrossRef]
- Trown, P.W. Antiviral activity of N,N′-dimethyl-epidithiapiperazinedione, a synthetic compound related to the gliotoxins, LL-S88α and β, chetomin and the sporidesmins. Biochem. Biophys. Res. Commun. 1968, 33, 402–407. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
