Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution
Abstract
:1. Introduction
2. Pyranoanthocyanins and Related Pigments in Wines

| Compounds | Molecular ion M+ (m/z) | Fragment ion M+ ( m/z) | λmax (nm) |
|---|---|---|---|
| Vitisin A type | |||
| Cyanidin-3-O-glucoside-pyruvic acid | 517 | 359 | 503 |
| Cyanidin-3-O-acetylglucoside-pyruvic acid | 559 | 359 | 505 |
| Cyanidin-coumaroylglucoside-pyruvic acid | 661 | 359 | 507 |
| Delphinidin-3-O-glucoside-pyruvic acid | 533 | 371 | 507 |
| Delphinidin-3-O-acetylglucoside-pyruvic acid | 575 | 371 | 509 |
| Delphinidin-3-O-coumaroylglucoside-pyruvic acid | 679 | 371 | 511 |
| Peonidin-3-O-glucoside-pyruvic acid | 531 | 369 | 509 |
| Peonidin-3-O-acetylglucoside-pyruvic acid | 573 | 369 | 510 |
| Peonidin-3-O-coumaroylglucoside-pyruvic acid | 677 | 369 | 511 |
| Petunidin-3-O-glucoside-pyruvic acid | 547 | 385 | 508 |
| Petunidin-3-O-acetylglucoside-pyruvic acid | 589 | 385 | 509 |
| Petunidin-3-O-coumaroylglucoside-pyruvic acid | 693 | 385 | 510 |
| Malvidin-3-O-glucoside-pyruvic acid | 561 | 399 | 513 |
| Malvidin-3-O-acetylglucoside-pyruvic acid | 603 | 399 | 516 |
| Malvidin-3-O-coumaroylglucoside-pyruvic acid | 707 | 399 | 513 |
| Vitisin B type | |||
| Malvidin-3-O-glucoside-acetaldehyde | 517 | 355 | 490 |
| Malvidin-3-O-acetylglucoside-acetaldehyde | 559 | 355 | 494 |
| Malvidin-3-O-coumaroylglucoside-acetaldehyde | 663 | 355 | 497 |
| Pinotin type | |||
| Delphinidin-3-O-glucoside-4-vinylcatechol | 597 | 435 | 510 |
| Delphinidin-3-O-acetylglucoside-4-vinylcatechol | 639 | 435 | 512 |
| Delphinidin-3-O-coumaroylglucoside-4-vinylcatechol | 743 | 435 | 514 |
| Peonidin-3-O-glucoside-4-vinylcatechol | 595 | 433 | 504 |
| Peonidin-3-O-acetylglucoside-4-vinylcatechol | 637 | 433 | 506 |
| Peonidin-3-O-coumaroylglucoside-4-vinylcatechol | 741 | 433 | 508 |
| Petunidin-3-O-glucoside-4-vinylcatechol | 611 | 449 | 510 |
| Petunidin-3-O-acetylglucoside-4-vinylcatechol | 653 | 449 | 512 |
| Petunidin-3-O-coumaroylglucoside-4-vinylcatechol | 757 | 449 | 516 |
| Malvidin-3-O-glucoside-4-vinylcatechol | 625 | 463 | 512 |
| Malvidin-3-O-acetylglucoside-4-vinylcatechol | 667 | 463 | 514 |
| Malvidin-3-O-coumaroylglucoside-4-vinylcatechol | 771 | 463 | 514 |
| Delphinidin-3-O-glucoside-4-vinylphenol | 581 | 419 | 504 |
| Delphinidin-3-O-acetylglucoside-4-vinylphenol | 623 | 419 | 506 |
| Delphinidin-3-O-coumaroylglucoside-4-vinylphenol | 727 | 419 | 506 |
| Peonidin-3-O-glucoside-4-vinylphenol | 579 | 417 | 499 |
| Peonidin-3-O-acetylglucoside-4-vinylphenol | 621 | 417 | 504 |
| Peonidin-3-O-coumaroylglucoside-4-vinylphenol | 725 | 417 | 505 |
| Petunidin-3-O-glucoside-4-vinylphenol | 595 | 433 | 504 |
| Petunidin-3-O-acetylglucoside-4-vinylphenol | 636 | 433 | 506 |
| Petunidin-3-O-coumaroylglucoside-4-vinylphenol | 741 | 433 | 507 |
| Malvidin-3-O-glucoside-4-vinylphenol | 609 | 447 | 504 |
| Malvidin-3-O-acetylglucoside-4-vinylphenol | 651 | 447 | 507 |
| Malvidin-3-O-coumaroylglucoside-4-vinylphenol | 755 | 447 | 509 |
| Malvidin-3-O-caffeoylglucoside-4-vinylphenol | 771 | 447 | 532 |
| Delphinidin-3-O-glucoside-4-vinylguaiacol | 611 | 451 | 502 |
| Peonidin-3-O-glucoside-4-vinylguaiacol | 609 | 447 | 499 |
| Petunidin-3-O-glucoside-4-vinylguaiacol | 625 | 463 | 502 |
| Malvidin-3-O-glucoside-4-vinylguaiacol | 639 | 477 | 504 |
| Malvidin-3-O-acetylglucoside-4-vinylguaiacol | 681 | 477 | 506 |
| Malvidin-3-O-coumaroylglucoside-vinylguaiacol | 755 | 477 | 508 |
| Flavanyl-pyranoanthocyanin type | |||
| Delphinidin-3-O-glucoside-4-vinyl(epi)catechin | 777 | 615 | 501 |
| Delphinidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 819 | 615 | 503 |
| Peonidin-3-O-glucoside-4-vinyl(epi)catechin | 775 | 613 | 199 |
| Peonidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 817 | 613 | 501 |
| Petunidin-3-O-glucoside-4-vinyl(epi)catechin | 791 | 629 | 502 |
| Petunidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 833 | 629 | 504 |
| Malvidin-3-O-glucoside-4-vinyl(epi)catechin | 805 | 643 | 503 |
| Malvidin-3-O-acetylglucoside-4-vinyl(epi)catechin | 847 | 643 | 508 |
| Malvidin-3-O-coumaroylglucoside-4-vinyl(epi)catechin | 951 | 643 | 503 |
2.1. Structures and Formation of Vitisins

2.2. Structures and Formation of Pinotins

2.3. Structures and Formation of Flavanyl-Pyranoanthocyanins

2.4. Structures and Formation of Portisins

2.5. Structures and Formation of Oxovitisins

2.6. Pyranoanthocyanin Dimers
3. Polymeric Anthocyanins

3.1. Directly Condensed Products of Anthocyanins and Flavanols

| Compounds | Molecular ion M+ (m/z) | Fragment ion M+ (m/z) |
|---|---|---|
| Delphinidin-glucoside-(epi)catechin | 753 | 591,573,465,439,303 |
| Petunidin-glucoside-(epi)catechin | 767 | 605,587,453,359,329 |
| Peonidin-glucoside-(epi)catechin | 751 | 589,571,463,437 |
| Malvidin-glucoside-gallocatechin | 797 | 635,617,509,467,373 |
| Malvidin-glucoside-(epi)catechin | 781 | 619,601,467,373,331 |
| Malvidin-acetylglucoside-(epi)catechin | 823 | 619,601,467,493,331 |
| Malvidin-coumaroylglucoside-(epi)catechin | 927 | 619,493,467,451,331 |
| Malvidin-glucoside-PC dimer | 1069 | 907,781,619 |
| Malvidin-glucoside-malvidin-glucoside | 985 | 823,661,535,331 |
| Malvidin-glucoside-malvidin-acetylglucoside | 1027 | 865,823,661,331 |
| Malvidin-glucoside-petunidin-acetylglucoside | 1013 | 851,809,647,331 |
| Malvidin-glucoside-delphinidin-acetylglucoside | 999 | 837,795,633,331,303 |
| Malvidin-acetylglucoside-malvidin-acetylglucoside | 1069 | 865,661,331 |
| Malvidin-glucoside-peonidin-acetylglucoside | 997 | 835,631,303 |
| Malvidin-acetylglucoside-petunidin-acetylglucoside | 1055 | 851,647,521,317 |
| Malvidin-glucoside-malvidin-coumaroylglucoside | 1131 | 969,823,661,535,331 |
| Malvidin-acetylglucoside-malvidin-coumaroylglucoside | 1173 | 969,865,661,535,331 |
| Malvidin-glucoside-delphinidin-coumaroylglucoside | 1103 | 941,795,633,507,331 |
| Malvidin-glucoside-petunidin-coumaroylglucoside | 1117 | 955,809,647,317 |
| Malvidin-glucoside-peonidin-coumaroylglucoside | 1101 | 939,793,631,505,331 |
| Malvidin-coumaroylglucoside-malvidin-coumaroylglucoside | 1277 | 969,661,639 |
| Malvidin-glucoside-delphinidin-glucoside | 957 | 795,633,507,331,303 |
| Malvidin-glucoside-cyanidin-glucoside | 941 | 779,617,491,449 |
| Malvidin-glucoside-petunidin-glucoside | 971 | 809,647,521,331,317 |
| Malvidin-glucoside-peonidin-glucoside | 955 | 793,631,505,331 |
| Malvidin-glucoside-cyanidin-coumaroylglucoside | 1087 | 925,779,617,493,287 |
| (Epi)catechin-delphinidin-glucoside-malvidin-glucoside | 1245 | 1083,795,921,903,633 |
| (Epi)catechin-cyanidin-glucoside-malvidin-glucoside | 1229 | 1067,917,904 |
| (Epi)catechin-petunidin-glucoside-malvidin-glucoside | 1259 | 1097,971,935,747,671 |
| (Epi)catechin-peonidin-glucoside-malvidin-glucoside | 1243 | 1081,1063,919 |
| (Epi)catechin-malvidin-glucoside-malvidin-glucoside | 1273 | 1111,949,931,823,661 |
| (Epi)gallocatechin-delphinidin-glucoside-malvidin-glucoside | 1261 | 1099,937 |
| (Epi)gallocatechin-petunidin-glucoside-malvidin-glucoside | 1275 | 1113,951,647 |
| (Epi)gallocatechin-malvidin-glucoside-malvidin-glucoside | 1289 | 1127,965,823,535,331 |
3.2. Acetaldehyde or Glyoxylic Acid Mediated Polymeric Products
| Compounds | Molecular ion M+ (m/z) | Fragment ion M+ (m/z) |
|---|---|---|
| Petunidin-glucoside-8-ethyl-(epi)catechin | 795 | 633,505,481,435,328 |
| Malvidin-glucoside-8-ethyl-gallocatechin | 825 | |
| Malvidin-glucoside-8-ethyl-(epi)catechin | 809 | 657,517,357,341,331 |
| Malvidin-acetylglucoside-8-ethyl-(epi)catechin | 851 | |
| Malvidin-coumaroylglucoside-8-ethyl-(epi)catechin | 955 | 803,665,647,357,341 |
| Peonidin-glucoside-8-ethyl-(epi)catechin | 779 | |
| Peonidin-coumaroylglucoside-8-ethyl-(epi)catechin | 925 | 635,617,327 |
| Malvidin-glucoside-4-methyl-(epi)catechin | 795 | 505 |
| Malvidin-glucoside-4-2methylpropyl-(epi)catechin | 837 | 547 |
| Malvidin-glucoside-4-3methylbutyl-(epi)catechin | 851 | 561 |
| Malvidin-glucoside-4-2methylbutyl-(epi)catechin | 851 | 561 |
| Malvidin-glucoside-4-benzyl-(epi)catechin | 871 | 581 |
| Malvidin-glucoside-4-propyl-(epi)catechin | 823 | 533 |
| Malvidin-glucoside-4-ethyl-PC dimer | 1097 | 519 |
| Malvidin-glucoside-4-3methylbutyl-PC dimer | 1139 | |
| Malvidin-glucoside-4-benzyl-PC dimer | 1159 | 707 |
| Malvidin-glucoside-4-propyl-PC dimer | 1111 | 959 |
| Malvidin-glucoside-4-ethyl-epicatechin-3-O-gallate | 961 | 799,519 |
| Malvidin-glucoside-4-ethyl-B2-3'-O-gallate | 1249 | |
| Malvidin-glucoside-4-3methylbutyl-epicatechin-3-O-gallate | 1003 | |
| Malvidin-glucoside-4-3methylbutyl-B2-3'-O-gallate | 1291 | |
| Malvidin-glucoside-4-benzyl-epicatechin-3-O-gallate | 1023 | 719 |
| Malvidin-glucoside-4-benzyl-PB2-3'-O-gallate | 1311 | 419,581 |
| Malvidin-glucoside-4-propyl-epicatechin-3-O-gallate | 975 | 813,671 |
| Malvidin-glucoside-4-propyl-PB2-3'-O-gallate | 1263 | 821 |
| Malvidin-glucoside-4-methyl-epicatechin-3-O-gallate | 947 | 785,343 |
| Malvidin-glucoside-4-methyl-PB2-3'-O-gallate | 1235 | 1073,793 |
| Malvidin-glucoside-4-isobutyl-epicatechin-3-O-gallate | 989 | 385 |
| Malvidin-glucoside-4-isobutyl-PB2-3'-O-gallate | 1277 | 835 |
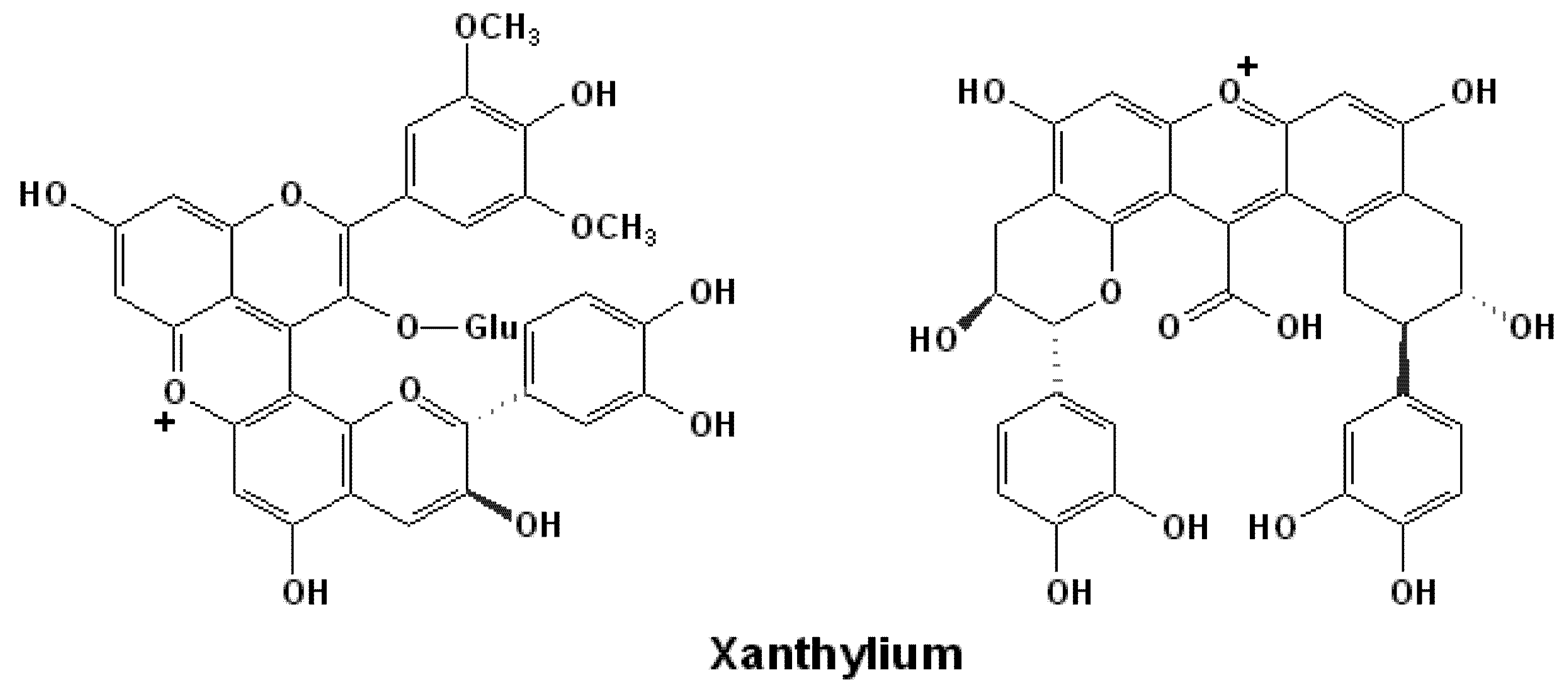
3.3. Xanthylium Pigments
4. Influence of Aging Practices on Anthocyanins and Their Derivatives in Red Wines
5. Conclusions
- (1) Identification of new pyranoanthocyanins in aged red wines, especially the new pigments from second generation of carboxy- or methyl-pyranoanthocyanins.
- (2) Identification of more complicated polymeric anthocyanins in aged red wines, especially the ones with higher polymerization degree and new configurations.
- (3) Quantification of the amounts of such pigments and their extinction coefficients to enable the assessment of their contribution to visual wine color.
- (4) New enology practices to improve the production and stability of anthocyanin derivatives, as well as total wine color.
Acknowledgements
- Sample Availability: Not available.
References and Notes
- Mazza, G.; Francis, F.J. Anthocyanins in grapes and grape products. Crit. Rev. Food Sci. Nutr. 1995, 35, 341–371. [Google Scholar] [CrossRef]
- Wrolstad, R.E.; Durst, R.W.; Lee, J. Tracking color and pigment changes in anthocyanin products. Trends Food Sci. Tech. 2005, 16, 423–428. [Google Scholar] [CrossRef]
- Degenhardt, A.; Hofmann, S.; Knapp, H.; Winterhalter, P. Preparative isolation of anthocyanins by high-speed countercurrent chromatography and application of the color activity concept to red wine. J. Agric. Food Chem. 2000, 48, 5812–5818. [Google Scholar]
- Robinson, W.B.; Weirs, L.D.; Bertino, J.J.; Mattick, L.R. The relation of anthocyanin composition to color stability of New York State wines. Am. J. Enol. Vitic. 1966, 17, 178–184. [Google Scholar]
- Busse-Valverde, N.; Gómez-Plaza, E.; López-Roc, J.M.; Gil-Muňoz, R.; Bautista-Ortín, A.B. The extraction of anthocyanins and proanthocyanidins from grapes to wine during fermentative maceration is affected by the enological technique. J. Agric. Food Chem. 2011, 59, 5450–5455. [Google Scholar] [CrossRef]
- Jensen, J.S.; Demiray, S.; Egebo, M.; Meyer, A.S. Prediction of wine color attributes from the phenolic profiles of red grapes (Vitis vinifera). J. Agric. Food Chem. 2008, 56, 1105–1115. [Google Scholar] [CrossRef]
- González-Manzano, S.; Santos-Buelga, C.; Dueñas, M.; Rivas-Gonzalo, J.C.; Escribano-Bailón, T. Colour implications of self-association processes of wine anthocyanins. Eur. Food Res. Technol. 2008, 226, 483–490. [Google Scholar] [CrossRef]
- Miniati, E.; Damiani, P.; Mazza, G. Copigmentation and self-association of anthocyanins in food model systems. Ital. J. Food Sci. 1992, 4, 109–116. [Google Scholar]
- Dangles, O.; Saito, N.; Brouillard, R. Anthocyanin intramolecular copigment effect. Phytochemistry 1993, 34, 119–124. [Google Scholar]
- Malien-Aubert, C.; Dangles, O.; Amiot, M.J. Color stability of commercial anthocyanin-based extracts in relation to the phenolic composition. Protective effects by intra- and intermolecular copigmentation. J. Agric. Food Chem. 2001, 49, 170–176. [Google Scholar] [CrossRef]
- Eiro, M.J.; Heinonen, M. Anthocyanin color behavior and stability during storage: Effect of intermolecular copigmentation. J. Agric. Food Chem. 2002, 50, 7461–7466. [Google Scholar] [CrossRef]
- Boulton, R. The copigmentation of anthocyanins and its role in the color of red wine: A critical review. Am. J. Enol. Vitic. 2001, 52, 67–87. [Google Scholar]
- Cavalcanti, R.N.; Santos, D.T.; Meireles, M.A.A. Non-thermal stabilization mechanisms of anthocyanins in model and food systems-An overview. Food Res. Int. 2011, 44, 499–509. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principle and Applications, 3rd ed; Elsevier-Academic Press: Oxford, UK, 2008; pp. 287–295. [Google Scholar]
- Brouillard, R.; Chassaing, S.; Fougerousse, A. Why are grape/fresh wine anthocyanins so simple and why is it that red wine color lasts so long? Phytochemistry 2003, 64, 1179–1186. [Google Scholar]
- Monagas, M.; Bartolomé, B.; Gómez-Cordovés, C. Evolution of polyphenols in red wines from Vitis vinifera L. during aging in the bottle. Eur. Food Res. Technol. 2005, 220, 331–340. [Google Scholar] [CrossRef]
- Gómez-Míguez, M.; González-Miret, M.L.; Heredia, F.J. Evolution of colour and anthocyanin composition of Syrah wines elaborated with pre-fermentative cold maceration. J. Food Eng. 2007, 79, 271–278. [Google Scholar] [CrossRef]
- Vasserot, Y.; Caillet, S.; Maujean, A. Study of anthocyanin adsorption by yeast lees. Effect of some physicochemical parameters. Am. J. Enol. Vitic. 1997, 48, 433–437. [Google Scholar]
- Morata, A.; Gómez-Cordovés, M.C.; Suberviola, J.; Bartolomé, B.; Colomo, B.; Suárez, J.A. Adsorption of anthocyanins by yeast cell walls during the fermentation of red wines. J. Agric. Food Chem. 2003, 51, 4084–4088. [Google Scholar]
- Tseng, K.-C.; Chang, H.-M.; Wu, J.S.-B. Degradation kinetics of anthocyanin in ethanolic solutions. J. Food Process. Preserv. 2006, 30, 503–514. [Google Scholar] [CrossRef]
- Kader, F.; Irmouli, M.; Zitouni, N.; Nicolas, J.-P.; Metche, M. Degradation of cyanidin 3-glucoside by caffeic acid o-quinone. Determination of the stoichiometry and characterization of the degradation products. J. Agric. Food Chem. 1999, 47, 4625–4630. [Google Scholar] [CrossRef]
- Cheynier, V.; Fulcrand, H.; Guyot, S.; Oszmianski, J.; Moutounet, M. Reactions of enzymically generated quinones in relation to browning in grape musts and wines. In Enzymatic Browning and Its Prevention, 1st; Lee, C.Y., Whitaker, J.R., Eds.; ACS Publications: Washington, DC, USA, 1995; pp. 130–143. [Google Scholar]
- Sarni-Manchado, P.; Cheynierm, V.; Moutounet, M. Reaction of polyphenoloxidase generated caftaric acid o-quinone with malvidin 3-O-glucoside. Phytochemistry 1997, 45, 1365–1369. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B. Anthocyanins and anthocyanin-derived compounds. In Wine Chemistry and Biochemistry, 1st; Victoria Moreno-Arribas, M., Carmen Polo, M., Eds.; Springer Science+Business Media, LLC: 233 Spring Street, New York, NY, USA, 2009; pp. 439–462. [Google Scholar]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology Volume 2 The Chemistry of Wine Stabilization and Treatments, 2nd ed; John Wiley & Sons Ltd: Chichester, UK, 2005; pp. 141–204. [Google Scholar]
- Guadalupe, Z.; Ayestarán, B. Changes in the color components and phenolic content of red wines from Vitis vinifera L. Cv. “Tempranillo” during vinification and aging. Eur. Food Res. Technol. 2008, 228, 29–38. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Noble, A.; Kwiatkowski, M.; Cheynier, V.; Waters, E. Taste and mouth-feel properties of different types of tannin-like polyphenolic compounds and anthocyanins in wine. Anal. Chim. Acta 2004, 513, 57–65. [Google Scholar] [CrossRef]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Dufour, C.; Sauvaitre, I. Interactions between anthocyanins and aroma substances in a model system. Effect on the flavor of grape-derived beverages. J. Agric. Food Chem. 2000, 48, 1784–1788. [Google Scholar]
- Picinelli, A.; Bakker, J.; Bridle, P. Model wine solutions: Effect of sulphur dioxide on colour and composition during ageing. Vitis 1994, 33, 31–35. [Google Scholar]
- Saucier, C.; Bourgeois, G.; Vitry, C.; Roux, D.; Glories, Y. Characterization of (+)-catechin-acetaldehyde polymers: A model for colloidal state of wine polyphenols. J. Agric. Food Chem. 1997, 45, 1045–1049. [Google Scholar] [CrossRef]
- Pissarra, J.; Lourenço, S.; González-Paramás, A.M.; Mateus, N.; Santos-Buelga, C.; De Freitas, V. Formation of new anthocyanin-alkyl/aryl-flavanol pigments in model solutions. Anal. Chim. Acta 2004, 513, 215–221. [Google Scholar] [CrossRef]
- Laurie, V.F.; Waterhouse, A.L. Glyceraldehyde bridging between flavanols and malvidin-3-glucoside in model solutions. J. Agric. Food Chem. 2006, 54, 9105–9111. [Google Scholar] [CrossRef]
- Sun, B.; Leandro, M.C.; De Freitas, V.; Spranger, M.I. Fractionation of red wine polyphenols by solid-phase extraction and liquid chromatography. J. Chromatogr. A 2006, 1128, 27–38. [Google Scholar] [CrossRef]
- Jeffery, D.W.; Mercurio, M.D.; Herderich, M.J.; Hayasaka, Y.; Smith, P.A. Rapid isolation of red wine polymeric polyphenols by solid-phase extraction. J. Agric. Food Chem. 2008, 56, 2571–2580. [Google Scholar] [CrossRef]
- Dugo, P.; Favoino, O.; Lo Presti, M.; Luppino, R.; Dugo, G.; Mondello, L. Determination of anthocyanins and related components in red wines by micro- and capillary HPLC. J. Sep. Sci. 2004, 27, 1458–1466. [Google Scholar]
- Vergara, C.; Mardones, C.; Hermosín-Gutiérrez, I.; Von Baer, D. Comparison of high-performance liquid chromatography separation of red wine anthocyanins on a mixed-mode ion-exchange reversed-phase and on a reversed-phase column. J. Chromatogr. A 2010, 1217, 5710–5717. [Google Scholar] [CrossRef]
- Mazzuca, P.; Ferranti, P.; Picariello, G.; Chianese, L.; Addeo, F. Mass spectrometry in the study of anthocyanins and their derivatives: Differentiation of Vitis vinifera and hybrid grapes by liquid chromatography/electrospray ionization mass spectrometry and tandem mass spectrometry. J. Mass Spectrom. 2005, 40, 83–90. [Google Scholar] [CrossRef]
- Monagas, M.; Núñez, V.; Bartolomé, B.; Gómez-Cordovés, C. Anthocyanin-derived pigments in Graciano, Tempranillo, and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003, 54, 163–169. [Google Scholar]
- Wang, J.; Sporns, P. Analysis of anthocyanins in red wine and fruit juice using MALDI-MS. J. Agric. Food Chem. 1999, 47, 2009–2015. [Google Scholar]
- Ivanova, V.; Dörnyei, Á.; Stefova, M.; Stafilov, T.; Vojnoski, B.; Kilár, F.; Márk, L. Rapid MALDI-TOF-MS detection of anthocyanins in wine and grape using different matrices. Food Anal. Methods 2011, 4, 108–115. [Google Scholar] [CrossRef]
- Gómez-Ariza, J.L.; García-Barrera, T.; Lorenzo, F. Anthocyanins profile as fingerprint of wines using atmospheric pressure photoionisation coupled to quadrupole time-of-flight mass spectrometry. Anal. Chim. Acta 2006, 570, 101–108. [Google Scholar] [CrossRef]
- Ferrari, E.; Foca, G.; Vignali, M.; Tassi, L.; Ulrici, A. Adulteration of the anthocyanin content of red wines: Perspectives for authentication by Fourier Transform-Near InfraRed and 1H NMR spectroscopies. Anal. Chim. Acta 2011, 701, 139–151. [Google Scholar] [CrossRef]
- Košir, I.J.; Lapornik, B.; Andrenšek, S.; Wondra, A.G.; Vrhovšek, U.; Kidrič, J. Identification of anthocyanins in wines by liquid chromatography, liquid chromatography-mass spectrometry and nuclear magnetic resonance. Anal. Chim. Acta 2004, 513, 277–282. [Google Scholar] [CrossRef]
- Castañeda-Ovando, A.; Pacheco-Hernández, Ma.d.L.; Páez-Hernández, Ma.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Nave, F.; Teixeira, N.; Mateus, N.; De Freitas, V. The fate of flavanol-anthocyanin adducts in wines: Study of their putative reaction patterns in the presence of acetaldehyde. Food Chem. 2010, 121, 1129–1138. [Google Scholar] [CrossRef]
- De Freitas, V.; Mateus, N. Formation of pyranoanthocyanins in red wines: A new and diverse class of anthocyanin derivatives. Anal. Bioanal. Chem. 2011, 401, 1463–1473. [Google Scholar] [CrossRef]
- Fulcrand, H.; Benabdeljalil, C.; Rigaud, J.; Chenyier, V.; Moutounet, M. A new class of wine pigments generated by reaction between pyruvic acid and grape anthocyanins. Phytochemistry 1998, 47, 1401–1407. [Google Scholar]
- Canals, R.; Llaudy, M.C.; Valls, J.; Canals, J.M.; Zamora, F. Influence of ethanol concentration on the extraction of color and phenolic compounds from the skin and seeds of Tempranillo grapes at different stages of ripening. J. Agric. Food Chem. 2005, 53, 4019–4025. [Google Scholar] [CrossRef]
- Bakker, J.; Timberlake, C.F. Isolation, identification, and characterization of new color-stable anthocyanins occurring in some red wines. J. Agric. Food Chem. 1997, 45, 35–43. [Google Scholar] [CrossRef]
- Schwarz, M.; Wabnitz, T.C.; Winterhalter, P. Pathway leading to the formation of anthocyanin-vinylphenol adducts and related pigments in red wines. J. Agric. Food Chem. 2003, 51, 3682–3687. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P. Pyranoanthocyanins-An overview on structures, occurrence, and pathways of formation. Trends Food Sci. Technol. 2007, 18, 526–534. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Francia-Aricha, E.; Rivas-Gonzalo, J.C. Formation of anthocyanin-derived pigments in experimental red wines. Food Sci. Tech. Int. 1999, 5, 347–352. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Vercauteren, J.; De Freitas, V. Occurrence of anthocyanin-derived pigments in red wines. J. Agric. Food Chem. 2001, 49, 4836–4840. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Asenstorfer, R.E. Screening for potential pigments derived from anthocyanins in red wine using nanoelectrospray tandem mass spectrometry. J.Agric. Food Chem. 2002, 50, 756–761. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Separation of pyranoanthocyanins from red wine by column chromatography. Anal. Chim. Acta 2004, 513, 305–318. [Google Scholar] [CrossRef]
- Von Baer, D.; Rentzsch, M.; Hitschfeld, M.A.; Mardones, C.; Vergara, C.; Winterhalter, P. Relevance of chromatographic efficiency in varietal authenticity verification of red wines based on their anthocyanin profiles: Interference of pyranoanthocyanins formed during wine ageing. Anal. Chim. Acta 2008, 621, 52–56. [Google Scholar] [CrossRef]
- He, J.; Carvalho, A.R.F.; Mateus, N.; De Freitas, V. Spectral features and stability of oligomeric pyranoanthocyanin-flavanol pigments isolated from red wines. J. Agric. Food Chem. 2010, 58, 9249–9258. [Google Scholar] [CrossRef]
- Mateus, N.; Carvalho, E.; Carvalho, A.R.F.; Melo, A.; González-Paramás, A.M.; Santos-Buelga, C.; Silva, A.M.S.; De Freitas, V. Isolation and structural characterization of new acylated anthocyanin-vinyl-flavanol pigments occurring in aging red wines. J. Agric. Food Chem. 2003, 51, 277–282. [Google Scholar]
- Pozo-Baón, M.Á.; Monagas, M.; Polo, M.C.; Gómez-Cordovés, C. Occurrence of pyranoanthocyanins in sparkling wines manufactured with red grape varieties. J. Agric. Food Chem. 2004, 52, 1300–1306. [Google Scholar] [CrossRef]
- Mateus, N.; De Pascual-Teresa, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; De Freitas, V. Structural diversity of anthocyanin-derived pigments in port wines. Food Chem. 2002, 76, 335–342. [Google Scholar] [CrossRef]
- Monagas, M.; Núñez, V.; Bartolomé, B.; Gómez-Cordovés, C. Anthocyanin-derived pigments in Graciano, Tempranillo, and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003, 54, 163–169. [Google Scholar]
- De Villiers, A.; Vanhoenacker, G.; Majek, P.; Sandra, P. Determination of anthocyanins in wine by direct injection liquid chromatography-diode array detection-mass spectrometry and classification of wines using discriminant analysis. J. Chromatogr. A 2004, 1054, 195–204. [Google Scholar]
- Boido, E.; Alcalde-Eon, A.; Carrau, F.; Dellacassa, E.; Rivas-Gonzalo, J.C. Aging effect on the pigment composition and color of Vitis vinifera L. cv. Tannat wines. Contribution of the main pigment families to wine color. J. Agric. Food Chem. 2006, 54, 6692–6704. [Google Scholar] [CrossRef]
- He, J.; Santos-Buelga, C.; Mateus, N.; De Freitas, V. Isolation and quantification of oligomeric pyranoanthocyanin-flavanol pigments from red wines by combination of column chromatographic techniques. J. Chromatogr. A 2006, 1134, 215–225. [Google Scholar] [CrossRef]
- Chinnici, F.; Sonni, F.; Natali, N.; Galassi, S.; Riponi, C. Colour features and pigment composition of Italian carbonic macerated red wines. Food Chem. 2009, 113, 651–657. [Google Scholar] [CrossRef]
- Aguirre, M.J.; Isaacs, M.; Matsuhiro, B.; Mendoza, L.; Santos, L.S.; Torres, S. Anthocyanin composition in aged Chilean Cabernet Sauvignon red wines. Food Chem. 2011, 129, 514–519. [Google Scholar] [CrossRef]
- Romero, C.; Bakker, J. Interactions between grape anthocyanins and pyruvic acid, with effect of pH and acid concentration on anthocyanin composition and color in model solutions. J. Agric. Food Chem. 1999, 47, 3130–3139. [Google Scholar] [CrossRef]
- Bakker, J.; Bridle, P.; Honda, T.; Kuwano, H.; Saito, N.; Terahara, N.; Timberlake, C.F. Identification of an anthocyanin occurring in some red wines. Phytochemistry 1997, 44, 1375–1382. [Google Scholar]
- Jordheim, M.; Fossen, T.; Andersen, Ø.M. Preparative isolation and NMR characterization of carboxypyranoanthocyanins. J. Agric. Food Chem. 2006, 54, 3572–3577. [Google Scholar] [CrossRef]
- Oliveira, J.; Mateus, N.; Silva, A.M.S.; De Freitas, V. Equilibrium forms of Vitisin B pigments in an aqueous system studied by NMR and visible spectroscopy. J. Phys. Chem. B 2009, 113, 11352–11358. [Google Scholar] [CrossRef]
- Lu, Y.; Foo, L.Y. Unusual anthocyanin reaction with acetone leading to pyranoanthocyanin formation. Tetrahedron Lett. 2001, 42, 1371–1373. [Google Scholar] [CrossRef]
- Carvalho, A.R.F.; Oliveira, J.; De Freitas, V.; Mateus, N.; Melo, A. A theoretical interpretation of the color of two classes of pyranoanthocyanins. J. Mol. Struct. Theochem. 2010, 948, 61–64. [Google Scholar] [CrossRef]
- Morata, A.; Calderón, F.; González, M.C.; Gómez-Cordovés, M.C.; Suárez, J.A. Formation of the highly stable pyranoanthocyanins (vitisins A and B) in red wines by the addition of pyruvic acid and acetaldehyde. Food Chem. 2007, 100, 1144–1152. [Google Scholar] [CrossRef]
- Romero, C.; Bakker, J. Effect of acetaldehyde and several acids on the formation of vitisin A in model wine anthocyanin and colour evolution. Int. J. Food Sci. Technol. 2000, 35, 129–140. [Google Scholar] [CrossRef]
- Romero, C.; Bakker, J. Effect of storage temperature and pyruvate on kinetics of anthocyanin degradation, vitisin A derivative formation, and color characteristics of model solutions. J. Agric. Food Chem. 2000, 48, 2135–2141. [Google Scholar] [CrossRef]
- Morata, A.; Gómez-Cordovés, M.C.; Calderón, F.; Suárez, J.A. Effects of pH, temperature and SO2 on the formation of pyranoanthocyanins during red wine fermentation with two species of Saccharomyces. Int. J. Food Microbiol. 2006, 106, 123–129. [Google Scholar] [CrossRef]
- Monagas, M.; Gómez-Cordovés, C.; Bartolomé, B. Evaluation of different Saccharomyces cerevisiae strains for red winemaking. Influence on the anthocyanin, pyranoanthocyanin and non-anthocyanin phenolic content and colour characteristics of wines. Food Chem. 2007, 104, 814–823. [Google Scholar] [CrossRef]
- Revilla, I.; Pérez-Magariño, S.; González-SanJosé, M.L.; Beltrán, S. Identification of anthocyanin derivatives in grape skin extracts and red wines by liquid chromatography with diode array and mass spectrometric detection. J. Chromatogr. A 1999, 847, 83–90. [Google Scholar] [CrossRef]
- Wang, H.; Race, E.J.; Shrikhande, A. Characterization of anthocyanins in grape juices by ion trap liquid chromatography−mass spectrometry. J. Agric. Food Chem. 2003, 51, 1839–1844. [Google Scholar] [CrossRef]
- Nave, F.; Teixeira, N.; Mateus, N.; De Freitas, V. Hemisynthesis and structural characterization of flavanol-(4, 8)-vitisins by mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 1964–1970. [Google Scholar] [CrossRef]
- Schwarz, M.; Jerz, G.; Winterhalter, P. Isolation and structure of pinotin A, a new anthocyanin derivative from pinotage wine. Vitis 2003, 42, 105–106. [Google Scholar]
- Hakånsson, A.E.; Pardon, K.; Hayasaka, Y.; De Sa, M.; Herderich, M. Structures and colour properties of new red wine pigments. Tetrahedron Lett. 2003, 44, 4887–4891. [Google Scholar] [CrossRef]
- Fulcrand, H.; Dos Santos, P.-J.C.; Sarni-Manchado, P.; Cheynier, V.; Favre-Bonvin, J. Structure of new anthocyanin-derived wine pigments. J. Chem. Soc. Perkin Trans. 1 1996, 7, 735–739. [Google Scholar]
- Chatonnet, P.; Dubourdieu, D.; Boidron, J.-N.; Lavigne, V. Synthesis of volatile phenols by Saccharomyces cerevisiae in wines. J. Sci. Food Agric. 1993, 62, 191–202. [Google Scholar] [CrossRef]
- Morata, A.; González, C.; Suárez-Lepe, J.A. Formation of vinylphenolic pyranoanthocyanins by selected yeasts fermenting red grape musts supplemented with hydroxycinnamic acids. Int. J. Food Microbiol. 2007, 116, 144–152. [Google Scholar] [CrossRef]
- Schwarz, M.; Winterhalter, P. A novel synthetic route to substituted pyranoanthocyanins with unique colour properties. Tetrahedron Lett. 2003, 44, 7583–7587. [Google Scholar] [CrossRef]
- Cameira-dos-Santos, P.J.; Brillouet, J.-M.; Cheynier, V.; Moutounet, M. Detection and partial characterisation of new anthocyanins derived pigments in wine. J. Sci. Food Agric. 1996, 70, 204–208. [Google Scholar] [CrossRef]
- Rentzsch, M.; Schwarz, M.; Winterhalter, P.; Hermosín-Guitiérrez, I. Formation of hydroxyphenyl-pyranoanthocyanins in Grenache wines: Precursor levels and evolution during aging. J. Agric. Food Chem. 2007, 55, 4883–4888. [Google Scholar] [CrossRef]
- Schwarz, M.; Hofmann, G.; Winterhalter, P. Investigations on anthocyanins in wines from Vitis vinifera cv. pinotage: Factors influencing the formation of pinotin A and its correlation with wine age. J. Agric. Food Chem. 2004, 52, 498–504. [Google Scholar] [CrossRef]
- Francia-Aricha, E.M.; Guerra, M.T.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. New anthocyanin pigments formed after condensation with flavanols. J. Agric. Food Chem. 1997, 45, 2262–2266. [Google Scholar]
- Cruz, L.; Teixeira, N.; Silva, A.M.S.; Mateus, N.; Borges, J.; De Freitas, V. Role of vinylcatechin in the formation of pyranomalvidin-3-glucoside-(+)-catechin. J. Agric. Food Chem. 2008, 56, 10980–10987. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Hayasaka, Y.; Jones, G.P. Isolation and structures of oligomeric wine pigments by bisulfite-mediated ion-exchange chromatography. J Agric. Food Chem. 2001, 49, 5957–5963. [Google Scholar] [CrossRef]
- Cruz, L.; Borges, E.; Silva, A.M.S.; Mateus, N.; De Freitas, V. Synthesis of a new (+)-catechin-derived compound: 8-Vinylcatechin. Lett. Org. Chem. 2008, 5, 530–536. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Studies on the acetaldehyde-induced condensation of (-)-epicatechin and malvidin 3-O-glucoside in a model solution system. J. Agric. Food Chem. 1999, 47, 2096–2102. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; De Freitas, V. A new class of blue anthocyanin-derived pigments isolated from red wines. J. Agric. Food Chem. 2003, 51, 1919–1923. [Google Scholar] [CrossRef]
- Mateus, N.; Oliveira, J.; Santos-Buelga, C.; Silva, A.M.S.; De Freitas, V. NMR structure characterization of a new vinylpyranoanthocyanin-catechin pigment (a portisin). Tetrahedron Lett. 2004, 45, 3455–3457. [Google Scholar] [CrossRef]
- Mateus, N.; Oliveira, J.; Pissarra, J.; González-Paramás, A.M.; Rivas-Gonzalo, J.C.; Santos-Buelga, C.; Silva, A.M.S.; De Freitas, V. A new vinylpyranoanthocyanin pigment occurring in aged red wine. Food Chem. 2006, 97, 689–695. [Google Scholar] [CrossRef]
- Oliveira, J.; Santos-Buelga, C.; Silva, A.M.S.; De Freitas, V.; Mateus, N. Chromatic and structural features of blue anthocyanin-derived pigments present in Port wine. Anal. Chim. Acta 2006, 563, 2–9. [Google Scholar] [CrossRef]
- Mateus, N.; De Freitas, V. Evolution and stability of anthocyanin-derived pigments during port wine aging. J. Agric. Food Chem. 2001, 49, 5217–5222. [Google Scholar] [CrossRef]
- Oliveira, J.; De Freitas, V.; Silva, A.M.S.; Mateus, N. Reaction between hydroxycinnamic acids and anthocyanin-pyruvic acid adducts yielding new portisins. J. Agric. Food Chem. 2007, 55, 6349–6356. [Google Scholar] [CrossRef]
- Mateus, N.; Oliveira, J.; González-Paramás, A.M.; Santos-Buelga, C.; De Freitas, V. Screening of portisins (vinylpyranoanthocyanin pigments) in port wine by LC/DAD-MS. Food Sci. Technol. Int. 2005, 5, 353–358. [Google Scholar]
- Carvalho, A.R.F.; Oliveira, J.; De Freitas, V.; Mateus, N.; Melo, A. Unusual color change of vinylpyranoanthocyanin-phenolic pigments. J. Agric. Food. Chem. 2010, 58, 4292–4297. [Google Scholar] [CrossRef]
- He, J.; Oliveira, J.; Silva, A.M.S.; Mateus, N.; De Freitas, V. Oxovitisins: A new class of neutral pyranone-anthocyanin derivatives in red wines. J. Agric. Food. Chem. 2010, 58, 8814–8819. [Google Scholar] [CrossRef]
- Asenstorfer, R.E.; Lee, D.F.; Jones, G.P. Influence of structure on the ionisation constants of anthocyanin and anthocyanin-like wine pigments. Anal. Chim. Acta 2006, 563, 10–14. [Google Scholar] [CrossRef]
- Oliveira, J.; Azevedo, J.; Silva, A.M.S.; Teixeira, N.; Cruz, L.; Mateus, N.; de Freitas, V. Pyranoanthocyanin dimers: A new family of turquoise blue anthocyanin-derived pigments found in port wine. J. Agric. Food Chem. 2010, 58, 5154–5159. [Google Scholar]
- Chassaing, S.; Isorez, G.; Kueny-Stotz, M.; Brouillard, R. En route to color-stable pyranoflavylium pigments-a systematic study of the reaction between 5-hydroxy-4-methylflavylium salts and aldehydes. Tetrahedron Lett. 2008, 49, 6999–7004. [Google Scholar] [CrossRef]
- Liao, H.; Cai, Y.; Haslam, E. Polyphenol interactions. Anthocyanins: Co-pigmentation and colour changes in red wines. J. Sci. Food Agric. 1992, 59, 299–305. [Google Scholar] [CrossRef]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem. Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef]
- Jurd, L. Review of polyphenol condensation reactions and their possible occurrence in the aging of wines. Am. J. Enol. Vitic. 1969, 20, 191–195. [Google Scholar]
- Somers, T.C. The polymeric nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Salas, E.; Le Guernevé, C.; Fulcrand, H.; Poncet-Legrand, C.; Cheynier, V. Structure determination and colour properties of a new directly linked flavanol-anthocyanin dimer. Tetrahedron Lett. 2004, 45, 8725–8729. [Google Scholar] [CrossRef]
- Somers, T.C. Pigment phenomena-From grapes to wine. In Grape and Wine Centennial Symposium Proceedings; Webb, A.D., Ed.; University of California: Davis, CA, USA, 1982; pp. 254–257. [Google Scholar]
- Puértolas, E.; Saldaña, G.; Alvarez, I.; Raso, J. Effect of pulsed electric field processing of red grapes on wine chromatic and phenolic characteristics during aging in oak barrel. J. Agric. Food Chem. 2010, 58, 2351–2357. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Francia-Aricha, E.M.; De Pascual-Teresa, S.; Rivas-Gonzalo, J.C. Contribution to the identification of the pigments responsible for the browning of anthocyanin-flavanol solutions. Eur. Food Res. Technol. 1999, 209, 411–415. [Google Scholar]
- Remy, S.; Fulcrand, H.; Labarbe, B.; Cheynier, V.; Moutounet, M. First confirmation in red wine of products resulting from direct anthocyanin-tannin reactions. J. Sci. Food Agric. 2000, 80, 745–751. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Dangles, O.; Brouillard, R. Coupling reactions between flavylium ions and catechin. Phytochemistry 1996, 41, 1583–1592. [Google Scholar]
- Salas, E.; Fulcrand, H.; Meudec, E.; Cheynier, V. Reactions of anthocyanins and tannins in model solutions. J. Agric. Food Chem. 2003, 51, 7951–7961. [Google Scholar] [CrossRef]
- Salas, E.; Atanasova, V.; Poncet-Legrand, C.; Meudec, E.; Mazauric, J.P.; Cheynier, V. Demonstration of the occurrence of flavanol-anthocyanin adducts in wine and in model solutions. Anal. Chim. Acta 2004, 513, 325–332. [Google Scholar] [CrossRef]
- He, F.; Pan, Q.-P.; Shi, Y.; Duan, C.-Q. Chemical synthesis of proanthocyanidins in vitro and their reactions in aging wines. Molecules 2008, 13, 3007–3032. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Effect of oxygenation on polyphenol changes occurring in the course of wine-making. Anal. Chim. Acta 2002, 458, 15–27. [Google Scholar] [CrossRef]
- Sáenz-López, R.; Fernández-Zurbano, P.; Tena, M.T. Analysis of aged red wine pigments by capillary zone electrophoresis. J. Chromatogr. A 2004, 1052, 191–197. [Google Scholar] [CrossRef]
- Hayasaka, Y.; Kennedy, J.A. Mass spectrometric evidence for the formation of pigmented polymers in red wine. Aust. J. Grape Wine Res. 2003, 9, 210–220. [Google Scholar] [CrossRef]
- Vivar-Quintana, A.M.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Anthocyanin-derived pigments and colour of red wines. Anal. Chim. Acta 2002, 458, 147–155. [Google Scholar] [CrossRef]
- Monagas, M.; Núñez, V.; Bartolomé, B.; Gómez-Cordovés, C. Anthocyanin-derived pigments in Graciano, Tempranillo and Cabernet Sauvignon wines produced in Spain. Am. J. Enol. Vitic. 2003, 54, 163–169. [Google Scholar]
- Santos-Buelga, C.; Francia-Aricha, E.M.; De Pascual-Teresa, S.; Rivas-Gonzalo, J.C. Contribution to the identification of the pigments responsible for the browning of anthocyaninflavanol solutions. Eur. Food Res. Technol. 1999, 209, 411–415. [Google Scholar] [CrossRef]
- Mateus, N.; Silva, A.M.S.; Santos-Buelga, C.; Rivas-Gonzalo, J.C.; De Freitas, V. Identification of anthocyanin-flavanol pigments in red wines by NMR and mass spectrometry. J. Agric. Food Chem. 2002, 50, 2110–2116. [Google Scholar] [CrossRef]
- Pati, S.; Losito, I.; Gambacorta, G.; La Notte, E.; Palmisano, F.; Zambonin, P.G. Simultaneous separation and identification of oligomeric procyanidins and anthocyanin-derived pigments in raw red wine by HPLC-UV-ESI-MSn. J. Mass Spectrom. 2006, 41, 861–871. [Google Scholar] [CrossRef]
- Remy-Tanneau, S.; Le Guernevé, C.; Meudec, E.; Cheynier, V. Characterization of a colorless anthocyanin-flavan-3-ol dimer containing both carbon-carbon and ether interflavanoid linkages by NMR and mass spectrometry. J. Agric. Food Chem. 2003, 51, 3592–3597. [Google Scholar] [CrossRef]
- Bishop, P.D.; Nagel, C.W. Characterization of the condensation product of malvidin 3,5-diglucoside and catechin. J. Agric. Food Chem. 1984, 32, 1022–1026. [Google Scholar] [CrossRef]
- Berke, B.; Chèze, C.; Vercauteren, J.; Deffieux, G. Bisulfite addition to anthocyanins: Revisited structures of colorless adducts. Tetrahedron Lett. 1998, 39, 5771–5774. [Google Scholar] [CrossRef]
- Kalbasi, A.; Cisneros-Zevallos, L. Fractionation of monomeric and polymeric anthocyanins from concord grape (Vitis labrusca L.) juice by membrane ultrafiltration. J. Agric. Food Chem. 2007, 55, 7036–7042. [Google Scholar] [CrossRef]
- Salas, E.; Dueñas, M.; Schwarz, M.; Winterhalter, P.; Cheynier, V.; Fulcrand, H. Characterization of pigmets from different high speed countercurrent chromatography wine fractions. J. Agric. Food Chem. 2005, 53, 4536–4546. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Identification of dimeric anthocyanins and new oligomeric pigments in red wine by means of HPLC-DAD-ESI/MSn. J. Mass Spectrom. 2007, 42, 735–748. [Google Scholar] [CrossRef]
- Pati, S.; Liberatore, M.T.; Gambacorta, G.; Antonacci, D.; La Notte, E. Rapid screening for anthocyanins and anthocyanin dimers in crude grape extracts by high performance liquid chromatography coupled with diode array detection and tandem mass spectrometry. J. Chromatogr. A 2009, 1216, 3864–3868. [Google Scholar] [CrossRef]
- Vidal, S.; Meudec, E.; Cheynier, V.; Skouroumounis, G.; Hayasaka, Y. Mass spectrometric evidence for the existence of oligomeric anthocyanins in grape skins. J. Agric. Food Chem. 2004, 52, 7144–7151. [Google Scholar] [CrossRef]
- Vidal, S.; Cartalade, D.; Souquet, J.M.; Fulcrand, H.; Cheynier, V. Changes in proanthocyanidin chain length in winelike model solutions. J. Agric. Food Chem. 2002, 50, 2261–2266. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- Timberlake, C.F.; Bridle, P. Interactions between anthocyanins, phenolic compounds and acetaldehyde and their significance in red wines. Am. J. Enol. Vitic. 1976, 27, 97–105. [Google Scholar]
- Rivas-Gonzalo, J.C.; Bravo-Haro, S.; Santos-Buelga, C. Detection of compounds formed through the reaction of malvidin 3-monoglucoside and catechin in the presence of acetaldehyde. J. Agric. Food Chem. 1995, 43, 1444–1449. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. New phenolic compounds formed by evolution of (+)-catechin and glyoxylic acid in hydroalcoholic solution and their implication in color changes of grape-derived foods. J. Agric. Food Chem. 2000, 48, 4233–4240. [Google Scholar] [CrossRef]
- Sousa, C.; Mateus, N.; Silva, A.M.S.; González-Paramás, A.M.; Santos-Buelga, C.; De Freitas, V. Structural and chromatic characterization of a new Malvidin 3-glucoside-vanillyl-catechin pigment. Food Chem. 2007, 102, 1344–1351. [Google Scholar] [CrossRef]
- Escribano-Bailón, T.; Áivarez-García, M.; Rivas-Gonzalo, J.C.; Heredia, F.J.; Santos-Buelga, C. Color and stability of pigments derived from the acetaldehyde-mediated condensation between malvidin 3-O-glucoside and (+)-catechin. J. Agric. Food Chem. 2001, 49, 1213–1217. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Cheynier, V.; Moutounet, M. Role of aldehydic derivatives in the condensation of phenolic compounds with emphasis on the sensorial properties of fruit-derived foods. J. Agric. Food Chem. 2002, 50, 5571–5585. [Google Scholar] [CrossRef]
- Sun, B.; Barradas, T.; Leandro, C.; Santos, C.; Spranger, I. Formation of new stable pigments from condensation reaction between malvidin 3-glucoside and (-)-epicatechin mediated by acetaldehyde: Effect of tartaric acid concentration. Food Chem. 2008, 110, 344–351. [Google Scholar] [CrossRef]
- Bakker, J.; Picinelli, A.; Bridle, P. Model wine solutions: Colour and composition changes during ageing. Vitis 1993, 32, 111–118. [Google Scholar]
- Lee, D.F.; Swinny, E.E.; Jones, G.P. NMR identification of ethyl-linked anthocyanin-flavanol pigments formed in model wine ferments. Tetrahedron Lett. 2004, 45, 1671–1674. [Google Scholar] [CrossRef]
- Atanasova, V.; Fulcrand, H.; Le Guernevé, C.; Cheynier, V.; Moutounet, M. Structure of new dimeric acetaldehyde malvidin-3-glucoside condensation product. Tetrahedron Lett. 2002, 43, 6151–6153. [Google Scholar] [CrossRef]
- Dallas, C.; Ricardo-da-Silva, J.M.; Laureano, O. Products formed in model wine solutions involving anthocyanins, procyanidin B2, and acetaldehyde. Agric. Food Chem. 1996, 44, 2402–2407. [Google Scholar] [CrossRef]
- Dueñas, M.; Salas, E.; Cheynier, V.; Dangles, O.; Fulcrand, H. UV-Visible spectroscopic investigation of the 8-8-methylmethine catechin-malvidin 3-glucoside pigments in aqueous solution: Structural transformations and molecular complexation with chlorogenic acid. J. Agric. Food Chem. 2006, 54, 189–196. [Google Scholar]
- Dallas, C.; Ricardo da Silva, J.M.; Laureano, O. Interactions of oligomeric procyanidins in model wine solutions containing malvidin-3-glucoside and acetaldehyde. J. Sci. Food Agric. 1996, 70, 493–500. [Google Scholar] [CrossRef]
- Baranowski, E.S.; Nagel, C.W. Kinetics of malvidin-3-glucoside condensation in wine model systems. J. Food Sci. 1983, 48, 419–429. [Google Scholar] [CrossRef]
- García-Viguera, C.; Bridle, P.; Bakker, J. The effect of pH on the formation of coloured compounds in model solutions containing anthocyanins, catechin and acetaldehyde. Vitis 1994, 33, 37–40. [Google Scholar]
- Es-Safi, N.-E.; Cheynier, V.; Moutounet, M. Study of the reactions between (+)-catechin and furfural derivatives in the presence or absence of anthocyanins and their implication in food color change. J. Agric. Food Chem. 2000, 48, 5946–5954. [Google Scholar] [CrossRef]
- Pissarra, J.; Mateus, N.; Rivas-Gonzalo, J.; Santos Buelga, C.; De Freitas, V. Reaction between malvidin 3-glucoside and (+)-catechin in model solutions containing different aldehydes. J. Food Sci. 2003, 68, 476–481. [Google Scholar]
- Drinkine, J.; Lopes, P.; Kennedy, J.A.; Teissedre, P.-L.; Saucier, C. Ethylidene-bridged flavan-3-ols in red wine and correlation with wine age. J. Agric. Food Chem. 2007, 55, 6292–6299. [Google Scholar]
- Fulcrand, H.; Cheynier, V.; Oszmiansky, J.; Moutounet, M. An oxidised tartaric acid residue as a new bridge potentially competing with acetaldehyde in flavan-3-ol condensation. Phytochemistry 1997, 46, 223–227. [Google Scholar]
- Drinkine, J.; Glories, Y.; Saucier, C. (+)-Catechin-aldehyde condensations: Competition between acetaldehyde and glyoxylic acid. J. Agric. Food Chem. 2005, 53, 7552–7558. [Google Scholar] [CrossRef]
- Es-Safi, N.-E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Tech. 2000, 35, 63–74. [Google Scholar] [CrossRef]
- Santos-Buelga, C.; Bravo-Haro, S.; Rivas-Gonzalo, J.C. Interactions between catechin and malvidin-3-monoglucoside in model solutions. Eur. Food Res. Technol. 1995, 201, 269–274. [Google Scholar]
- Es-Safi, N.-E.; Le Gueraevé, C.; Labarbe, B.; Fulerand, H.; Cheyuier, V.; Moutouaet, M. Structure of a new xanthyfium salt derivative. Tetrahedron Lett. 1999, 40, 5869–5872. [Google Scholar] [CrossRef]
- Es-Safi, N.; Le Guernevé, C.; Labarbe, B.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Structure of a new xanthylium salt derivative. Tetrahedron Lett. 1999, 40, 5869–5872. [Google Scholar]
- Somers, T.C. The phenolic nature of wine pigments. Phytochemistry 1971, 10, 2175–2186. [Google Scholar] [CrossRef]
- Dueñas, M.; Fulcrand, H.; Cheynier, V. Formation of anthocyanin-flavanol adducts in model solutions. Anal. Chim. Acta 2006, 563, 15–25. [Google Scholar]
- Jurd, L.; Somers, T.C. The formation of xanthylium salts from proanthocyanidins. Phytochemistry 1970, 9, 419–427. [Google Scholar]
- George, N.; Clark, A.C.; Prenzler, P.D.; Scollary, G.R. Factors influencing the production and stability of xanthylium cation pigments in a model white wine system. Aust. J. Grape Wine Res. 2006, 12, 57–68. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. New polyphenolic compounds with xanthylium skeletons formed through reaction between (+)-catechin and glyoxylic acid. J. Agric. Food Chem. 1999, 47, 5211–5217. [Google Scholar] [CrossRef]
- Alcalde-Eon, C.; Escribano-Bailón, M.T.; Santos-Buelga, C.; Rivas-Gonzalo, J.C. Changes in the detailed pigment composition of red wine during maturity and ageing: A comprehensive study. Anal. Chim. Acta 2006, 563, 238–254. [Google Scholar] [CrossRef]
- Cano-López, M.; Pardo-Mínguez, F.; Schmauch, G.; Saucier, C.; Teissedre, P.-L.; López-Roca, J.M.; Gómez-Plaza, E. Effect of micro-oxygenation on color and anthocyanin-related compounds of wines with different phenolic contents. J. Agric. Food Chem. 2008, 56, 5932–5941. [Google Scholar]
- Revilla, E.; López, J.F.; Ryan, J.-M. Anthocyanin pattern of Tempranillo wines during ageing in oak barrels and storage in stainless-steel tanks. Eur. Food Res. Technol. 2005, 220, 592–596. [Google Scholar] [CrossRef]
- Cano-López, M.; Pardo-Minguez, F.; López-Roca, J.M.; Gómez-Plaza, E. Effect of the microoxygenation on anthocyanin and derived pigment content and chromatic characteristics of red wines. Am. J. Enol. Vitic. 2006, 57, 325–331. [Google Scholar]
- Sartini, E.; Arfelli, G.; Fabiani, A.; Piva, A. Influence of chips, lees and micro-oxygenation during aging on the phenolic composition of a red Sangiovese wine. Food Chem. 2007, 104, 1599–1604. [Google Scholar] [CrossRef]
- Cano-López, M.; Pardo-Mínguez, F.; López-Roca, J.M.; Gómez-Plaza, E. Chromatic characteristics and anthocyanin profile of a micro-oxygenated red wine after oak or bottle maturation. Eur. Food Res. Technol. 2007, 225, 127–132. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Ortega-Heras, M.; Cano-Mozo, E.; González-Sanjosé, M.L. The influence of oak wood chips, micro-oxygenation treatment, and grape variety on colour, and anthocyanin and phenolic composition of red wines. J. Food Compos. Anal. 2009, 22, 204–211. [Google Scholar]
- Cano-López, M.; López-Roca, J.M.; Pardo-Mínguez, F.; Gómez-Plaza, E. Oak barrel maturation vs. micro-oxygenation: Effect on the formation of anthocyanin-derived pigments and wine colour. Food Chem. 2010, 119, 191–195. [Google Scholar] [CrossRef]
- Del Álamo Sanza, M.; Domínguez, I.N.; Merino, S.G. Influence of different aging systems and oak woods on aged wine color and anthocyanin composition. Eur. Food Res. Technol. 2004, 219, 124–132. [Google Scholar]
- Del Álamo Sanza, M.; Nevares Domínguez, I. Wine aging in bottle from artificial systems (staves and chips) and oak woods Anthocyanin composition. Anal. Chim. Acta 2006, 563, 255–263. [Google Scholar] [CrossRef]
- Gambuti, A.; Capuano, R.; Lisanti, M.T.; Strollo, D.; Moio, L. Effect of aging in new oak, one-year-used oak, chestnut barrels and bottle on color, phenolics and gustative profile of three monovarietal red wines. Eur. Food Res. Technol. 2010, 231, 455–465. [Google Scholar] [CrossRef]
- Palomero, F.; Morata, A.; Benito, S.; Gonzalez, M.C.; Suárez-Lepe, J.A. Conventional and enzyme-assisted autolysis during ageing over lees in red wines: Influence on the release of polysaccharides from yeast cell walls and on wine monomeric anthocyanin content. Food Chem. 2007, 105, 838–846. [Google Scholar]
- Moreno-Arribas, M.V.; Gómez-Cordovés, C.; Martín-Álvarez, P.J. Evolution of red wine anthocyanins during malolactic fermentation, postfermentative treatments and ageing with lees. Food Chem. 2008, 109, 149–158. [Google Scholar]
- Feuillat, M. Yeast macromolecules: Origin, composition and enological interest. Am. J. Enol. Vitic. 2003, 54, 211–213. [Google Scholar]
- Baptista, A.S.; Horii, J.; Calori-Domingues, M.A.; Micotti da Glória, E.; Salgado, J.M.; Vizioli, M.R. The capacity of manno-oligosaccharides, thermolysed yeast and active yeast to attenuate aflatoxicosis. World J. Microb. Biot. 2004, 20, 475–481. [Google Scholar] [CrossRef]
- Guilloux-Benatier, M.; Guerreau, J.; Feuillat, M. Influence of initial colloid content on yeast macromolecule production and on the metabolism of wine microorganisms. Am. J. Enol. Vitic. 1995, 46, 486–492. [Google Scholar]
- Moine-Ledoux, V.; Perrin, A.; Paladin, I.; Dubourdieu, D. Premiers résultats de stabilisation tartrique des vins par addition de mannoprotéines purifies (Mannostab). J. Int. Sci. Vigne Vin 1997, 31, 23–31. [Google Scholar]
- Waters, E.; Pellerin, P.; Brillouet, J. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohyd. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Escot, S.; Feulliat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Vitic. 2007, 58, 87–91. [Google Scholar]
- Guadalupe, Z.; Palacios, A.; Ayestarán, B. Maceration enzymes and mannoproteins: A possible strategy to increase colloidal stability and color extraction in red wines. J. Agric. Food Chem. 2007, 55, 4854–4862. [Google Scholar]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and colour composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez, L.; Ayestarán, B. Yeast mannoproteins in red winemaking. Effect on polysaccharide, polyphenolic and colour composition. Am. J. Enol. Vitic. 2010, 61, 191–200. [Google Scholar]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Brouillard, R.; Dangles, O. Anthocyanin molecular interactions: The first step in the formation of new pigments during wine aging? Food Chem. 1994, 51, 265–371. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
He, F.; Liang, N.-N.; Mu, L.; Pan, Q.-H.; Wang, J.; Reeves, M.J.; Duan, C.-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules 2012, 17, 1483-1519. https://doi.org/10.3390/molecules17021483
He F, Liang N-N, Mu L, Pan Q-H, Wang J, Reeves MJ, Duan C-Q. Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules. 2012; 17(2):1483-1519. https://doi.org/10.3390/molecules17021483
Chicago/Turabian StyleHe, Fei, Na-Na Liang, Lin Mu, Qiu-Hong Pan, Jun Wang, Malcolm J. Reeves, and Chang-Qing Duan. 2012. "Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution" Molecules 17, no. 2: 1483-1519. https://doi.org/10.3390/molecules17021483
APA StyleHe, F., Liang, N.-N., Mu, L., Pan, Q.-H., Wang, J., Reeves, M. J., & Duan, C.-Q. (2012). Anthocyanins and Their Variation in Red Wines II. Anthocyanin Derived Pigments and Their Color Evolution. Molecules, 17(2), 1483-1519. https://doi.org/10.3390/molecules17021483