Abstract
A simple procedure for SnCl2/TiCl3-mediated deoximation of ketoximes in an aqueous solvent is reported. Under the conditions developed in this effort, various ketones and aldehydes are produced in good to excellent yields.
1. Introduction
Oximes are often used in organic synthesis as protected forms of carbonyl compounds [1] or as carbonyl derivatives for purification and characterization purposes [2]. Furthermore, oximes can be prepared from noncarbonyl compounds, such as nitroalkanes [3,4,5], or primary amines [6], thus deoximation provides an alternative approach for the syntheses of aldehydes and ketones. A plethora of examples of procedures for the regeneration of carbonyl compounds from oximes have been reported. So far a good number of deoximation methods based on hydrolytic [7], reductive [8,9,10,11,12], oxidative [13,14,15,16,17,18,19], and transoximation [20,21,22] reactions have been developed. Among them some require strong acidic conditions; some take long reaction times, and give low product yields, while some are performed at higher temperatures. For example, regeneration of carbonyl compounds from oximes via direct hydrolysis usually involves strong acidic conditions due to the relatively high hydrolytic stability of oximes, and thus leads to the damage of acid-sensitive groups and the formation of amides as byproducts by Beckmann rearrangement [23,24]. Besides, most of reductive and oxidative methods require reagents that are often hazardous or very toxic, expensive or not readily available. Therefore, a milder, high yielding, and inexpensive method is still in demand.
Tin and tin-containing Lewis acids have been extensively used in organic chemistry owing to their low cost, commercial availability and modestly low toxicity [25,26]. Deoximation of oximes by using SnCl2-SiO2 has been reported. However, harsh reaction conditions are applied such as reflux or microwave irradiation at high temperature [27,28]. Several examples of mild methods that use tin metal and stannic chloride to promote allylation reactions of protected carbonyls such as enol ethers and acetals in aqueous media have been described [29,30,31,32]. Apparently, the protected carbonyls are hydrolyzed to the corresponding aldehydes, which then undergo allylation in the presence of allyl anion equivalents. In this regard, we envisioned that deoximation of oximes by tin-mediated hydrolysis reactions under aqueous and mild conditions should be possible. Below, we describe the results of a study which demonstrate that oximes serve as starting materials for tin promoted hydrolysis reactions.
2. Results and Discussion
The effort began with an investigation of metal- or metal halide-mediated aqueous deoximation of acetophenone oxime (1a). The conditions employed and the results of the reactions are presented in Table 1 [33].

Table 1.
Metal- or metal halide-mediated deoximation of ketoxime a. 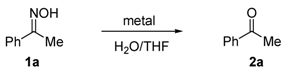

| Entry | Metal | Time, conversion b |
|---|---|---|
| 1 | TiCl3 | 4 h, 12% |
| 2 | SnCl2 | 4 h, 14% |
| 3 | SnCl2/TiCl3 | 4 h, 99+% |
| 4 | SnCl2/KI | 4 h, 14% |
| 5 | Sn/TiCl3 | 4 h, 80% |
| 6 | Mn/TiCl3 | 4 h, 23% |
| 7 | In/TiCl3 | 4 h, 55% |
| 8 | Fe/TiCl3 | 4 h, 53% |
| 9 | Cu/TiCl3 | 4 h, 39% |
| 10 | Zn/TiCl3 | 4 h, 56% c |
| 11 d | TiCl3 | 4 h, 92% |
| 12 d | SnCl2 | 4 h, 26% |
a Conditions: 1a (1.0 mmol) and indicated metal or metal halide (1.5 mmol) in THF (1.0 mL)/water (1.0 mL) at r.t.; b conversion was determined by 1H-NMR; c Isolated yield; d Conditions: 1a (1.0 mmol) and indicated metal halide (3.0 mmol) in THF (1.0 mL)/water (1.0 mL) at r.t.
The results show that SnCl2/TiCl3 serves as a superior reagent for the deoximation of acetophenone oxime (1a) in aqueous medium (Table 1, entry 3). It is reported that SnCl2 is prone to partial hydrolysis in water and generates an acidic solution [34]. However, the results showed that the acidic conditions generated are not sufficient to promote regeneration of carbonyl compounds from ketoxime in aqueous solvent (Table 1, entries 2, 4 and 12). The reduction of oximes to form imines, using trivalent titanium reductant or utilizing a low-valent titanium reagent generated from TiCl4/SnCl2 has been described [35,36,37,38]. However, these reactions are performed under anhydrous conditions and excess amounts of reagents are employed. Thus, we envisaged that imines formed in this manner would be susceptible to rapid hydrolysis to produce ketones. Herein, a commercially available titanium trichloride 20% in 3% hydrochloric acid was employed in this study. The intervention and effect of this reduction process are seen by comparing the rates of reactions promoted by using various low-valent titanium reagents (Table 1, entries 1, 3 and 5–11). It is important to note that the deoximation process was deteriorated by using low-valent titanium generated from zinc (Table 1, entry 10).

Table 2.
SnCl2/TiCl3-mediated deoximation of ketoximes a. 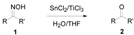

| Entry | Substrate | Time, yield | Entry | Substrate | Time, yield |
|---|---|---|---|---|---|
| 1 |  | 4 h, 96% | 9 |  | 5 h, 89% |
| 2 |  | 6 h, 92% | 10 | 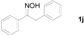 | 9 h, 97% |
| 3 | 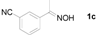 | 9 h, 92% | 11 |  | 6 h, 97% |
| 4 | 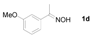 | 4 h, 84% | 12 | 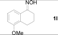 | 9 h, 99% |
| 5 |  | 9 h, 82% | 13 |  | 9 h, 83% |
| 6 | 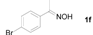 | 9 h, 98% | 14 | 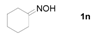 | 3 h, 91% |
| 7 | 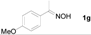 | 3 h, 95% | 15 | 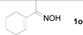 | 7 h, 92% |
| 8 |  | 2.5 h, 94% |
a Conditions: ketoxime 1 (1.0 mmol), SnCl2 (1.5 mmol) and TiCl3 (1.5 mmol) in THF (1.0 mL)/water (1.0 mL) at r.t.
Next, the substrate scope of the deoximation reactions of ketoximes was probed under the SnCl2/TiCl3-mediated conditions (Table 2). It is found that the amount of TiCl3 could be lowered but it depends on the substrate. For example, a catalytic amount of TiCl3 (0.25 equivalent) accompanied with SnCl2 (1.0 equivalent) was able to deoximate acetophenone oxime (1a) but at least one equivalent of TiCl3 was required to deoximate oxime 1k. Therefore, we chose fixed amounts of SnCl2 and TiCl3 (1.5 equivalents each) as a general procedure for deoximation of various ketoximes shown in Table 2. As can be seen by viewing the results displayed in Table 2, the yields of these processes starting with both aromatic (Table 2, entries 1–13) and aliphatic (Table 2, entries 14 and 15) oximes, are good to excellent. Interestingly, the presence of an amide group that is present in 1e (Table 2, entry 5) and a free phenol group that is present in 1h (Table 2, entry 8) do not alter the efficiency of the reaction. In addition, aromatic halogen (1b, 1f), nitrile (1c), and alkoxyl (1d, 1g, 1l) as well as olefin (1o) were tolerated under this mild condition.
With the excellent results from deoximation of ketoximes, we turned our attention to deoximation of aldoximes with the same approach and the results are shown in Table 3. Deoximation worked well for aldoximes 1p, 1q, and 1r with the SnCl2/TiCl3 system (entries 3, 6 and 9, Table 3) and deoximation products were obtained in excellent yields under this mild condition. Due to poor solubility of aldoxime 1r in water, THF is added to boost the reaction to complete (entry 9 vs. entry 10, Table 3).

Table 3.
Metal halide-mediated deoximation of aldoxime a. 

| Entry | Substrate | Metal | Solvent | Time | Conversion b (%) |
|---|---|---|---|---|---|
| 1 | 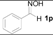 | TiCl3 | H2O | 2 h | 33 |
| 2 | 1p | SnCl2 | H2O | 2 h | 4 |
| 3 | 1p | SnCl2/TiCl3 | H2O | 2 h | 99 (96) c |
| 4 |  | TiCl3 | H2O | 5 h | 12 |
| 5 | 1q | SnCl2 | H2O | 5 h | <1 |
| 6 | 1q | SnCl2/TiCl3 | H2O | 5 h | 99 (93) c |
| 7 | 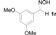 | TiCl3 | THF/H2O (1/3) | 4 h | 17 |
| 8 | 1r | SnCl2 | THF/H2O (1/3) | 4 h | 9 |
| 9 | 1r | SnCl2/TiCl3 | THF/H2O (1/3) | 4 h | 99 (97) c |
| 10 | 1r | SnCl2/TiCl3 | H2O | 6 h | 71 |
a Conditions: aldoxime 1 (1.0 mmol) and indicated metal halide (1.0 mmol) in solvent (2.0 mL) at r.t.; b Conversion was determined by 1H-NMR; c Isolated yield of 2.
3. Experimental
3.1. General Information and Materials
All commercially available chemicals were used without further purification. Oximes were prepared according to the reported procedures [39]. TLC analyses were run on a glass plate (Silica gel 60 F254) and were visualized using UV or a solution of phosphomolybdic acid in ethanol (5 wt%) or p-anisaldehyde stain. Flash chromatography was performed using silica gel (70–230 mesh). 1H and 13C-NMR spectra were recorded on a 300 MHz spectrometer (Bruker AV-300). Chemical shifts are reported relative to CHCl3 [δH 7.24, δC (central line) 77.0]. Mass spectra and high-resolution mass spectra were recorded under electron spray interface (ESI) conditions (Finnigan/Thermo Quest MAT).
3.2. General Procedure for SnCl2/TiCl3-Mediated Deoximation of Ketoximes in an Aqueous Solvent
Tin chloride dihydrate (345 mg, 1.5 mmol) and titanium(III) chloride, (0.96 mL, 1.5 mmol, 20% in 3% hydrochloric acid) were added successively to a solution of oxime 1 (1.0 mmol) in THF (1 mL)/water (1 mL) at r.t. The mixture was stirred at ambient temperature until all the starting material was consumed, the solution was extracted with Et2O (3 × 5 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo, giving a residue which was subjected to silica gel chromatography to furnish the pure ketone 2.
Acetophenone (2a). Following the general procedure, the title compound was obtained (115 mg, 96%). Oil; TLC (EtOAc/hexanes (1:2)) R f= 0.58; 1H-NMR (CDCl3): δ 2.58 (s, 3H), 7.41–7.54 (m, 3H), 7.93 (dd, J = 8.4, 1.2 Hz, 2H). Data are in good agreement with the literature [40].
1-(3-Bromophenyl)ethanone (2b). Following the general procedure, the title compound was obtained (183 mg, 92%). Oil; TLC (EtOAc/hexanes (1:4)) R f= 0.68; 1H-NMR (CDCl3): δ 2.57 (s, 3H), 7.33 (t, J = 7.8 Hz, 1H), 7.65–7.69 (m, 1H), 7.84–7.87 (m, 1H), 8.06 (d, J = 2.1 Hz, 1H). Data are in good agreement with the literature [41].
3-Acetylbenzonitrile (2c). Following the general procedure, the title compound was obtained (134 mg, 92%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.25; 1H-NMR (CDCl3): δ 2.62 (s, 3H), 7.59 (t, J = 7.8 Hz, 1H), 7.80–7.84 (m, 1H), 8.14–8.21 (m, 2H). Data are in good agreement with the literature [42].
1-(3-Methoxyphenyl)ethanone (2d). Following the general procedure, the title compound was obtained (126 mg, 84%). Oil; TLC (EtOAc/hexanes (1:4)) R f= 0.75; 1H-NMR (CDCl3): δ 2.58 (s, 3H), 3.84 (s, 3H), 7.07–7.11 (m, 1H), 7.35 (t, J = 7.8 Hz, 1H), 7.46–7.53 (m, 2H). Data are in good agreement with the literature [43].
N-(3-Acetylphenyl)acetamide (2e). Following the general procedure, the title compound was obtained (145 mg, 82%). Oil; TLC (EtOAc/hexanes (1:2)) R f= 023; 1H-NMR (CDCl3): δ 2.17 (s, 3H), 2.52 (s, 3H), 7.33 (t, J = 7.8 Hz, 1H), 7.59 (d, J = 7.8 Hz, 1H), 7.91 (d, J = 7.8 Hz, 1H), 8.05 (s, 1H), 8.85 (s, 1H). Data are in good agreement with the literature [44].
1-(4-Bromophenyl)ethanone (2f). Following the general procedure, the title compound was obtained (195 mg, 98%). Oil; TLC (EtOAc/hexanes (1:4)) R f= 0.53; 1H-NMR (CDCl3): δ 2.56 (s, 3H), 7.57 (d, J = 7.8 Hz, 2H), 7.78 (d, J = 7.8 Hz, 2H). Data are in good agreement with the literature [45].
1-(4-Methoxyphenyl)ethanone (2g). Following the general procedure, the title compound was obtained (143 mg, 95%). Oil; TLC (EtOAc/hexanes (1:9)) R f= 0.43; 1H-NMR (CDCl3): δ 2.52 (s, 3H), 3.81 (s, 3H), 6.86 (d, J = 7.8 Hz, 2H), 7.87 (d, J = 7.8 Hz, 2H). Data are in good agreement with the literature [46].
1-(4-Hydroxyphenyl)ethanone (2h). Following the general procedure, the title compound was obtained (128 mg, 94%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.58; 1H-NMR (CDCl3): δ 2.54 (s, 3H), 6.08 (s, 1H), 6.87 (d, J = 8.4 Hz, 2H), 7.89 (d, J = 8.4 Hz, 2H). Data are in good agreement with the literature [45].
Propiophenone (2i). Following the general procedure, the title compound was obtained (119 mg, 89%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.58; 1H-NMR (CDCl3): δ 1.20 (t, J = 7.5 Hz, 3H), 2.97 (q, J = 7.5 Hz, 2H), 7.40–7.53 (m, 3H), 7.93 (d, J = 7.8 Hz, 2H). Data are in good agreement with the literature [45].
1,2-Diphenylethanone (2j). Following the general procedure, the title compound was obtained (190 mg, 97%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.63; 1H-NMR (CDCl3): δ 4.28 (s, 2H), 7.25–7.35 (m, 5H), 7.42–7.57 (m, 3 H), 8.00 (t, J = 2.1 Hz, 2H). Data are in good agreement with the literature [47].
1-(Naphthalen-2-yl)ethanone (2k). Following the general procedure, the title compound was obtained (165 mg, 97%). Oil; TLC (Et2O/hexanes (1:4)) R f= 0.45; 1H-NMR (CDCl3): δ 2.71 (s, 3H), 7.54–7.59 (m, 2H), 7.85–8.03 (m, 4H), 8.45 (s, 1H). Data are in good agreement with the literature [40].
5-Methoxy-3,4-dihydronaphthalen-1(2H)-one (2l). Following the general procedure, the title compound was obtained (174 mg, 99%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.30; 1H-NMR (CDCl3): δ 2.05–2.15 (m, 2H), 2.61–2.65 (m, 2H), 2.87 (t, J = 6.3 Hz, 2H), 3.84 (s, 3H), 7.01 (d, J = 7.8 Hz, 1H), 7.24 (d, J = 7.8 Hz, 1H), 7.61 (d, J = 7.8 Hz, 1H). Data are in good agreement with the literature [48].
1-(Benzofuran-2-yl)ethanone (2m). Following the general procedure, the title compound was obtained (133 mg, 83%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.40; 1H-NMR (CDCl3): δ 2.59 (s, 3H), 7.24–7.32 (m, 1H), 7.45–7.49 (m, 2H), 7.54–7.58 (m, 1H), 7.67–7.71 (m, 1H). Data are in good agreement with the literature [49].
Cyclohexanone (2n). Following the general procedure, the title compound was obtained (89 mg, 91%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.45; 1H-NMR (CDCl3): δ 1.64–1.87 (m, 6H), 2.28–2.32 (m, 4H). Data are in good agreement with the literature [50].
1-Cyclohexenylethanone (2o). Following the general procedure, the title compound was obtained (114 mg, 92%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.53; 1H-NMR (CDCl3): δ 1.58–1.61 (m, 4H), 2.19–2.24 (m, 4H), 2.25 (s, 3H), 6.85–6.88 (m, 1H). Data are in good agreement with the literature [51].
3.3. General Procedure for SnCl2/TiCl3-Mediated Deoximation of Aldoximes in an Aqueous Solvent
Tin chloride dihydrate (230 mg, 1.0 mmol) and titanium(III) chloride, (0.64 mL, 1.0 mmol, 20% in 3% hydrochloric acid) were added successively to a solution of aldoxime 1 (1.0 mmol) in H2O (2 mL) or THF/water (1/3, 2 mL) at rt. The mixture was stirred at ambient temperature until all the starting material was consumed, the solution was extracted with Et2O (3 × 5 mL). The combined organic layers were dried over MgSO4, filtered, and concentrated in vacuo, giving a residue which was subjected to silica gel chromatography to furnish the pure aldehyde 2.
Benzaldehyde (2p). Following the general procedure, the title compound was obtained (102 mg, 96%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.63; 1H-NMR (CDCl3): δ 7.47–7.52 (m, 2H), 7.57–7.63 (m, 1H), 7.83–7.87 (m, 2H), 9.99 (s, 1H). Data are in good agreement with the literature [52].
4-Chlorobenzaldehyde (2q). Following the general procedure, the title compound was obtained (131 mg, 93%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.45; 1H-NMR (CDCl3): δ 7.48 (d, J = 9.0 Hz, 2H), 7.80 (d, J = 9.0 Hz, 2H), 9.96 (s, 1H). Data are in good agreement with the literature [52].
3,5-Dimethoxybenzaldehyde (2r). Following the general procedure, the title compound was obtained (161 mg, 97%). Oil; TLC (Et2O/hexanes (1:2)) R f= 0.43; 1H-NMR (CDCl3): δ 3.64 (s, 3H), 3.65 (s, 3H), 6.51 (d, J = 2.4 Hz, 1H), 6.81 (d, J = 2.4 Hz, 2H), 9.70 (s, 1H). Data are in good agreement with the literature [53].
4. Conclusions
In summary, we report a simple, mild and inexpensive method for deoximation of oximes in an aqueous solvent. This procedure appears to be advantageous in terms of chemical yield, reaction conditions, and functional group compatibility.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/17/3/2464/s1.
Acknowledgments
Financial support from the National Science Council of the Republic of China, Taiwan (NSC100-2113-M-018-004-MY2) is gratefully acknowledged.
- Samples Availability: Samples of the all compounds are available from the authors.
References and Notes
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 4th ed; John Wiley: New York, NY, USA, 2007; pp. 506–527. [Google Scholar]
- Shriner, R.L.; Fuson, R.C.; Curtin, D.Y.; Morrill, T.C. The Systematic Identification of Organic Compounds, 6th ed; John Wiley: New York, NY, USA, 1980. [Google Scholar]
- Wang, K.; Qian, X.; Cui, J. One step from nitro to oxime: A convenient preparation of unsaturated oximes by the reduction of the corresponding vinylnitro compounds. Tetrahedron 2009, 65, 10377–10382. [Google Scholar] [CrossRef]
- Domingo, L.R.; Picher, M.T.; Arroyo, P.; Sez, J.A. 1,3-Dipolar cycloadditions of electrophilically activated benzonitrile N-oxides. Polar cycloaddition versus oxime formation. J. Org. Chem. 2006, 71, 9319–9330. [Google Scholar]
- Czekelius, C.; Carreira, E.M. Convenient transformation of optically active nitroalkanes into chiral aldoximes and nitriles. Angew. Chem. Int. Ed. 2005, 44, 612–615, and references cited therein. [Google Scholar]
- Suzuki, K.; Watanabe, T.; Murahashi, S.-I. Aerobic oxidation of primary amines to oximes catalyzed by DPPH and WO3/Al2O3. Angew. Chem. Int. Ed. 2008, 47, 2079–2081. [Google Scholar]
- Quan, N.; Shi, X.-X.; Nie, L.-D.; Dong, J.; Zhu, R.-H. A green chemistry method for the regeneration of carbonyl compounds from oximes by using cupric chloride dihydrate as a recoverable promoter for hydrolysis. Synlett 2011, 7, 1028–1032, and references cited therein. [Google Scholar]
- Majireck, M.M.; Witek, J.A.; Weinreb, S.M. An expedient reductive method for conversion of ketoximes to the corresponding carbonyl compounds. Tetrahedron Lett. 2010, 51, 3555–3557. [Google Scholar] [CrossRef]
- Martin, M.; Martinez, G.; Urpi, F.; Vilarrasa, J. Conversion of ketoximes to ketones with trimethylphosphine and 2,2’-dipyridyl diselenide. Tetrahedron Lett. 2004, 45, 5559–5561. [Google Scholar] [CrossRef]
- Lukin, K.A.; Narayanan, B.A. The reaction of oximes with tributylphosphine-phenyldisulfide: Mechanistic insight and new synthetic possibilities. Tetrahedron 2002, 58, 215–219. [Google Scholar] [CrossRef]
- Akazome, M.; Tsuji, Y.; Watanabe, Y. Ruthenium complex-catalyzed selective deoxygenation of ketoximes to ketimines. Chem. Lett. 1990, 635–638. [Google Scholar]
- Curran, D.P.; Brill, J.F.; Rakiewicz, D.M. A mild reductive conversion of oximes to ketones. J. Org.Chem. 1984, 49, 1654–1656. [Google Scholar] [CrossRef]
- Zhou, X.-T.; Yuan, Q.-L.; Ji, H.-B. Highly efficient aerobic oxidation of oximes to carbonyl compounds catalyzed by metalloporphyrins in the presence of benzaldehyde. Tetrahedron Lett. 2010, 51, 613–617. [Google Scholar]
- Shaabani, A.; Farhangi, E. Cobalt(II) phthalocyanine catalyzed aerobic regeneration of carbonyl compounds from the corresponding oximes in 1-butyl-3-methylimidazolium bromide. Appl. Catal. A 2009, 371, 148–152. [Google Scholar] [CrossRef]
- Ganguly, N.C.; Barik, S.K. A facile, catalytic deoximation method using potassium bromide and ammonium heptamolybdate in the presence of hydrogen peroxide in an aqueous medium. Synthesis 2008, 425–428. [Google Scholar] [CrossRef]
- Gupta, P.K.; Manral, L.; Ganesan, K. An efficient approach for the conversion of oximes into carbonyl compounds using dichloramine-T. Synthesis 2007, 1930–1932. [Google Scholar]
- Gogoi, P.; Hazarika, P.; Konwar, D. Surfactant/I2/Water: An efficient system for deprotection of oximes and imines to carbonyls under neutral conditions in water. J. Org. Chem. 2005, 70, 1934–1936. [Google Scholar] [CrossRef]
- Shaabani, A.; Naderi, S.; Rahmati, A.; Badri, Z.; Darvishi, M.; Lee, D.G. Cleavage of oximes, semicarbazones, and phenylhydrazones with supported potassium permanganate. Synthesis 2005, 3023–3025. [Google Scholar]
- Khazaei, A.; Manesh, A.A. Microwave-assisted chemoselective cleavage of oximes to their corresponding carbonyl compounds using 1,3-dichloro-5,5-dimethylhydantoin (DCDMH) as a new deoximating reagent. Synthesis 2005, 1929–1931. [Google Scholar] [CrossRef]
- Chavan, S.P.; Soni, P. A facile deprotection of oximes using glyoxylic acid in an aqueous medium. Tetrahedron Lett. 2004, 45, 3161–3162. [Google Scholar] [CrossRef]
- Maynez, S.R.; Pelavin, L.; Erker, G. Regeneration of ketones from tosylhydrazones, arylhydrazones, and oximes by exchange with acetone. J. Org. Chem. 1975, 40, 3302–3303. [Google Scholar] [CrossRef]
- DePuy, C.H.; Ponder, B.W. Levulinic acid as a reagent for the hydrolysis of oximes and 2,4-dinitrophenylhydrazones. J. Am. Chem. Soc. 1959, 81, 4629–4631. [Google Scholar] [CrossRef]
- Luca, L.D.; Giacomelli, G.; Porcheddu, A. Beckmann rearrangement of oximes under very mild conditions. J. Org. Chem. 2002, 67, 6272–6274. [Google Scholar] [CrossRef]
- Gawley, R.E. The Beckmann reactions: Rearrangements, elimination-additions, fragmentations, and rearrangement-cyclizations. Org. React. 1988, 35, 1–420, and references cited therein. [Google Scholar]
- Pereyre, M.; Quintard, J.-P.; Rahm, A. Tin in Organic Synthesis; Butterworth: London, UK, 1987. [Google Scholar]
- Smith, P.J. Toxicological Data on Organotin Compounds; International Tin Research Inst.: London, UK, 1978. [Google Scholar]
- Das, N.B.; Nayak, A.; Nanda, B. A general approach for the microwave-assisted regeneration of carbonyl compounds from their nitrogenous derivatives. J. Chem. Res. 2004, 712–713. [Google Scholar]
- Das, N.B.; Nanda, B.; Nayak, A. SnCl2-SiO2: A selective reagent for efficient regeneration of carbonyls from nitrogenous derivatives. Synth. Commun. 2002, 32, 3647–3651. [Google Scholar] [CrossRef]
- Lin, M.-H.; Hung, S.-F.; Lin, L.-Z.; Tsai, W.-S.; Chuang, T.-H. Tin mediated allylation reactions of enol ethers in water. Org. Lett. 2011, 13, 332–335. [Google Scholar] [CrossRef]
- Gremyachinskiy, D.E.; Smith, L.L.; Gross, P.H.; Samoshin, V.V. Facile synthesis of homoallylic alcohols from aldehyde acetals in water. Green Chem. 2002, 4, 317–318. [Google Scholar]
- Samoshin, V.V.; Gremyachinskiy, D.E.; Smith, L.L.; Bliznets, I.V.; Gross, P.H. Practical synthesis of bis-homoallylic alcohols from dialdehydes or their acetals. Tetrahedron Lett. 2002, 43, 6329–6330. [Google Scholar] [CrossRef]
- Juan, S.; Hua, Z.-H.; Qi, S.; Ji, S.-J.; Loh, T.-P. Indium trichloride-catalyzed indium-mediated allylation of dihydropyrans and dihydrofurans in water. Synlett 2004, 5, 829–830. [Google Scholar]
- Due to poor solubility of ketoximes in water, THF is added to increase the reaction rate. Based on screening results, amounts of SnCl2 and TiCl3 (1.5 equivalents each) are chosen as the general quantity for deoximation of various ketoximes.
- Houllemare, D.; Outurquin, F.; Paulmier, C. Synthesis of homoallylic (but-3-enylic) alcohols from aldehydes with allylic chlorides, tin(II) chloride and potassium iodide in water. J. Chem. Soc. Perkin Trans. 1 1997, 1629–1632. [Google Scholar]
- Lee, S.Y.; Lee, B.S.; Lee, C.-W.; Oh, D.Y. Synthesis of 4-oxo-2-alkenylphosphonates via nitrile oxide cycloaddition: γ-Acylation of allylic phosphonates. J. Org. Chem. 2000, 65, 256–257. [Google Scholar] [CrossRef]
- Timms, G.H.; Wildsmith, E. Reduction of oximes with tervalent titanium, a mild deoximation procedure and the partial synthesis of erythromycylamine. Tetrahedron Lett. 1971, 2, 195–198. [Google Scholar]
- Balicki, R.; Kaczmarek, Ł.; Malinowski, M. Facile reductive cleavage of oximes with a low-valent titanium reagent. Liebigs Ann. Chem. 1989, 1139–1140. [Google Scholar]
- McMurry, J.E. Organic chemistry of low-valent titanium. Acc. Chem. Res. 1974, 7, 281–286. [Google Scholar] [CrossRef]
- Ramón, R.S.; Bosson, J.; Díez-González, S.; Marion, N.; Nolan, S.P. Au/Ag-cocatalyzed aldoximes to amides rearrangement under solvent- and acid-free conditions. J. Org. Chem. 2010, 75, 1197–1202. [Google Scholar]
- Nobuta, T.; Hirashima, S.-I.; Tada, N.; Miura, T.; Itoh, A. One-pot metal-free syntheses of acetophenones from styrenes through aerobic photo-oxidation and deiodination with iodine. Org. Lett. 2011, 13, 2576–2579. [Google Scholar] [CrossRef]
- Bonvin, Y.; Callens, E.; Larrosa, I.; Henderson, D.A.; Oldham, J.; Burton, A.J.; Barrett, A.G.M. Bismuth-catalyzed benzylic oxidations with tert-butyl hydroperoxide. Org. Lett. 2005, 7, 4549–4552. [Google Scholar] [CrossRef]
- Ushkov, A.V.; Grushin, V.V. Rational catalysis design on the basis of mechanistic understanding: Highly efficient Pd-catalyzed cyanation of aryl bromides with NaCN in recyclable solvents. J. Am. Chem. Soc. 2011, 133, 10999–11005. [Google Scholar] [CrossRef]
- Ruan, J.; Iggo, J.A.; Berry, N.G.; Xiao, J. Hydrogen-bonding-promoted oxidative addition and regioselective arylation of olefins with aryl chlorides. J. Am. Chem. Soc. 2010, 132, 16689–16699. [Google Scholar]
- Sarvari, H.M.; Sharghi, H. Zinc oxide (ZnO) as a new, highly efficient, and reusable catalyst for acylation of alcohols, phenols and amines under solvent free conditions. Tetrahedron 2005, 61, 10903–10907. [Google Scholar] [CrossRef]
- Zhang, G.; Wen, X.; Wang, Y.; Mo, W.; Ding, C. Sodium nitrite catalyzed aerobic oxidative deoximation under mild conditions. J. Org. Chem. 2011, 76, 4665–4668. [Google Scholar] [CrossRef]
- Liu, Y.; Yao, B.; Deng, C.-L.; Tang, R.-Y.; Zhang, X.-G.; Li, J.-H. Palladium-catalyzed oxidative coupling of trialkylamines with aryl iodides leading to alkyl aryl ketones. Org. Lett. 2011, 13, 2184–2187. [Google Scholar] [CrossRef]
- Zhu, F.-X.; Wang, W.; Li, H.-X. Water-medium and solvent-free organic reactions over a bifunctional catalyst with Au nanoparticles covalently bonded to HS/SO3H functionalized periodic mesoporous organosilica. J. Am. Chem. Soc. 2011, 133, 11632–11640. [Google Scholar]
- Huffman, J.W. A new synthetic route to methoxytetralones. J. Org. Chem. 1959, 24, 1759–1763. [Google Scholar] [CrossRef]
- Shang, Y.; Wang, C.; He, X.; Ju, K.; Zhang, M.; Yu, S.; Wu, J. DMAP-catalyzed cascade reaction: one-pot synthesis of benzofurans in water. Tetrahedron 2010, 66, 9629–9633. [Google Scholar] [CrossRef]
- Fujita, K.-I.; Yoshida, T.; Imori, Y.; Yamaguchi, R. Dehydrogenative oxidation of primary and secondary alcohols catalyzed by a Cp*Ir complex having a functional C,N-chelate ligand. Org. Lett. 2011, 9, 2278–2281. [Google Scholar]
- Schultz, M.J.; Hamilton, S.S.; Jensen, D.R.; Sigman, M.S. Development and comparison of the substrate scope of Pd-catalysts for the aerobic oxidation of alcohols. J. Org. Chem. 2005, 70, 3343–3352. [Google Scholar]
- Shen, Z.; Dai, J.; Xiong, J.; He, X.; Mo, W.; Hu, B.; Sun, N.; Hu, X. 2,3-Dichloro-5,6-dicyano-1,4-benzoquinone (DDQ)/tert-butyl nitrite/oxygen: A versatile catalytic oxidation system. Adv. Synth. Catal. 2011, 353, 3031–3038. [Google Scholar] [CrossRef]
- Kompis, I.; Wick, A. Synthesis of 4-halo-substituted analogs of trimethoprim. Helv. Chim. Acta 1977, 60, 3025–3034. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).