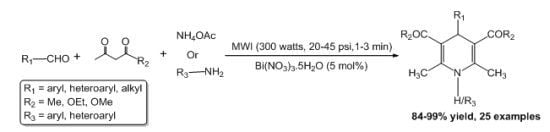
A Microwave-Assisted Bismuth Nitrate-Catalyzed Unique Route Toward 1,4-Dihydropyridines
Abstract
:1. Introduction

2. Results and Discussion
2.1. Results
| Entry | Bi-salt (10 mol%) | Yield (%) a |
|---|---|---|
| 1 | BiCl3 | 81 |
| 2 | Bi(OTf)3 | 79 |
| 3 | BiI3 | 72 |
| 4 | Bi5O(OH)9(NO3)3 | 67 |
| 5 | BiBr3 | 73 |
| 6 | Bi(NO3)3.5H2O | 96 |
| 7 | No catalyst | 52 |
| Entry | Bi (NO3)3.5H2O (mol%) | Yield (%) a |
|---|---|---|
| 1 | 30 | 88 |
| 2 | 25 | 87 |
| 3 | 20 | 93 |
| 4 | 15 | 91 |
| 5 | 10 | 96 |
| 6 | 5 | 99 |
| 7 | 2 | 86 |
| 8 | 1 | 73 |
| Entry | Solvent (1 mL) | Yield (%) a |
|---|---|---|
| 1 | Water | 91 |
| 2 | THF | 77 |
| 3 | Ethanol | 79 |
| 4 | Toluene | 62 |
| 5 | Methanol | 76 |
| 6 | Dichloromethane | 71 |
| 7 | DMSO | 76 |
| 8 | Neat | 99 |
2.2. Discussion
| Entry | Aldehyde | 1,3-Dicarbonyl | Ammonia | Product | Time | Yield (%) a |
|---|---|---|---|---|---|---|
| compounds | source | (min) | ||||
| 1 | 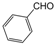 |  |  | 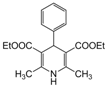 | 1 | 99 |
| 2 | 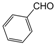 | 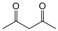 | 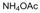 | 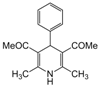 | 2.5 | 91 |
| 3 | 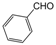 | 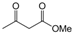 | 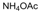 | 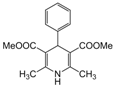 | 2 | 93 |
| 4 | 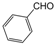 |  | 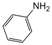 | 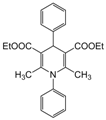 | 1.5 | 94 |
| 5 |  |  | 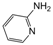 | 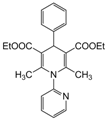 | 2.5 | 90 |
| 6 | 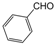 | 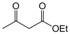 | 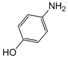 | 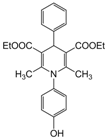 | 3 | 91 |
| 7 |  |  |  |  | 11.5 | 99 |
| 8 | 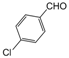 | 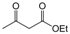 | 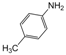 |  | 2 | 89 |
| 9 | 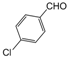 |  |  | 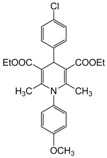 | 2.5 | 94 |
| 10 | 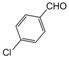 | 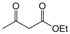 | 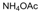 | 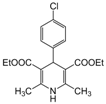 | 2 | 97 |
| 11 |  |  | 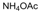 | 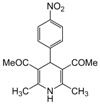 | 3 | 84 |
| 12 | 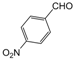 | 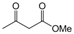 |  | 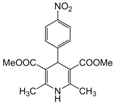 | 3 | 91 |
| 13 |  | 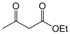 |  |  | 3 | 93 |
| 14 | 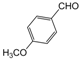 | 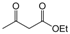 | 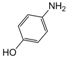 | 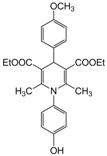 | 3 | 89 |
| 15 | 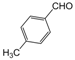 |  | 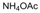 | 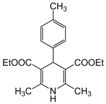 | 2.5 | 98 |
| 16 | 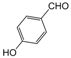 | 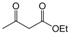 |  | 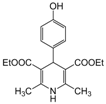 | 3 | 95 |
| 17 | 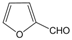 | 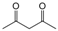 | 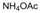 | 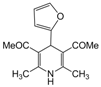 | 2 | 95 |
| 18 | 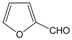 | 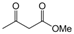 | 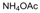 | 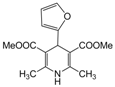 | 2 | 93 |
| 19 |  |  |  | 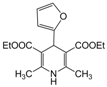 | 2 | 98 |
| 20 | 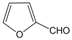 |  |  | 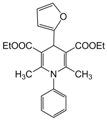 | 2.5 | 97 |
| 21 | 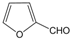 | 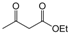 | 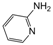 | 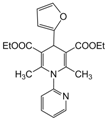 | 3 | 90 |
| 22 | 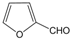 | 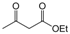 | 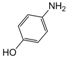 |  | 2.5 | 92 |
| 23 |  | 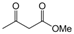 |  | 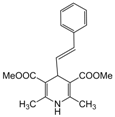 | 3 | 91 |
| 24 |  | 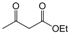 | 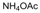 | 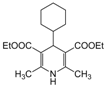 | 3 | 86 |
| 25 | 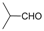 | 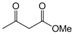 | 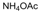 | 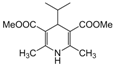 | 3 | 87 |
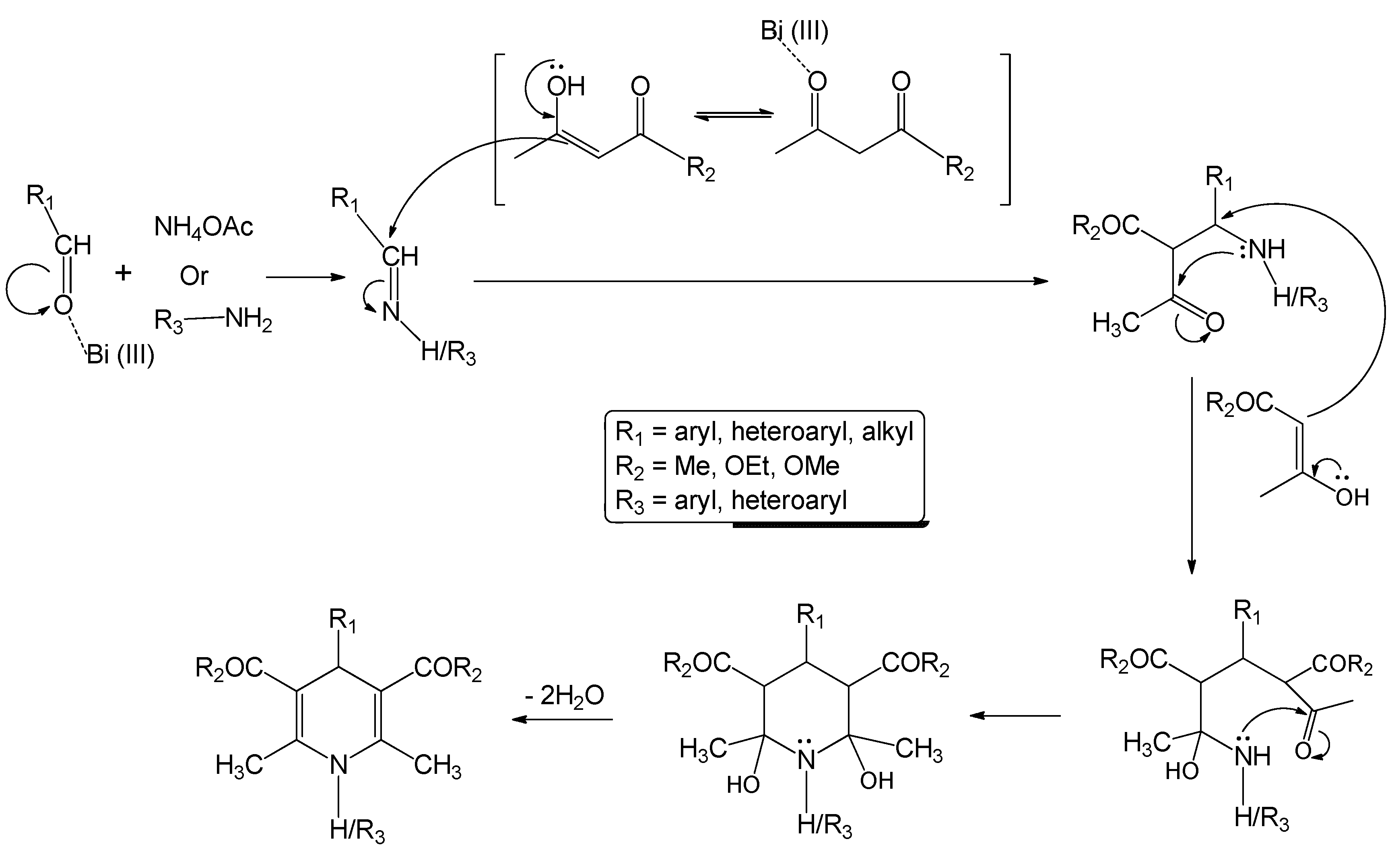
3. Experimental
3.1. General
3.2. General Procedure for the One-Pot, Three-Component Synthesis of 1,4-Dihydropyridines
4. Conclusions
Acknowledgments
References and Notes
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Ramón, D.J.; Yus, M. Asymmetric multicomponent reactions (AMCRs): The new frontier. Angew. Chem. Int. Ed. 2005, 44, 1602–1634. [Google Scholar] [CrossRef]
- Guillena, G.; Ramón, D.J.; Yus, M. Organocatalytic enantioselective multicomponent reactions (OEMCRs). Tetrahedron: Asymmetry 2007, 18, 693–700. [Google Scholar] [CrossRef]
- Isambert, N.; Duque, M.M.S.; Plaquevent, J.-C.; Genisson, Y.; Rodriguez, J.; Constantieux, T. Multicomponent reactions and ionic liquids: A perfect synergy for eco-compatible heterocyclic synthesis. Chem. Soc. Rev. 2007, 40, 1347–1357. [Google Scholar]
- Duque, M.M.S.; Allais, C.; Isambert, N.; Constantieux, T.; Rodriguez, J. β-Diketo building blocks for MCRs-based syntheses of heterocycles. Top. Heterocycl. Chem. 2010, 23, 227–277. [Google Scholar] [CrossRef]
- Bonne, D.; Coquerel, Y.; Constantieux, T.; Rodriguez, J. 1,3-Dicarbonyl compounds in stereoselective domino and multicomponent reactions. Tetrahedron: Asymmetry 2010, 21, 1085–1109. [Google Scholar] [CrossRef]
- Hantzsch, A. Condensationprodukte aus Aldehydammoniak und ketoniartigen Verbindungen. Chem. Ber. 1881, 14, 1637–1638. [Google Scholar] [CrossRef]
- The Merck Index, 12th ed; Merck Research Laboratories: Rahway, NJ, USA, 1996; p. 1121.
- Boström, S.L.; Ljung, B.; Mårdh, S.; Forsen, S.; Thulin, E. Interaction of the antihypertensive drug felodipine with cal-modulin. Nature 1981, 292, 777–778. [Google Scholar] [CrossRef]
- Iwanami, M.; Shibanuma, T.; Fujimoto, M.; Kawai, R.; Tamazawa, K.; Takenaka, T.; Takahashi, K.; Murakami, M. Synthesis of new water-soluble dihydropyridine vasodilatators. Chem. Pharm. Bull. 1979, 27, 1426–1440. [Google Scholar]
- Arrowsmith, J.E.; Campbell, S.F.; Cross, P.E.; Stubbs, J.K.; Burges, R.A.; Gardiner, D.G.; Blackburn, K.J. Long-acting dihydropyridine calcium antagonists. 1, 2-alkoxymethyl derivatives incorporating basic substituents. J. Med. Chem. 1986, 29, 1696–1702. [Google Scholar] [CrossRef]
- Harper, J.L.; Camerini-Otero, C.S.; Li, A.H.; Kim, S.A.; Jacobson, K.A.; Daly, J.W. Dihydropyridines as inhibitors of capacitative calcium entry in Leukemic HL-60 cells. Biochem. Pharmacol. 2003, 65, 329–338. [Google Scholar]
- Zarghi, A.; Sadeghi, H.; Fassihi, A.; Faizi, M.; Shafiee, A. Synthesis and calcium antagonist activity of 1,4-dihydropyridines containing phenylaminoimidazolyl substituents. Farmaco 2003, 58, 1077–1081. [Google Scholar] [CrossRef]
- Peri, R.; Padmanabhan, S.; Singh, S.; Rutledge, A.; Triggle, D.J. Permanently charged chiral 1,4-dihydropyridines: Molecular probes of L-type calcium channels. Synthesis and pharmacological characterization of methyl(-trimethylalkylammonium) 1,4-dihydro-2,6-dimethyl-4-(3-nitrophenyl)-3,5- pyridinedicarboxylate iodide, calcium channel antagonists. J. Med. Chem. 2000, 43, 2906–2914. [Google Scholar] [CrossRef]
- Berkels, B.; Roesen, R.; Dhein, S.; Fricke, U.; Klaus, W. Dihydropyridine calcium antagonist-induced modulation of endothelial function: A review. Cardiovasc. Drug Rev. 1999, 17, 179–186. [Google Scholar]
- Tsuruo, T.; Iida, H.; Nojiri, M.; Tsukagoshi, S.; Sakurai, Y. Circumvention of vincristine and adriamycin resistance in vitro and in vivo by calcium influx blockers. Cancer Res. 1983, 43, 2905–2910. [Google Scholar]
- Krauze, A.; Germane, S.; Eberlins, O.; Sturms, I.; Klusa, V.; Duburs, G. Derivatives of 3-cyano-6-phenyl-4-(3′-pyridyl)pyridine-2(1H)-thione and their neurotropic activity. Eur. J. Med. Chem. 1999, 34, 301–310. [Google Scholar] [CrossRef]
- Zhou, X.; Zhang, L.; Tseng, E.; Scott-Ramsay, E.; Schentag, J.J.; Coburn, R.A.; Morris, M.E. New 4-aryl-1,4-dihydropyridines and 4-arylpyridines as P-glycoprotein inhibitors. Drug Metab. Dispos. 2005, 33, 321–328. [Google Scholar]
- Chapman, R.W.; Danko, G.; Siegels, M.I. Effect of extra- and intracellular calcium blockers on histamine and antigen-induced bronchospasm in guinea pigs and rats. Pharmacology 1984, 29, 282–291. [Google Scholar] [CrossRef]
- Malaise, W.J.; Mathias, P.C.F. Stimulation of insulin release by organic calcium-agonist. Diabetologia 1985, 28, 153–156. [Google Scholar]
- Long, S.; Panunzio, M.; Petroli, A.; Qin, W.; Xia, Z. The use of magnesium nitride for the synthesis of enantiomerically pure 1,4-dihydropyridines via the Hantzsch reaction. Synthesis 2011, 7, 1071–1078. [Google Scholar]
- Vijesh, A.M.; Isloor, A.M.; Peethambar, S.K.; Shivananda, K.N.; Arulmoli, T.; Isloor, N.A. Hantzsch reaction. Synthesis and characterization of some new 1,4-dihydropyridine derivatives as potent antimicrobial and antioxidant agents. Eur. J. Med. Chem. 2011, 46, 5591–5597. [Google Scholar] [CrossRef]
- Saini, A.; Kumar, S.; Sandhu, J.S. Hantzsch reaction: Recent advances in Hantzsch 1,4-dihydropyridines. J. Sci. Ind. Res. 2008, 67, 95–111. [Google Scholar]
- Bhatti, R.S.; Krishan, P.; Suresh; Sandhu, J.S. Bismuth trichloride, a clean, green catalyst for the synthesis of Hantzsch 1,4- dihydropyridines (DHPs). J. Indian Chem. Soc. 2010, 87, 707–710. [Google Scholar]
- Kolvari, E.; Zolfigol, M.A.; Koukabi, N.; Shirmardi-Shaghasemi, B. A simple and efficient one-pot synthesis of Hantzsch 1,4-dihydropyridines using silica sulphuric acid as a heterogeneous and reusable catalyst under solvent-free conditions. Chem. Papers 2011, 65, 898–902. [Google Scholar] [CrossRef]
- Koukabi, N.; Kolvari, E.; Khazaei, A.; Zolfigol, M.A.; Shirmardi-Shaghasemi, B.; Khavasi, H.R. Hantzsch reaction on free nano-Fe2O3 catalyst: Excellent reactivity combined with facile catalyst recovery and recyclability. Chem. Commun. 2011, 47, 9230–9232. [Google Scholar]
- Ladani, N.K.; Mungra, D.C.; Patel, M.P.; Patel, R.G. Microwave-assisted synthesis of novel Hantzsch 1,4-dihydropyridines, acridine-1,8-diones and polyhydroquinolines bearing the tetrazolo[1,5-a]quinoline moiety and their antimicrobial activity assess. Chin. Chem. Lett. 2011, 22, 1407–1410. [Google Scholar] [CrossRef]
- Akbari, J.D.; Tala, S.D.; Dhaduk, M.F.; Joshi, H.S. Molecular iodine-catalyzed one-pot synthesis of some new Hantzsch 1,4-dihydropyridines at ambient temperature. ARKIVOC 2008, 12, 126–135. [Google Scholar]
- Sureshkumar, D.; Sandhu, J.S. New efficient protocol for the production of Hantzsch 1,4-dihydropyridines using RuCl3. Synth. Commun. 2009, 39, 1957–1965. [Google Scholar] [CrossRef]
- Yadav, D.K.; Patel, R.; Srivastava, V.P.; Watal, G.; Yadav, L.D.S. LiBr as an efficient catalyst for one-pot synthesis of Hantzsch 1,4-dihydropyridines under mild conditions. Chin. J. Chem. 2011, 29, 118–122. [Google Scholar] [CrossRef]
- Kumar, B.R.P.; Masih, P.; Lukose, C.R.; Abraham, N.; Priya, D.; Xavier, R.M.; Saji, K.; Adhikary, L. Facile, microwave-assisted parallel syntheses of N-substituted 1,4-dihydropyridines of biological interest. J. Heterocycl. Chem. 2009, 46, 336–339. [Google Scholar]
- Ghorbani-Choghamarani, A.; Zolfigol, M.A.; Hajjami, M.; Goudarziafshar, H.; Nikoorazm, M.; Yousefi, S.; Tahmasbi, B. Nano aluminium nitride as a solid source of ammonia for the preparation of Hantzsch 1,4-dihydropyridines and bis-(1,4-dihydropyridines) in water via one pot multicomponent reaction. J. Braz. Chem. Soc. 2011, 22, 525–531. [Google Scholar] [CrossRef]
- Lee, J.H. Synthesis of Hantsch 1,4-dihydropyridines by fermenting bakers’ yeast. Tetrahedron Lett. 2005, 46, 7329–7330. [Google Scholar] [CrossRef]
- Sathicq, A.G.; Romanelli, G.P.; Ponzinibbio, A.; Baronetti, G.T.; Thomas, H.J. An efficient one-step Hantzsch multicomponent synthesis of 1,4-dihydropyridines via a Wells-Dawson heteropolyacid catalyst under solvent-free conditions. Lett. Org. Chem. 2010, 7, 511–518. [Google Scholar] [CrossRef]
- Chari, M.A.; Syamasundar, K. Silica gel/NaHSO4 catalyzed one-pot synthesis of Hantzsch 1,4-dihydropyridines at ambient temperature. Catal. Commun. 2005, 6, 624–626. [Google Scholar] [CrossRef]
- Heydari, A.; Khaksar, S.; Tajbakhsh, M.; Bijanzadeh, H.R. One-step, synthesis of Hantzsch esters and polyhydroquinoline derivatives in fluoro alcohols. J. Fluorine Chem. 2009, 130, 609–614. [Google Scholar] [CrossRef]
- Legeay, J.-C.; Eyndeb, J.J.V.; Bazureaua, J.P. Ionic liquid phase technology supported the three component synthesis of Hantzsch 1,4-dihydropyridines and Biginelli 3,4-dihydropyrimidin-2(1H)-ones under microwave dielectric heating. Tetrahedron 2005, 61, 12386–12397. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Banik, B.K. Microwave-induced stereoselectivity of β-lactam formation with dihydrophenanthrenyl imines via Staudinger cycloaddition. Helv. Chim. Acta 2010, 93, 298–301. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Yañez, M.; Banik, B.K. Microwave-induced stereoselectivity of β-lactam formation: effects of solvents. Heterocycl. Lett. 2011, 1, 65–67. [Google Scholar]
- Bandyopadhyay, D.; Rivera, G.; Salinas, I.; Aguilar, H.; Banik, B.K. Iodine-catalyzed remarkable synthesis of novel N-polyaromatic β-lactams bearing pyrroles. Molecules 2010, 15, 1082–1088. [Google Scholar]
- Bandyopadhyay, D.; Mukherjee, S.; Banik, B.K. An expeditious synthesis of N-substituted pyrroles via microwave-induced iodine-catalyzed reaction under solventless conditions. Molecules 2010, 15, 2520–2525. [Google Scholar] [CrossRef]
- Andoh-Baidoo, R.; Danso, R.; Mukherjee, S.; Bandyopadhyay, D.; Banik, B.K. Microwave-induced N-bromosuccinimide-mediated novel synthesis of pyrroles via Paal-Knorr reaction. Heterocycl. Lett. 2011, 1, 107–109. [Google Scholar]
- Bandyopadhyay, D.; Banik, A.; Bhatta, S.; Banik, B.K. Microwave-assisted ruthenium trichloride catalyzed synthesis of pyrroles fused with indole systems. Heterocycl. Commun. 2009, 15, 121–122. [Google Scholar]
- Abrego, D.; Bandyopadhyay, D.; Banik, B.K. Microwave-induced indium-catalyzed synthesis of pyrrole fused with indolinone in water. Heterocycl. Lett. 2011, 1, 94–95. [Google Scholar]
- Kall, A.; Bandyopadhyay, D.; Banik, B.K. Microwave-induced aza-Michael reaction in water: A remarkable simple procedure. Synth. Commun. 2010, 42, 1730–1735. [Google Scholar]
- Bandyopadhyay, D.; Mukherjee, S.; Rodriguez, R.R.; Banik, B.K. An effective microwave-induced iodine-catalyzed method for the synthesis of quinoxalines via condensation of 1,2-dicarbonyl compounds. Molecules 2010, 15, 4207–4212. [Google Scholar] [CrossRef]
- Bothwell, J.M.; Krabbe, S.W.; Mohan, R.S. Applications of bismuth(iii) compounds in organic synthesis. Chem. Soc. Rev. 2011, 40, 4649–4707. [Google Scholar] [CrossRef]
- Hua, R. Recent advances in bismuth-catalyzed organic synthesis. Curr. Org. Synth. 2008, 1, 1–27. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Silvestre, S.M. Recent advances of bismuth(III) salts in organic chemistry: Application to the synthesis of aliphatics, alicyclics, aromatics, amino acids and peptides, terpenes and steroids of pharmaceutical interest. Mini Rev. Org. Chem. 2009, 6, 241–274. [Google Scholar] [CrossRef]
- Salvador, J.A.R.; Pinto, R.M.A.; Silvestre, S.M. Recent advances of bismuth(III) salts in organic chemistry: Application to the synthesis of heterocycles of pharmaceutical interest. Curr. Org. Synth. 2009, 6, 426–470. [Google Scholar] [CrossRef]
- Canales, L.; Bandyopadhyay, D.; Banik, B.K. Bismuth nitrate pentahydrate-induced novel nitration of Eugenol. Org. Med. Chem. Lett. 2011, 1, 9. [Google Scholar] [CrossRef]
- Banik, B.K.; Samajdar, S.; Banik, I.; Ng, S.; Hann, J. Montmorillonite impregnated with bismuth nitrate: Microwave-assisted facile nitration of β-lactams. Heterocycles 2003, 61, 97–100. [Google Scholar] [CrossRef]
- Bose, A.; Sanjoto, W.P.; Villarreal, S.; Aguilar, H.; Banik, B.K. Novel nitration of estrone by metal nitrates. Tetrahedron Lett. 2007, 48, 3945–3947. [Google Scholar]
- Srivastava, N.; Banik, B.K. Bismuth nitrate-catalyzed versatile Michael reactions. J. Org. Chem. 2003, 68, 2109–2114. [Google Scholar] [CrossRef]
- Srivastava, N.; Dasgupta, S.K.; Banik, B.K. A remarkable bismuth nitrate-catalyzed protection of carbonyl compounds. Tetrahedron Lett. 2003, 44, 1191–1193. [Google Scholar]
- Banik, B.K.; Adler, D.; Nguyen, P.; Srivastava, N. A new bismuth nitrate-induced stereospecific glycosylation of alcohols. Heterocycles 2003, 61, 101–104. [Google Scholar] [CrossRef]
- Rivera, S.; Bandyopadhyay, D.; Banik, B.K. Facile synthesis of N-substituted pyrroles via microwave-induced bismuth nitrate-catalyzed reaction under solventless conditions. Tetrahedron Lett. 2009, 50, 5445–5448. [Google Scholar]
- Bandyopadhyay, D.; Fonseca, R.S.; Banik, B.K. Microwave-induced bismuth nitrate-mediated selective hydrolysis of amide. Heterocycl. Lett. 2011, 1, 75–77. [Google Scholar]
- Iglesias, L.; Aguilar, C.; Bandyopadhyay, D.; Banik, B.K. A new bismuth nitrate-catalyzed electrophilic substitution of indoles with carbonyl compounds under solventless conditions. Synth. Commun. 2010, 40, 3678–3682. [Google Scholar] [CrossRef]
- Rivera, S.; Bandyopadhyay, D.; Banik, B.K. Microwave-induced bismuth nitrate-catalyzed electrophilic substitution of 7-aza indole with activated carbonyl compound under solvent-free conditions. Heterocycl. Lett. 2011, 1, 43–46. [Google Scholar]
- Banik, A.; Bhatta, S.; Bandyopadhyay, D.; Banik, B.K. A highly efficient bismuth salts-catalyzed route for the synthesis of α-aminophosphonates. Molecules 2010, 15, 8205–8213. [Google Scholar] [CrossRef]
- Banik, B.K.; Reddy, A.T.; Datta, A.; Mukhopadhyay, C. Microwave-induced bismuth nitrate-catalyzed synthesis of dihydropyrimidones via Biginelli condensation under solventless conditions. Tetrahedron Lett. 2007, 48, 7392–7394. [Google Scholar]
- Tajbakhsh, M.; Heravi, M.M.; Hosseini, A.; Shahrezaiee, A. Old reagent, new results: Aromatization of Hantzsch 1,4-dihydropyridines with supported bismuth nitrate under microwave irradiation in solventless system. Phosphor. Sulfur Silicon 2003, 178, 773–776. [Google Scholar] [CrossRef]
- Becker, F.F.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: Synthesis and biological evaluation of some chrysene derivatives. Bioorg. Med. Chem. Lett. 1998, 8, 2877–2880. [Google Scholar] [CrossRef]
- Becker, F.F.; Mukhopadhyay, C.; Hackfeld, L.; Banik, I.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: synthesis and biological evaluation of dibenzofluorene derivatives. Bioorg. Med. Chem. 2000, 8, 2693–2699. [Google Scholar] [CrossRef]
- Banik, B.K.; Becker, F.F. Polycyclic aromatic compounds as anticancer agents. 4. Structure-activity relationships of chrysene and pyrene derivatives. Bioorg. Med. Chem. 2001, 9, 593–605. [Google Scholar] [CrossRef]
- Banik, B.K.; Becker, F.F. Synthesis, electrophilic substitution and structure-activity relationship studies of polycyclic aromatic compounds towards the development of anticancer agents. Curr. Med. Chem. 2001, 8, 1513–1533. [Google Scholar]
- Banik, B.K.; Becker, F.F.; Banik, I. Synthesis of anticancer β-lactams: Mechanism of action. Bioorg. Med. Chem. 2004, 12, 2523–2528. [Google Scholar] [CrossRef]
- Banik, I.; Becker, F.F.; Banik, B.K. Stereoselective synthesis of β-lactams with polyaromatic imines: Entry to new and novel anticancer agents. J. Med. Chem. 2003, 46, 12–15. [Google Scholar] [CrossRef]
- Bandyopadhyay, D.; Granados, J.C.; Short, J.D.; Banik, B.K. Polycyclic aromatic compounds as anticancer agents: evaluation of synthesis and in vitro cytotoxicity. Oncol. Lett. 2012, 3, 45–49. [Google Scholar]
- Blackwell, H.E. Out of the oil bath and into the oven—Microwave-assisted combinatorial chemistry heats up. Org. Biomol. Chem. 2003, 1, 1251–1255. [Google Scholar] [CrossRef]
- Kidwai, M. Green chemistry trends toward sustainability. Pure Appl. Chem. 2006, 78, 1983–1992. [Google Scholar] [CrossRef]
- Kranjc, K.; Kočevar, M. Microwave-assisted organic synthesis: General considerations and transformations of heterocyclic compounds. Curr. Org. Chem. 2010, 14, 1050–1074. [Google Scholar]
- Martins, M.A.P.; Frizzo, C.P.; Moreira, D.N.; Buriol, L.; Machado, P. Solvent-free heterocyclic synthesis. Chem. Rev. 2009, 109, 4140–4182. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the all compounds (mg quantity) are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bandyopadhyay, D.; Maldonado, S.; Banik, B.K. A Microwave-Assisted Bismuth Nitrate-Catalyzed Unique Route Toward 1,4-Dihydropyridines. Molecules 2012, 17, 2643-2662. https://doi.org/10.3390/molecules17032643
Bandyopadhyay D, Maldonado S, Banik BK. A Microwave-Assisted Bismuth Nitrate-Catalyzed Unique Route Toward 1,4-Dihydropyridines. Molecules. 2012; 17(3):2643-2662. https://doi.org/10.3390/molecules17032643
Chicago/Turabian StyleBandyopadhyay, Debasish, Stephanie Maldonado, and Bimal K. Banik. 2012. "A Microwave-Assisted Bismuth Nitrate-Catalyzed Unique Route Toward 1,4-Dihydropyridines" Molecules 17, no. 3: 2643-2662. https://doi.org/10.3390/molecules17032643
APA StyleBandyopadhyay, D., Maldonado, S., & Banik, B. K. (2012). A Microwave-Assisted Bismuth Nitrate-Catalyzed Unique Route Toward 1,4-Dihydropyridines. Molecules, 17(3), 2643-2662. https://doi.org/10.3390/molecules17032643