Synthesis and Antimicrobial Activity of N′-Heteroarylidene-1-adamantylcarbohydrazides and (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines
Abstract
:1. Introduction
2. Results and Discussion
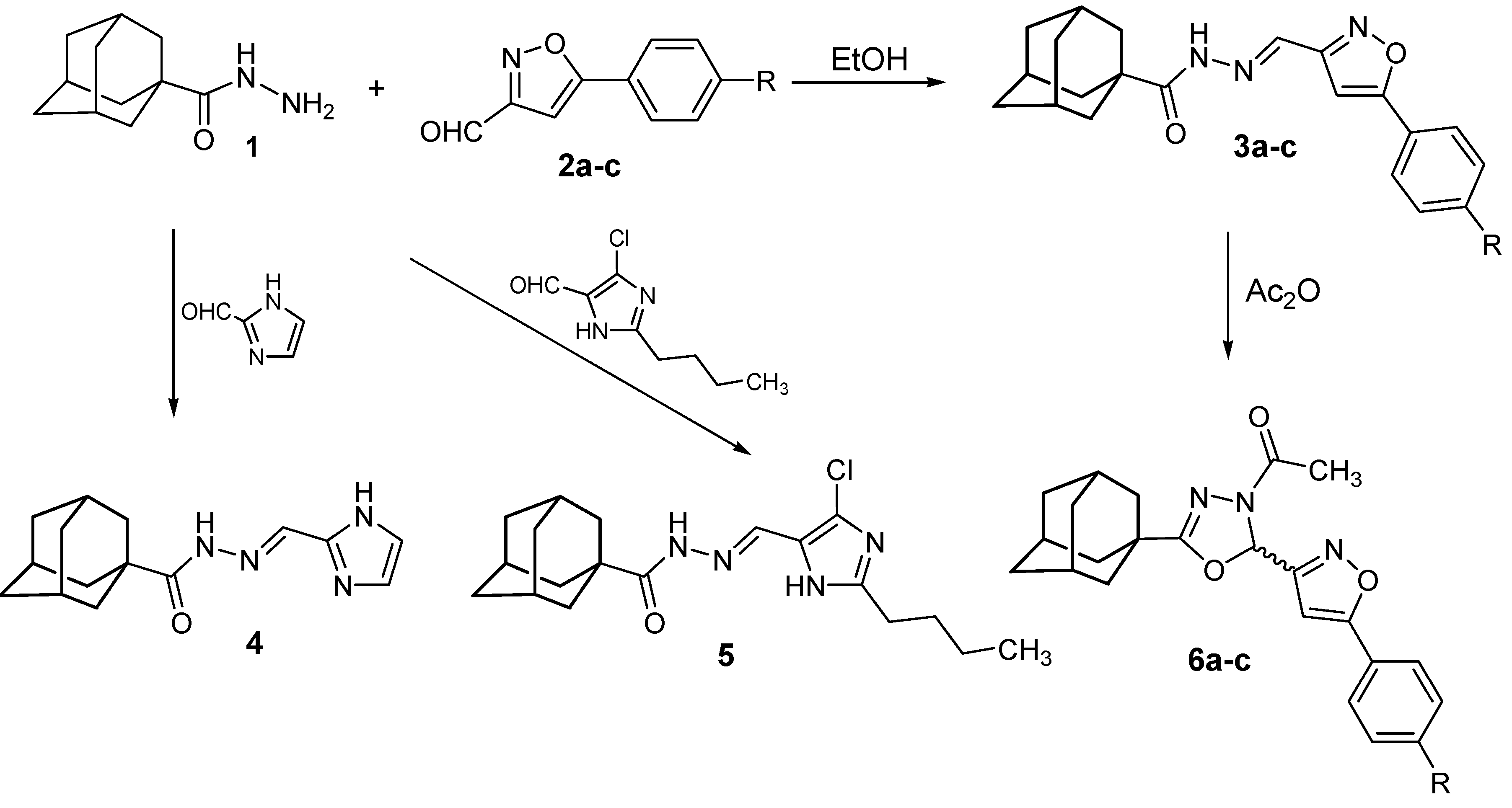
2.1. Chemistry
2.2. Antimicrobial Testing
| Comp. No. | Clog P | Diameter of Growth Inhibition Zone (mm) * | |||||
|---|---|---|---|---|---|---|---|
| SA | BS | ML | EC | PA | C A | ||
| 3a | 4.96 | 19(16) † | 22(8) † | 17 | 11 | - | - |
| 3b | 4.74 | 18(16) † | 18(16) † | 14 | - | - | - |
| 3c | 4.24 | 17 | 18(16) † | 13 | - | - | - |
| 4 | 2.30 | 32(0.5) † | 26(0.5) † | 23(8) † | 16 | 11 | 17 |
| 5 | 3.89 | 28(1) † | 30(1) † | 22(4) † | 19(8) † | 14 | 16 |
| 6a | 5.20 | 12 | 11 | 11 | - | - | - |
| 6b | 4.95 | - | - | - | - | - | - |
| 6c | 4.48 | - | - | - | - | - | - |
| Gentamicin | 26(2) † | 25(2) † | 18(2) † | 20(0.5) † | 19(1) † | NT | |
| Ampicillin | 23(2) † | 21(0.5) † | 19(2) † | 17(2) † | 16(2) † | NT | |
| Clotrimazole | NT | NT | NT | NT | NT | 21 | |
3. Experimental
3.1. General
3.2. N′-Heteroarylidene-1-adamantylcarbohydrazides 3a–c, 4 and 5
3.3. (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines 6a–c
3.4. Determination of the Antimicrobial Activity by the Agar Disc-Diffusion Method [30]
3.5. Determination of Minimal Inhibitory Concentrations (MICs) [31]
4. Conclusions
Acknowledgements
- Sample Availability: Contact the corresponding author.
References
- Lamoureux, G.; Artavia, G. Use of the adamantane structure in medicinal chemistry. Curr. Med. Chem. 2010, 17, 2967–2978. [Google Scholar] [CrossRef]
- Liu, J.; Obando, D.; Liao, V.; Lifa, T.; Codd, R. The many faces of the adamantyl group in drug design. Eur. J. Med. Chem. 2011, 46, 1949–1963. [Google Scholar] [CrossRef]
- Davies, W.L.; Grunnert, R.R.; Haff, R.F.; McGahen, J.W.; Neumeyer, E.M.; Paulshock, M.; Watts, J.C.; Wood, T.R.; Hermann, E.C.; Hoffmann, C.E. Antiviral activity of 1-adamantamine (amantadine). Science 1964, 144, 862–863. [Google Scholar]
- Vernier, V.G.; Harmon, J.B.; Stump, J.M.; Lynes, T.L.; Marvel, M.P.; Smith, D.H. The toxicologic and pharmacologic properties of amantadine hydrochloride. Toxicol. Appl. Pharmacol. 1969, 15, 642–665. [Google Scholar] [CrossRef]
- Togo, Y.; Hornick, R.B.; Dawkins, A.T. Studies on induced influenza in man. I. Double blind studies designed to assess prophylactic efficacy of amantadine hydrochloride against A2/Rockville/1/65 strain. J. Am. Med. Assoc. 1968, 203, 1089–1094. [Google Scholar]
- Zoidis, G.; Kolocouris, N.; Naesens, E.; De Clercq, E. Design and synthesis of 1,2-annulated adamantane piperidines with anti-influenza virus activity. Bioorg. Med. Chem. 2009, 17, 1534–1541. [Google Scholar] [CrossRef]
- Van Derpoorten, K.; Balzarini, J.; De Clercq, E.; Poupaert, J.H. Anti-HIV activity of N-1-adamantyl-4-aminophthalimide. Biomed. Pharmacother. 1997, 51, 464–468. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Al-Deeb, O.A.; Al-Omar, M.A.; Lehmann, J. Synthesis, antimicrobial, and anti-HIV-1 activity of certain 5-(1-adamantyl)-2-substituted thio-1,3,4-oxadiazoles and 5-(1-adamantyl)-3-substituted aminomethyl-1,3,4-oxadiazoline-2-thione. Bioorg. Med. Chem. 2004, 12, 5107–5113. [Google Scholar] [CrossRef]
- Balzarini, J.; Orzeszko, B.; Mauri, J.K.; Orzeszko, A. Synthesis and anti-HIV studies of 2-adamantyl-substituted thiazolidin-4-ones. Eur. J. Med. Chem. 2007, 42, 993–1003. [Google Scholar] [CrossRef]
- 10. Abou-Gharbia, M.A.; Childers, W.E., Jr.; Fletcher, H.; McGaughey, G.; Patel, U.; Webb, M.B.; Yardley, J.; Andree, T.; Boast, C.; Kucharik, R.J., Jr.; et al Synthesis and SAR of adatanserin: novel adamantly aryl- and heteroarylpiperazines with dual serotonin 5-HT1A and 5-HT2 activity as potential anxiolytic and antidepressant agents. J. Med. Chem. 1999, 42, 5077–5094. [Google Scholar] [CrossRef]
- Owen, J.C.E.; Whitton, P.S. Effect of amantadine and budipune on antidepressant drug-evoked changes in extracellular dopamine in the frontal cortex of freely moving rats. Brain Res. 2006, 1117, 206–212. [Google Scholar]
- Omar, K.; Geronikaki, A.; Zoumpoulakis, P.; Camoutsis, C.; Soković, M.; Ćirić, A.; Glamoćlija, J. Novel 4-thiazolidinone derivatives as potential antifungal and antibacterial drugs. Bioorg. Med. Chem. 2010, 18, 426–432. [Google Scholar]
- Orzeszko, A.; Kamińska, B.; Starościak, B.J. Synthesis and antimicrobial activity of new adamantane derivatives III. Il Farmaco 2002, 57, 619–624. [Google Scholar] [CrossRef]
- 14. Al-Deeb, O.A.; Al-Omar, M.A.; El-Brollosy, N.R.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]acetic acids, 2-[3-(1-adamantyl)-4-substituted-5-thioxo-1,2,4-triazolin-1-yl]propionic acids and related derivatives. Arzneim.-Forsch./Drug Res. 2006, 56, 40–47. [Google Scholar]
- Kadi, A.A.; El-Brollosy, N.R.; Al-Deeb, O.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 2-(1-adamantyl)-5-substituted-1,3,4-oxadiazoles and 2-(1-adamantylamino)-5-substituted-1,3,4-thiadiazole. Eur. J. Med. Chem. 2007, 42, 235–242. [Google Scholar] [CrossRef]
- Al-Omar, M.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial, and anti-inflammatory activities of novel 5-(1-adamantyl)-4-arylideneamino-3-mercapto-1,2,4-triazoles and related derivative. Molecules 2010, 15, 2526–2550. [Google Scholar] [CrossRef]
- Mamolo, M.G.; Zampieri, D.; Vio, L.; Fermeglia, M.; Ferrone, M.; Pricl, S.; Scialino, G.; Banfi, E. Antimycobacterial activity of new 3-substituted 5-(pyridin-4-yl)-3H-1,3,4-oxadiazol-2-one and 2-thione derivatives. Preliminary molecular modeling investigations. Bioorg. Med. Chem. 2005, 13, 3797–3809. [Google Scholar] [CrossRef]
- Macaev, F.; Rusu, G.; Bogrebnoi, S.; Gudima, A.; Stingaci, E.; Vlad, L.; Shvets, N.; Kandemirli, F.; Dimoglo, A.; Reynolds, R. Synthesis of novel 5-aryl-2-thio-1,3,4-oxadiazoles and the study of their structure-anti-mycobacterial activities. Bioorg. Med. Chem. 2005, 13, 4842–4850. [Google Scholar] [CrossRef]
- Andrzejak, V.; Muccioli, G.G.; Body-Malapel, M.; El Bakali, J.; Djouina, M.; Renault, N.; Chavatte, P.; Desreumaux, P.; Lambert, D.M.; Millet, R. New FAAH inhibitors based on 3-carboxamido-5-aryl-isoxazole scaffold that protect against experimental colitis. Bioorg. Med. Chem. 2011, 19, 3777–3786. [Google Scholar]
- Changtam, C.; Hongmanee, P.; Suksamrarn, A. Isoxazole analogs of curcuminoids with highly potent multidrug-resistant antimycobacterial activity. Eur. J. Med. Chem. 2010, 45, 4446–4457. [Google Scholar] [CrossRef]
- El-Emam, A.A.; Ibrahim, T.M. Synthesis, anti-inflammatory and analgesic activity of certain 3-(1-adamantyl)-4-substituted-5-mercapto-1,2,4-triazole derivatives. Arzneim.-Forsch./Drug Res. 1991, 41, 1260–1264. [Google Scholar]
- Kadi, A.A.; Al-Abdullah, E.S.; Shehata, I.A.; Habib, E.E.; Ibrahim, T.M.; El-Emam, A.A. Synthesis, antimicrobial and anti-inflammatory activities of novel 5-(1-adamantyl)-1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5006–5011. [Google Scholar] [CrossRef]
- Al-Abdullah, E.S.; Shehata, I.A.; Al-Deeb, O.A.; El-Emam, A.A. Microwave-assisted dehydrosulphurization: An efficient, solvent-free synthesis of 5-(1-adamantyl)-2-arylamino-1,2,4-triazolo[3,4-b][1,3,4]thiadiazoles. Heterocycles 2007, 71, 379–388. [Google Scholar] [CrossRef]
- Ficarra, R.; Ficarra, P.; Tommasini, A.; Fenech, G.; Pizzimenti, F.C.; Bisignano, G. 1-Adamantanecarboxylic acid hydrazides with presumed antimicrobial activity. Boll. Chim. Farmaceutico 1984, 123, 317–321. [Google Scholar]
- Hassan, G.S.; El-Emam, A.A.; Gad, L.M.; Barghash, A.M. Synthesis, antimicrobial and antiviral testing of some new 1-adamantyl analogues. Saudi Pharm. J. 2010, 18, 123–128. [Google Scholar] [CrossRef]
- Ishii, M.; Jorge, S.D.; de Oliveira, A.A.; Palace-Berl, F.; Sonehara, I.Y.; Pasqualoto, K.F.M.; Tavares, L.C. Synthesis, molecular modeling and preliminary biological evaluation of a set of 3-acetyl-2,5-disubstituted-2,3-dihydro-1,3,4-oxadiazole as potential antibacterial, anti-Trypanosomacruzi and antifungal agent. Bioorg. Med. Chem. 2011, 19, 6292–6301. [Google Scholar] [CrossRef]
- Hamdi, H.; Passarelli, V.; Romerosa, A. Synthesis, spectroscopy and electrochemistry of new 4-(4-acetyl-5-substituted-4,5-dihydro-1,3,4-oxodiazol-2-yl)methoxy)-2H-chromen-2-ones as a novel class of potential antibacterial and antioxidant derivatives. Compt. Rend. Chim. 2011, 14, 548–555. [Google Scholar]
- Joshi, S.D.; Vagdevi, H.M.; Vaidya, V.P.; Gadaginamath, G.S. Synthesis of new 4-pyrrol-1-yl benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: A novel class of potential antibacterial and antitubercular agents. Eur. J. Med. Chem. 2008, 43, 1989–1996. [Google Scholar] [CrossRef]
- Al-Tamimi, A.-M.S.; Bari, A.; Al-Omar, M.A.; El-Emam, A.A.; Ng, S.W. N’-[(2-n-Butyl-4-chloro-1H-imidazol-5-yl)methylidene]adamantane-1-carbohydrazide sesquihydrate ethanol hemisolvate. Acta Cryst. 2010, E66, o2131. [Google Scholar]
- Murray, P.R.; Baron, E.J.; Pfaller, M.A.; Tenover, F.C.; Yolken, R.H. Manual of Clinical Microbiology; Wood, G.L., Washington, J.A., Eds.; American Society for Microbiology: Washington, DC, USA, 1995. [Google Scholar]
- National Committee for Clinical Laboratory Standards (NCCLS). Approved standard document M7A ; NCCLS: Villanova, PA, USA, 1985.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
El-Emam, A.A.; Alrashood, K.A.; Al-Omar, M.A.; Al-Tamimi, A.-M.S. Synthesis and Antimicrobial Activity of N′-Heteroarylidene-1-adamantylcarbohydrazides and (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules 2012, 17, 3475-3483. https://doi.org/10.3390/molecules17033475
El-Emam AA, Alrashood KA, Al-Omar MA, Al-Tamimi A-MS. Synthesis and Antimicrobial Activity of N′-Heteroarylidene-1-adamantylcarbohydrazides and (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules. 2012; 17(3):3475-3483. https://doi.org/10.3390/molecules17033475
Chicago/Turabian StyleEl-Emam, Ali A., Khalid A. Alrashood, Mohamed A. Al-Omar, and Abdul-Malek S. Al-Tamimi. 2012. "Synthesis and Antimicrobial Activity of N′-Heteroarylidene-1-adamantylcarbohydrazides and (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines" Molecules 17, no. 3: 3475-3483. https://doi.org/10.3390/molecules17033475
APA StyleEl-Emam, A. A., Alrashood, K. A., Al-Omar, M. A., & Al-Tamimi, A.-M. S. (2012). Synthesis and Antimicrobial Activity of N′-Heteroarylidene-1-adamantylcarbohydrazides and (±)-2-(1-Adamantyl)-4-acetyl-5-[5-(4-substituted phenyl-3-isoxazolyl)]-1,3,4-oxadiazolines. Molecules, 17(3), 3475-3483. https://doi.org/10.3390/molecules17033475




