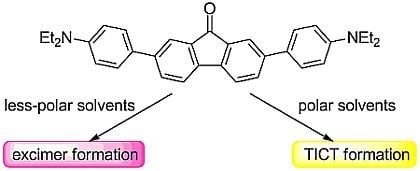
Selective Formation of Twisted Intramolecular Charge Transfer and Excimer Emissions on 2,7-bis(4-Diethylaminophenyl)-fluorenone by Choice of Solvent
Abstract
:1. Introduction
2. Results and Discussion




| Solvent (line color) | λabs/nm | λem/nm | ENT b | ||
|---|---|---|---|---|---|
| LE | TICT | excimer | |||
| MeCN (―) | 307, 369, 529 | 409 | - a | - | 0.460 |
DMF (  ) ) | 310, 372, 520 | 419 | 450 | - | 0.386 |
CHCl3 (  ) ) | 310, 366, 503 | 413 | 442 | - | 0.259 |
THF (  ) ) | 307, 371, 522 | 416 | 436 | 644 | 0.207 |
toluene (  ) ) | 308, 371, 496 | 417 | - | 619 | 0.099 |
3. Experimental
3.1. Instruments
3.2. Materials: Synthesis of 2,7-(4-Diethylaminophenyl)-fluorenone
4. Conclusions
Supplementary Materials
- Sample Availability: Samples of the compounds are available from G.K.
References and Notes
- Grabowski, Z.R.; Rotkiewicz, K.; Rettig, W. Structural changes accompanying intramolecular electron transfer: Focus on twisted intramolecular charge-transfer states and structures. Chem. Rev. 2003, 103, 3899–4032. [Google Scholar] [CrossRef]
- Rettig, W. Charge separation in excited states of decoupled systems—TICT compounds and implications regarding the development of new laser dyes and the primary process of vision and photosynthesis. Angew. Chem. Int. Ed. Engl. 1986, 25, 971–988. [Google Scholar] [CrossRef]
- Biczók, L.; Bérces, T.; Inoue, H. Effects of molecular structure and hydrogen bonding on the radiationless deactivation of singlet excited fluorenone derivatives. J. Phys. Chem. A 1999, 103, 3837–3842. [Google Scholar] [CrossRef]
- Bhattacharyya, K.; Chowdhury, M. Environmental and magnetic field effects on exciplex and twisted charge transfer emission. Chem. Rev. 1993, 93, 507–535. [Google Scholar] [CrossRef]
- Schuddeboom, W.; Jonker, S.A.; Warman, J.M.; Leinhos, U.; Kuehnle, W.; Zachariasse, K.A. Excited-state dipole moments of dual fluorescent 4-(dialkylamino)benzonitriles: Influence of alkyl chain length and effective solvent polarity. J. Phys. Chem. 1992, 96, 10809–10819. [Google Scholar] [CrossRef]
- Amdursky, N.; Gepshtein, R.; Erez, Y.; Huppert, D. Temperature dependence of the fluorescence properties of thioflavin-T in propanol, a glass-forming liquid. J. Phys. Chem. A 2011, 115, 2540–2548. [Google Scholar] [CrossRef]
- Gazi, H.A.R.; Biswas, R. Heterogeneity in binary mixtures of (water + tertiary butanol): Temperature dependence across mixture composition. J. Phys. Chem. A 2011, 115, 2447–2455. [Google Scholar]
- Thayer, M.P.; McGuire, C.; Stennett, E.M.S.; Lockhart, M.K.; Canache, D.; Novak, M.; Schmidtke, S.J. pH-Dependent spectral properties of para-aminobenzoic acid and its derivatives. Spectrochim. Acta A 2011, 84, 227–232. [Google Scholar] [CrossRef]
- Zhang, C.H.; Chena, Z.B.; Jiang., Y.B. Intramolecular charge transfer dual fluorescence of p-dimethylaminobenzoates. Spectrochim. Acta A 2004, 60, 2729–2732. [Google Scholar] [CrossRef]
- Hagfeldt, A.; Boschloo, G.; Sun, L.; Kloo, L.; Pettersson, H. Dye-sensitized solar cells. Chem. Rev. 2010, 110, 6595–6663. [Google Scholar] [CrossRef]
- Mishra, A.; Fischer, M.K.R.; Bäuerle, P. Metal-free organic dyes for dye-sensitized solar cells: From structure: Property relationships to design rules. Angew. Chem. Int. Ed. Engl. 2009, 48, 2474–2499. [Google Scholar] [CrossRef]
- Chang, Y.J.; Watanabe, M.; Chou, P.; Chow, T.J. [2.2]Paracyclophane as a bridging unit in the design of organic dyes for sensitized solar cells. Chem. Commun. 2012, 48, 726–728. [Google Scholar]
- Kulkarni, A.P.; Kong, X.; Jenekhe, S.A. High-performance organic light-emitting diodes based on intramolecular charge-transfer emission from donor-acceptor molecules: Significance of electron-donor strength and molecular geometry. Adv. Funct. Mater. 2006, 16, 1057–1066. [Google Scholar] [CrossRef]
- Cravino, A.; Leriche, P.; Alévêque, O.; Roquet, S.; Roncali, J. Light-emitting organic solar cells based on a 3D conjugated system with internal charge transfer. Adv. Mater. 2006, 18, 3033–3037. [Google Scholar] [CrossRef] [Green Version]
- Morozumi, T.; Anada, T.; Nakamura, H. New fluorescent “Off-On” behavior of 9-anthryl aromatic amides through controlling the twisted intramolecular charge transfer relaxation process by complexation with metal ions. J. Phys. Chem. B 2001, 105, 2923–2931. [Google Scholar] [CrossRef]
- Wu, J.; Liu, W.; Ge, J.; Zhang, H.; Wang, P. New sensing mechanisms for design of fluorescent chemosensors emerging in recent years. Chem. Soc. Rev. 2011, 40, 3483–3495. [Google Scholar] [CrossRef]
- Hall, M.J.; Allen, L.T.; O’Shea, D.F. PET modulated fluorescent sensing from the BF2 chelated azadipyrromethene platform. Org. Biomol. Chem. 2006, 4, 776–780. [Google Scholar] [CrossRef]
- Choi, L. Triple fluorescence of 4-(1,4,8,11-tetraazacyclotetradecyl)benzonitrile. Chem. Commun. 1998, 1998, 893–894. [Google Scholar] [CrossRef]
- Heldt, J.R.; Heldt, J.; Józefowicz, M.; Kamiński, J. Spectroscopic studies of fluorenone derivatives. J. Fluoresc. 2001, 11, 65–73. [Google Scholar] [CrossRef]
- Revill, J.A.T.; Browna, R.G. Excimer versus TICT state formation in polar solutions of methyl 4-(N,N-dimethylamino)benzoate. Chem. Phys. Lett. 1992, 188, 433–438. [Google Scholar] [CrossRef]
- Zhen, Z.; Tung, C. Hydrophobic effects on photophysical and photochemical processes: Excimer fluorescence and aggregate formation of long-chain alkyl 4-(N,N-dimethylamino) benzoate in water—Organic binary mixtures. Chem. Phys. Lett. 1991, 180, 211–215. [Google Scholar] [CrossRef]
- Estrada, L.A.; Yarnell, J.E.; Neckers, D.C. Revisiting fluorenone photophysics via dipolar fluorenone derivatives. J. Phys. Chem. A 2011, 115, 6366–6375. [Google Scholar]
- Sahoo, D.; Bhattacharya, P.; Chakravorti, S. Spectral signature of 2-[4-(dimethylamino)styryl]-1-methylquinolinium iodide: A case of negative solvatochromism in water. J. Phys. Chem. B 2011, 115, 10983–10989. [Google Scholar] [CrossRef] [Green Version]
- Kotaka, H.; Konishi, G.; Mizuno, K. Synthesis and photoluminescence properties of π-extended fluorene derivatives: The first example of a fluorescent solvatochromic nitro-group-containing dye with a high fluorescence quantum yield. Tetrahedron Lett. 2010, 51, 181–184. [Google Scholar] [CrossRef]
- Rani, S.A.; Sobhanadri, J.; Rao, T.A.P. Solvent and concentration effects on the steady state fluorescence of fluorenone. J. Photochem. Photobiol. A 1996, 94, 1–5. [Google Scholar] [CrossRef]
- Kuboyama, A. Electronic spectrum of fluorenone. Bull. Chem. Soc. Jpn. 1964, 37, 1540–1544. [Google Scholar] [CrossRef]
- Biczók, L.; Bérces, T.; Linschitz, H. Quenching processes in hydrogen-bonded pairs: Interactions of excited fluorenone with alcohols and phenols. J. Am. Chem. Soc. 1997, 119, 11071–11077. [Google Scholar] [CrossRef]
- Kobayashi, T.; Nagakura, S. Picosecond time-resolved spectroscopy and the intersystem crossing rates of anthrone and fluorenone. Chem. Phys. Lett. 1976, 43, 429–434. [Google Scholar] [CrossRef]
- Andrews, L.J.; Deroulede, A.; Linschitz, H. Photophysical processes in fluorenone. J. Phys. Chem. 1978, 82, 2304–2309. [Google Scholar] [CrossRef]
- Yoshihara, K.; Kearns, D.R. Spectroscopic properties of the lower lying excited states of fluorenone. J. Chem. Phys. 1966, 45, 1991–1999. [Google Scholar] [CrossRef]
- Ghosh, H.N.; Adamczyk, K.; Verma, S.; Dreyer, J.; Nibbering, E.T.J. On the role of hydrogen bonds in photoinduced electron-transfer dynamics between 9-fluorenone and amine solvents. Chem. Eur. J. 2012, 18, 4930–4937. [Google Scholar] [CrossRef]
- Zhao, G.; Han, K. Ultrafast hydrogen bond strengthening of the photoexcited fluorenone in alcohols for facilitating the fluorescence quenching. J. Phys. Chem. A 2007, 111, 9218–9223. [Google Scholar] [CrossRef]
- Zhao, G.; Han, K. Role of intramolecular and intermolecular hydrogen bonding in both singlet and triplet excited states of aminofluorenones on internal conversion, intersystem crossing, and twisted intramolecular charge transfer. J. Phys. Chem. A 2009, 113, 14329–14335. [Google Scholar] [CrossRef]
- Yatsuhashi, T.; Nakajima, Y.; Shimada, T.; Inoue, H. Photophysical properties of intramolecular charge-transfer excited singlet state of aminofluorenone derivatives. J. Phys. Chem. A 1998, 102, 3018–3024. [Google Scholar]
- Estrada, L.A.; Neckers, D.C. Synthesis and photophysics of ambipolar fluoren-9-ylidene malononitrile derivatives. J. Org. Chem. 2009, 74, 8484–8487. [Google Scholar] [CrossRef]
- Miyaura, N.; Suzuki, A. Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Grabowski, Z.R. Electron transfer and the structural changes in the excited state. Pure Appl. Chem. 1992, 64, 1249–1255. [Google Scholar] [CrossRef]
- Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Shigeta, M.; Morita, M.; Konishi, G.-i. Selective Formation of Twisted Intramolecular Charge Transfer and Excimer Emissions on 2,7-bis(4-Diethylaminophenyl)-fluorenone by Choice of Solvent. Molecules 2012, 17, 4452-4459. https://doi.org/10.3390/molecules17044452
Shigeta M, Morita M, Konishi G-i. Selective Formation of Twisted Intramolecular Charge Transfer and Excimer Emissions on 2,7-bis(4-Diethylaminophenyl)-fluorenone by Choice of Solvent. Molecules. 2012; 17(4):4452-4459. https://doi.org/10.3390/molecules17044452
Chicago/Turabian StyleShigeta, Masayuki, Mifumi Morita, and Gen-ichi Konishi. 2012. "Selective Formation of Twisted Intramolecular Charge Transfer and Excimer Emissions on 2,7-bis(4-Diethylaminophenyl)-fluorenone by Choice of Solvent" Molecules 17, no. 4: 4452-4459. https://doi.org/10.3390/molecules17044452
APA StyleShigeta, M., Morita, M., & Konishi, G. -i. (2012). Selective Formation of Twisted Intramolecular Charge Transfer and Excimer Emissions on 2,7-bis(4-Diethylaminophenyl)-fluorenone by Choice of Solvent. Molecules, 17(4), 4452-4459. https://doi.org/10.3390/molecules17044452