Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives
Abstract
:1. Introduction
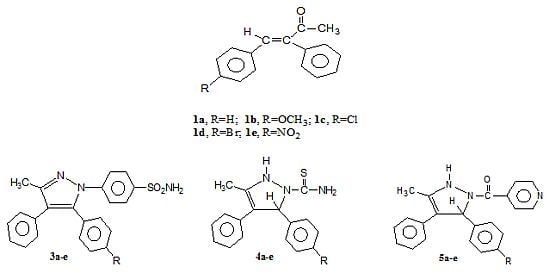
2. Results and Discussion

| Compound | R | Yield | M.P. | Molecular Formula | Calculated % | Found % | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (%) | (°C) | C | H | N | C | H | N | |||
| 2a | H | 67 | 147 | C22H21N3SO2 | 67.52 | 5.37 | 10.74 | 67.50 | 5.40 | 10.70 |
| 2b | OCH3 | 90 | 160 | C23H23N3SO3 | 65.56 | 5.46 | 9.98 | 65.60 | 5.44 | 9.95 |
| 2c | Cl | 87 | 103 | C22H20N3SO2Cl | 61.97 | 4.69 | 9.86 | 61.94 | 4.66 | 9.90 |
| 2d | Br | 93 | 110 | C22H20N3SO2Br | 56.17 | 4.26 | 8.94 | 56.20 | 4.30 | 8.97 |
| 2e | NO2 | 79 | 135 | C22H20N4SO4 | 60.55 | 4.59 | 12.84 | 60.50 | 4.63 | 12.80 |
| 3a | H | 69 | 187 | C22H19N3SO2 | 67.87 | 4.88 | 10.80 | 67.84 | 4.92 | 10.84 |
| 3b | OCH3 | 91 | 193 | C23H21N3SO3 | 65.87 | 5.01 | 10.02 | 65.90 | 5.05 | 10.06 |
| 3c | Cl | 76 | 172 | C22H18N3SO2Cl | 62.26 | 4.25 | 9.91 | 62.30 | 4.21 | 9.94 |
| 3d | Br | 78 | 177 | C22H18N3SO2Br | 56.41 | 3.85 | 8.97 | 56.45 | 3.90 | 8.95 |
| 3e | NO2 | 69 | 197 | C22H18N4SO4 | 60.83 | 4.15 | 12.90 | 60.80 | 4.15 | 12.94 |
| 4a | H | 81 | 149 | C17H17N3S | 69.15 | 5.76 | 14.24 | 69.10 | 5.76 | 14.20 |
| 4b | OCH3 | 83 | 156 | C18H19N3SO | 66.46 | 5.85 | 12.92 | 66.50 | 5.85 | 12.95 |
| 4c | Cl | 87 | 172 | C17H16N3SCl | 61.82 | 4.85 | 12.73 | 61.86 | 4.88 | 12.69 |
| 4d | Br | 79 | 146 | C17H16N3SBr | 54.55 | 4.28 | 11.23 | 54.50 | 4.29 | 11.20 |
| 4e | NO2 | 93 | 166 | C17H16N4SO2 | 60.00 | 4.71 | 16.47 | 59.99 | 4.66 | 16.43 |
| 5a | H | 81 | 159 | C22H19N3O | 77.42 | 5.57 | 12.32 | 77.45 | 5.59 | 12.36 |
| 5b | OCH3 | 69 | 166 | C23H21N3O2 | 74.39 | 5.66 | 11.32 | 74.35 | 5.69 | 11.30 |
| 5c | Cl | 89 | 142 | C22H18N3OCl | 70.21 | 4.79 | 11.17 | 70.25 | 4.74 | 11.24 |
| 5d | Br | 86 | 168 | C22H18N3OBr | 62.86 | 4.29 | 10.00 | 62.82 | 4.25 | 10.05 |
| 5e | NO2 | 97 | 171 | C22H18N4O3 | 68.39 | 4.66 | 14.51 | 68.44 | 4.60 | 14.55 |
| Compound | IR cm−1 (KBr) | 1H-NMR (δ / ppm) a | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Vinyl | C=N | C=O | C=S | NH and/or | Ar-H’S (m) | N=C-C=CH | pyrazoline- | CH3 and/or Ar- OCH3 (s) | NH and/or NH2 | |
| HC=CH | NH2 | (s) | C5-H (s) | |||||||
| 2a | 1603 | 1632 | - | - | 3391, 3291 | 7.33–7.82 | 6.91 | - | 2.33 | 8.21 and 9.23 |
| 2b | 1609 | 1629 | - | - | 3389, 3309 | 7.31–7.81 | 6.93 | - | 2.22 and 3.26 | 8.46 and 9.29 |
| 2c | 1612 | 1626 | - | - | 3382, 3289 | 7.36–7.61 | 6.97 | - | 2.22 | 8.54 and 9.31 |
| 2d | 1604 | 1633 | - | - | 3383, 3302 | 7.41–7.71 | 6.89 | - | 2.19 | 8.61 and 9.39 |
| 2e | 1619 | 1627 | - | - | 3379,3310 | 7.26–7.76 | 6.95 | - | 2.37 | 8.73 and 9.40 |
| 3a | 1515 | 1632 | - | - | 3386 | 7.24–8.29 | - | - | 2.25 | 9.33 |
| 3b | 1510 | 1641 | - | - | 3384 | 7.35–7.94 | - | - | 2.28 and 3.34 | 9.76 |
| 3c | 1500 | 1644 | - | - | 3378 | 7.29–7.86 | - | - | 2.22 | 9.92 |
| 3d | 1515 | 1654 | - | - | 3391 | 7.13–7.64 | - | - | 2.25 | 9.71 |
| 3e | 1520 | 1656 | - | - | 3393 | 7.29–8.10 | - | - | 2.28 | 10.37 |
| 4a | 1527 | - | - | 1246 | 3394, 3287 | 7.26–7.82 | - | 5.39 | 2.25 | 10.73 and 8.26 |
| 4b | 1522 | - | - | 1245 | 3390, 3293 | 7.27–7.77 | - | 5.42 | 2.32 and 3.39 | 10.77 and 8.37 |
| 4c | 1531 | - | - | 1248 | 3390, 3282 | 7.29–8.01 | - | 5.44 | 2.27 | 10.79 and 8.42 |
| 4d | 1527 | - | - | 1247 | 3393, 3311 | 7.23–7.61 | - | 5.46 | 2.29 | 10.93 and 8.43 |
| 4e | 1526 | - | - | 1232 | 3387, 3316 | 7.27–7.99 | – | 5.39 | 2.32 | 10.71 and 8.48 |
| 5a | 1517 | - | 1630 | - | 3291 | 6.81–7.09 and 7.32–7.48 | - | 5.40 | 2.32 | 8.31 |
| 5b | 1530 | - | 1628 | - | 3293 | 6.79–7.12 and 7.29–7.49 | - | 5.37 | 2.25 and 3.31 | 8.36 |
| 5c | 1522 | - | 1631 | - | 3287 | 6.83–7.08 and 7.27–7.51 | - | 5.46 | 2.27 | 8.45 |
| 5d | 1519 | - | 1633 | - | 3289 | 6.82–7.11 and 7.27–7.52 | - | 5.47 | 2.22 | 8.47 |
| 5e | 1524 | - | 1634 | - | 3311 | 6.84–7.10 and 7.26–7.71 | - | 5.42 | 2.39 | 8.51 |
2.1. Antimicrobial Activity
| Compound | Zone of inhibition (mm) | Minimum inhibition concentration (MIC) g/mL | ||
|---|---|---|---|---|
| S. aureus | C. albicans | S. aureus | C. albicans | |
| 2a | - | 20 | - | 250 |
| 2b | - | 15 | - | - |
| 2c | 17 | 20 | 100 | 50 |
| 2d | 14 | 20 | 120 | 500 |
| 2e | 12 | 15 | - | - |
| 3a | 19 | 22 | 63 | 31 |
| 3b | 18 | 25 | 125 | 31 |
| 3c | 22 | 26 | 50 | 50 |
| 3d | 18 | 20 | 63 | 125 |
| 3e | 17 | 17 | - | - |
| 4a | - | 15 | - | - |
| 4b | - | 15 | - | - |
| 4c | 21 | 20 | 100 | 50 |
| 4d | 15 | 18 | 63 | 63 |
| 4e | - | 15 | - | - |
| 5a | - | 15 | - | - |
| 5b | - | 15 | - | - |
| 5c | 21 | 24 | 50 | 50 |
| 5d | - | 15 | - | - |
| 5e | 17 | 17 | - | - |
| Rifampicin | 32 | - | - | - |
| Ampicillin | 30 | - | - | - |
| DMSO | - | 14 | - | - |
| Concentrations µg/mL | 1000 | 500 | 250 | 125 | 63 | 31 | 1000 | 500 | 250 | 125 | 63 | 31 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Microorganism Growth | S. aureus | C. albicans | ||||||||||
| 2c | - | - | - | + | + | + | - | - | - | - | + | + |
| 3c | - | - | - | + | + | + | - | - | - | + | + | + |
| 4c | - | - | - | + | + | + | - | - | + | + | + | + |
| 5c | - | - | + | + | + | + | - | - | - | - | + | + |
3. Experimental
3.1. General
3.2. General Procedure for Preparation of 3-Phenyl-4-(p-substituted phenyl)-3-buten-1-ones 1a–e
3.3. General Procedure for Preparation of 3-Phenyl-4-(p-substituted phenyl)-3-buten-1-(p-sulphamyl-phenyl)hydrazones 2a–e
3.4. General Procedure for Preparation of 3-Methyl-4-phenyl-5-(p-substituted phenyl)-1-(p-sulphamyl-phenyl)pyrazoles 3a–e
3.5. General Procedure for Preparation of 4,5-Dihydro-3-methyl-4-phenyl-5-(p-substituted phenyl)-pyrazole-1-thiocarboxamides 4a–e
3.6. General Procedure for Preparation of 4,5-Dihydro-3-methyl-4-phenyl-5-(p-substituted phenyl)-1-isonicotinoylpyrazoles 5a–e
3.7. Determination of Antimicrobial Activity
4. Conclusions
Acknowledgements
References and Notes
- Taylor, E.C.; Patel, H.; Kumar, H. Synthesis of pyrazolo 3,4-dpyrimidine analogues of the potent agent N-4-2-2-amino-43H-oxo-7H-pyrrolo2,3-dpyrimidin-5-yl ethylbenzoyl-L-glutamic acid (LY231514). Tetrahedron 1992, 48, 8089–8100. [Google Scholar] [CrossRef]
- Abdel-Rahman, A.A.-H.; Abdel-Megied, A.E.-S.; Hawata, M.A.M.; Kasem, E.R.; Shabaan, M.T. Synthesis and antimicrobial of some chalcones and their derived pyrazoles, pyrazolines, isoxzolines and 5,6-dihydropyrimidine-2-(1H)-thiones. Monatsh. Chem. 2007, 138, 889–897. [Google Scholar] [CrossRef]
- Sharshira, E.M.; Hamada, N.M. Synthesis and in vitro antimicrobial activity of some pyrazolyl-1-carboxamide derivatives. Molecules 2011, 16, 7736–7745. [Google Scholar] [CrossRef]
- Rashad, A.E.; Shamroukh, A.H.; Hegab, M.I.; Awad, H.M. Synthesis of some biologically active pyrazoles and C-nucleosides. Acta Chim. Slov. 2005, 52, 429–434. [Google Scholar]
- Rashad, A.E.; Hegab, M.I.; Abdel-Megeid, R.E.; Micky, J.A.; Abdel-Megeid, F.M.E. Synthesis and antiviral evaluation of some new pyrazole and fused pyrazolopyrimidine derivatives. Bioorg. Med. Chem. 2008, 16, 7102–7106. [Google Scholar] [CrossRef]
- Bhat, B.A.; Dhar, K.L.; Saxena, A.K.; Shanmugavel, M. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agent. Bioorg. Med. Chem. 2005, 15, 3177–3180. [Google Scholar] [CrossRef]
- Michael, L.E.; David, M.S.; Prasad, S.S. Chalcones: A new class of antimitotic agents. J. Med. Chem. 1990, 33, 1948–1954. [Google Scholar] [CrossRef]
- Kalirajan, R.; Sivakumar, S.U.; Jubie, S.; Gowramma, B.; Suresh, B. Synthesis and biological evaluation of some heterocyclic derivatives of chalcones. Int. J. ChemTech Res. 2009, 1, 27–34. [Google Scholar]
- Holla, B.S.; Akberali, P.M.; Sivanada, M.K. Studies on arylfuran derivatives: Part X. Synthesis and antibacterial properties of arylfuryl-Δ2-pyrazolines. Farmaco 2000, 55, 256–263. [Google Scholar] [CrossRef]
- Maggio, B.; Daidone, G.; Raffa, D.; Plescia, S.; Mantione, L.; Cutuli, V.M.C.; Mangano, N.G.; Caruso, A. Synthesis and pharmacological study of ethyl 1-methyl-5-(substituted-3,4-dihydro-4-oxoquinazolin-3-yl)-1H-pyrazole-4-acetates. Eur. J. Med. Chem. 2001, 36, 737–742. [Google Scholar] [CrossRef]
- Vibhute, Y.B.; Basser, M.A. Synthesis and activities of a new series of chalcones as antibacterial agents. Ind. J. Chem. 2003, 42B, 202–205. [Google Scholar]
- Clinton, R.O.; Manson, A.J.; Stonner, F.W.; Beyler, A.L.; Potts, G.O.; Arnold, A. Steroidal [3,2-c] pyrazoles. J. Am. Chem. Soc. 1959, 81, 1513–1514. [Google Scholar]
- Kalirajan, R.; Palanivelu, M.; Rajamanickam, V.; Vinothapooshan, G.; Andarajagopal, K. Synthesis and biological evaluation of some heterocyclic derivatives chalcones. Int. J. Chem. Sci. 2007, 5, 73–80. [Google Scholar]
- Urmila, G.; Vineeta, S.; Vineeta, K.; Sanjana, C. Synthesis and antifungal activity of new fluorine containing 4-(substituted phenylazo) pyrazoles and isoxazoles. Indian J. Heterocycl. Chem. 2005, 14, 265–266. [Google Scholar]
- Klimova, E.I.; Marcos, M.; Klimova, T.B.; Cecilio, A.T.; Ruben, A.T.; Lena, R.R. The structure of bicyclic ferrocenylmethylene substituted 2-pyrazolines and their reactions with azodicarboxylic acid N-phenylimide. J. Organomet. Chem. 1999, 585, 106–114. [Google Scholar]
- Padmavathi, V.; Sumathi, R.P.; Chandrasekhar, B.N.; Bhaskar, D. 1,3-Dipolar cycloaddition of dipolar reagents to bifunctional olefins in the presence of chloramine-T (CAT). J. Chem. Res. (S) 1999, 10, 610–611. [Google Scholar]
- Faidallah, M.H.; Sharshira, E.M.; Basaif, A.S.; A-Ba-Oum, K.A. Synthesis and spectral characterization of novel 1,3,4-oxadiazole and 1,2,4-triazole derivatives: Synthesis for potential pharmacological activities. Phosphor. Sulfur Silicon Relat. Elem. 2002, 177, 67–79. [Google Scholar] [CrossRef]
- Vibhute, Y.B.; Basser, M.A. Synthesis and activities of a new series of chalcones as anti bacterial agents. Ind. J. Chem. 2003, 42B, 202–205. [Google Scholar]
- Hamada, N.M.; Sharshira, E.M. Synthesis and antimicrobial evaluation of some heterocyclic chalcone derivatives. Molecules 2011, 16, 2304–2312. [Google Scholar] [CrossRef]
- Azarifar, D.; Shaabanzadeh, M. Synthesis and characterization of new 3,5-dinaphthyl substituted 2-pyrazolines and study of their antimicrobial activity. Molecules 2002, 7, 885–895. [Google Scholar] [CrossRef]
- Padmaia, A.; Payani, T.; Reddy, G.D.; Padmavathi, V. Synthesis, antimicrobial and antioxidant activities of substituted pyrazoles, isoxazoles, pyrimidine and thioxopyrimidine derivatives. Eur. J. Med. Chem. 2009, 44, 4557–4566. [Google Scholar] [CrossRef]
- Yale, H.L.; Lose, K.; Martins, J.; Holing, M.; Perry, F.M.; Bernstein, J. Chemotherapy of experimental tuberculosis. VIII. The synthesis of acid hydrazides, their derivatives and related compounds. J. Am. Chem. Soc. 1953, 75, 1933–1942. [Google Scholar]
- Abdelhamid, A.O.; Zohidi, H.F.; Sallam, M.M.M.; Ahmed, N.A. Reactions with hydrazonoyl halides. 31. Synthesis of some new pyrrolidino[3,4-c]pyrazolines, pyrazoles, and pyrazolo[3,4-d]pyridazines. Molecules 2000, 5, 967–973. [Google Scholar] [CrossRef]
- Rurack, K.; Bricks, J.L.; Schultz, B.; Maus, M.; Reck, G.; Resch-Genger, U.J. Substituted1,5 diphenyl-3-benzothiazol-2-yl-Δ2-pyrazolines: Synthesis, X-ray structure, photophysics and cation complexation properties. J. Phys. Chem. A 2000, 104, 6171–6188. [Google Scholar]
- Guerra, F.M.; Mish, M.R.; Carreira, E.M. Versatile, diastereoselective additions of silyl ketene acetals, allyl tributylstannane and Me3SiCN to N-acyl pyrazolines: Asymmetric synthesis of densely functionalized pyrazolidines. Org. Lett. 2000, 2, 4265–4267. [Google Scholar] [CrossRef]
- Vedso, P.; Begtrup, M. Synthesis of 5-substituted 1-hydroxypyrazoles through directed lithiation of 1-(benzyloxy) pyrazole. J. Org. Chem. 1995, 60, 4995–4998. [Google Scholar] [CrossRef]
- Gupta, R.; Gupta, N.; Jain, A. Improved synthesis of chalcones and pyrazolines under ultrasonic irradiation. Ind. J. Chem. 2010, 49B, 351–355. [Google Scholar]
- Nakamichi, N.; Kawashita, Y.; Hayashi, M. Oxidative aromatization of 1,3,5-trisubstituted pyrazolines and hantzsch 1,4-dihydropyridines by Pd/C in acetic acid. Org. Lett. 2002, 4, 3955–3957. [Google Scholar] [CrossRef]
- Wang, S.; Shi, B.; Li, Y.; Wang, Q.; Huang, R. A convenient synthesis of novel 4-(1,2, 4-triazol-1-yl)-2-pyrazolines and their derivatives. Synth. Commun. 2003, 33, 1449–1457. [Google Scholar] [CrossRef]
- Chen, Y.; Lam, Y.; Lai, Y.H. Solid-phase synthesis of pyrazolines and isoxazolines with sodium benzene sulfinate as a traceless linker. Org. Lett. 2003, 5, 1067–1069. [Google Scholar] [CrossRef]
- Stephen, A.F.; Philip, D.P. Reexamination of the Claisen-Schmidt condensation of phenylacetone with aromatic aldehydes. J. Org. Chem. 1973, 38, 1747–1749. [Google Scholar] [CrossRef]
- Hamed, E.A.; Sharaf, S.M.; Abdel-Baky, S.A.; Ibrahim, M.F.; Youssef, A.A. Stereochemistry and kinetics of addition of amines to acetylenic ketones. J. Phys. Org. Chem. 1990, 3, 627–634. [Google Scholar] [CrossRef]
- Ansari, F.L.; Nazir, S.; Noureen, H.; Miraza, B. Combinatorial synthesis and antibacterial evaluation of an indexed chalcone library. Chem. Biodivers. 2005, 2, 1656–1664. [Google Scholar] [CrossRef]
- Sample Availability: Samples of all the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sharshira, E.M.; Hamada, N.M.M. Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules 2012, 17, 4962-4971. https://doi.org/10.3390/molecules17054962
Sharshira EM, Hamada NMM. Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules. 2012; 17(5):4962-4971. https://doi.org/10.3390/molecules17054962
Chicago/Turabian StyleSharshira, Essam Mohamed, and Nagwa Mohamed Mahrous Hamada. 2012. "Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives" Molecules 17, no. 5: 4962-4971. https://doi.org/10.3390/molecules17054962
APA StyleSharshira, E. M., & Hamada, N. M. M. (2012). Synthesis and Antimicrobial Evaluation of Some Pyrazole Derivatives. Molecules, 17(5), 4962-4971. https://doi.org/10.3390/molecules17054962