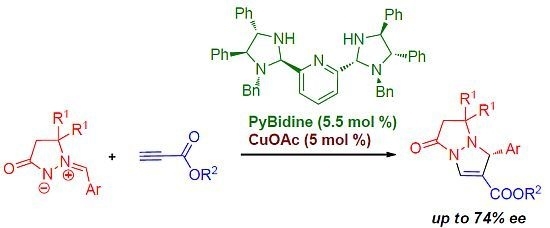
Chiral Bis(Imidazolidine)Pyridine-Cu Complex-Catalyzed Enantioselective [3+2]-Cycloaddition of Azomethine Imines with Propiolates
Abstract
:1. Introduction



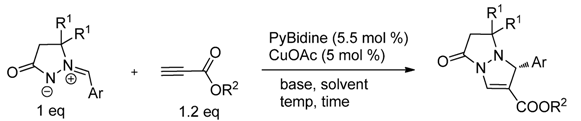
2. Results and Discussion

| Entry | Metal salt | Time (h) | Yield (%) | ee (%) |
|---|---|---|---|---|
| 1 | CuI | 3 | 99 | 44 |
| 2 | CuBr | 5 | 80 | 60 |
| 3 | CuOAc | 2 | 99 | 60 |
| 4 | Cu(OAc)2 | 3 | 99 | 46 |
| 5 | Cu(OTf)2 | 24 | 69 | 21 |
| 6 | Co(OAc)2 | 31 | trace | - |
| 7 | Ni(OAc)2 | 32 | trace | - |
| 8 | Zn(OAc)2 | 50 | trace | - |
| Entry | Ar | R1 | R2 | Base b | Solvent | Temp (°C) | Time (h) | Yield (%) | ee (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Ph | H | Me | - | CHCl3 | rt | 24 | 66 | 55 |
| 2 | Ph | H | Me | - | CH2Cl2 | rt | 4 | 95 | 67 |
| 3 | Ph | H | Me | - | PhMe | rt | 2.5 | 99 | 56 |
| 4 | Ph | H | Me | - | MeCN | rt | 27 | 39 | 52 |
| 5 | Ph | H | Me | - | THF | rt | 24 | 96 | 61 |
| 6 | Ph | H | Me | - | dioxane | rt | 24 | 95 | 35 |
| 7 | Ph | H | Me | - | EtOH | rt | 28 | 88 | 44 |
| 8 | Ph | Me | Me | - | CH2Cl2 | rt | 24 | 67 | 53 |
| 9 | Ph | H | Et | - | CH2Cl2 | rt | 2 | 99 | 60 |
| 10 | Ph | H | Et | - | CH2Cl2 | −10 | 45 | 97 | 65 |
| 11 | 4-IC6H4- | H | Et | - | CH2Cl2 | rt | 2 | 99 | 59 |
| 12 | 4-IC6H4- | H | Et | - | CH2Cl2 | −10 | 23 | 58 | 54 |
| 13 | 3-ClC6H4- | H | Et | - | CH2Cl2 | rt | 3 | 88 | 47 |
| 14 | 3-ClC6H4- | H | Et | - | CH2Cl2 | −10 | 23 | 62 | 62 |
| 15 | Ph | H | Et | DIPEA | CH2Cl2 | rt | 1 | 99 | 70 |
| 16 | Ph | H | Et | Pyridine | CH2Cl2 | rt | 1 | 99 | 40 |
| 17 | Ph | H | Et | K2CO3 | CH2Cl2 | rt | 16 | 90 | 32 |
| 18 | Ph | H | Et | DIPEA | CH2Cl2 | 0 | 25 | 78 | 72 |
| 19 | Ph | H | Et | DIPEA | CH2Cl2 | −20 | 58 | 68 | 74 |

3. Experimental
3.1. General
3.2. General Procedure
4. Conclusions
Acknowledgments
References and Notes
- Eicher, T.; Hauptmann, S. The Chemistry of Heterocycles, 2nd ed; Wiley-VCH: Weinheim, Germany, 2003. [Google Scholar]
- Varvounis, G.; Fiamegos, Y.; Pilidis, G. Pyrazol-3-ones part 1: Synthesis and applications. Adv. Heterocycl. Chem. 2001, 80, 73–156. [Google Scholar] [CrossRef]
- Elguero, J. Pyrazoles: Comprehensive Heterocyclic Chemistry II; Katritzky, A.R., Rees, C.W., Scriven, E.F.V., Eds.; Elsevier: Oxford, UK, 1996; Volume 3, pp. 1–75. [Google Scholar]
- Marchand-Brynaert, J.; Ghosez, L. Non β-Lactam Analogs of Penicillins and Cephalosporins. In Recent Progress in the Chemical Synthesis of Antibiotics; Lukacs, G., Ohno, M., Eds.; Springer: Berlin, Germany, 1990; pp. 727–794. [Google Scholar]
- Hanessian, S.; McNaughton-Smith, G.; Lombart, H.-G.; Lubell, W.D. Design and synthesis of conformationally constrained amino acids as versatile scaffolds and peptide mimetics. Tetrahedron 1997, 53, 12789–12854. [Google Scholar] [CrossRef]
- Konaklieva, M.I.; Plotkin, B.J. Bioisosters of β-Lactams as Anti-Infectives. Curr. Med. Chem. Anti-Infect. Agents 2003, 2, 287–302. [Google Scholar] [CrossRef]
- White, H.L.; Howard, J.L.; Cooper, B.R.; Soroko, F.E.; McDermed, J.D.; Ingold, K.J.; Maxwell, R.A. A Novel inhibitor of gamma-aminobutyrate aminotransferase with anorectic activity. J. Neurochem. 1982, 39, 271–273. [Google Scholar] [CrossRef]
- Cucurou, C.; Battioni, J.P.; Thang, D.C.; Nam, N.H.; Mansuy, D. Mechanisms of inactivation of lipoxygenases by phenidone and BW755C. Biochemistry 1991, 30, 8964–8970. [Google Scholar] [CrossRef]
- Kawai, K.; Hasegawa, J.; Shiojiri, H.; Nozawa, Y.; Tsurumi, K.; Fujimura, H. Interaction of BW755C, a potent inhibitor of lipoxygenase and cyclo-oxygenase, with mitochondrial cytochrome oxidase. Experientia 1985, 41, 490–492. [Google Scholar] [CrossRef]
- Jungheim, L.N.; Sigmund, S.K.; Fisher, J.W. Bicyclic pyrazolidinones, a new class of antibacterial agent based on the β-lactam model. Tetrahedron Lett. 1987, 28, 285–288. [Google Scholar] [CrossRef]
- Jungheim, L.N.; Sigmund, S.K. 1,3-Dipolar cycloaddition reactions of pyrazolidinium ylides with acetylenes. Synthesis of a new class of antibacterial agents. J. Org. Chem. 1987, 52, 4007–4013. [Google Scholar] [CrossRef]
- Ternansky, R.J.; Draheim, S.E.; Pike, A.J.; Counter, F.T.; Eudaly, J.A.; Kasher, J.S. Structure-activity relationship within a series of pyrazolidinone antibacterial agents. 2. Effect of side-chain modification on in vitro activity and pharmacokinetic parameters. J. Med. Chem. 1993, 36, 3224–3229. [Google Scholar] [CrossRef]
- Claramunt, R.M.; Elguero, J. The chemistry of pyrazolidinones. A review. Org. Prep. Proc. Int. 1991, 23, 273–320. [Google Scholar] [CrossRef]
- Kosower, E.M.; Radkowsky, A.E.; Fairlamb, A.H.; Croft, S.L.; Nea, R.A. Bimane cyclic esters, possible stereologues of trypanothione as antitrypanosomal agents. Bimanes 29. Eur. J. Med. Chem. 1995, 30, 659–671. [Google Scholar] [CrossRef]
- Karlsson, S.; Hogberg, H.-E. Asymmetric 1,3-Dipolar Cycloadditions for the Construction of Enantiomerically Pure Heterocycles. A Review. Org. Prep. Proc. Int. 2001, 33, 103–172. [Google Scholar] [CrossRef]
- Gothelf, K.V.; Jørgensen, K.A. Asymmetric 1,3-Dipolar cycloaddition reactions. Chem. Rev. 1998, 98, 863–909. [Google Scholar]
- Kanemasa, S. Cornerstone works for catalytic 1,3-Dipolar cycloaddition reactions. Heterocycles 2010, 82, 87–200. [Google Scholar] [CrossRef]
- Taylor, E.C.; Haley, N.F.; Clemens, R.J. Synthesis and Properties of 3-Oxo-1,2-diazetidinium Ylides. J. Am. Chem. Soc 1981, 103, 7743–7752. [Google Scholar] [CrossRef]
- Dorn, H. 1,3-Dipolar cycloaddition of pyrazolid-3-one azomethinimines and E-β-nitrostyrene—elucidation of the generation of seemingly “non cisoid” adducts. Tetrahedron Lett. 1985, 26, 5123–5126. [Google Scholar] [CrossRef]
- Huisgen, R.; Weinberger, R. Are any non-stereospecific 1,3-Dipolar cycloaddition known? A revision. Tetrahedron Lett. 1985, 26, 5119–5122. [Google Scholar] [CrossRef]
- Keller, M.; Sido, A.S.S.; Pale, P.; Sommer, J. Copper(I) Zeolites as Heterogeneous and Ligand-Free Catalysts: [3+2] Cycloaddition of Azomethine Imines. Chem. Eur. J. 2009, 15, 2810–2817. [Google Scholar] [CrossRef]
- Pezdirc, L.; Stanovnik, B.; Svete, J. Copper(I) Iodide-Catalyzed Cycloadditions of (1Z, 4R*,5R*)-4-Benzamido-5-phenylpyrazolidin-3-on-1-azomethine Imines to Ethyl Propiolate. Aust. J. Chem. 2009, 62, 1661–1666. [Google Scholar] [CrossRef]
- Yamashita, Y.; Kobayashi, S. NO cations as highly efficient catalysts for carbon-carbon bond forming reactions. Chem. Lett. 2009, 38, 678–679. [Google Scholar] [CrossRef]
- Shi, F.; Mancuso, R.; Larock, R.C. 1,3-Dipolar cycloaddition of arynes with azomethine imines: synthesis of 1,2-dihydropyrazolo[1,2-a]indazol-3(9H)-ones. Tetrahedron. Lett. 2009, 50, 4067–4070. [Google Scholar] [CrossRef]
- Oishi, T.; Yoshimura, K.; Yamaguchi, K.; Mizuno, N. An efficient copper-mediated 1,3-Dipolar Cycloaddition of pyrazolidinone-based dipoles to terminal alkynes to produce N,N-Bicyclic pyrazolidinone derivatives. Chem. Lett. 2010, 39, 1086–1087. [Google Scholar] [CrossRef]
- Na, R.; Jing, C.; Xu, Q.; Jiang, H.; Wu, X.; Shi, J.; Zhong, J.; Wang, M.; Benitez, D.; Tkatchouk, E.; et al. Phosphine-Catalyzed Annulations of Azomethine Imines: Allene-Dependent [3+2], [3+3], [4+3], and [3+2+3] Pathways. J. Am. Chem. Soc. 2011, 133, 13337–13348. [Google Scholar]
- Yoshimura, K.; Oishi, T.; Yamaguchi, K.; Mizuno, N. An efficient, ligand-free, heterogeneous supported copper hydroxide catalyst for the synthesis of N,N-Bicylic pyrazolidinone derivatives. Chem. Eur. J. 2011, 17, 3827–3831. [Google Scholar] [CrossRef]
- Xin, Y.; Zhao, J.; Gu, J.; Zhu, S. Catalyst free 1,3-dipolar cycloaddition of 3-oxo-1,2-pyrazolidinium ylides to β-trifluoroacetyl vinyl ethyl ether: Synthesis of 6-trifluoroacetyl substituted bicyclic pyrazolidinones. J. Fluorine Chem. 2011, 132, 402–408. [Google Scholar] [CrossRef]
- Koptelov, Y.B.; Sednev, M.V. Stable azomethine imines derived from pyrazolidin-3-one and their cycloaddition to N-Arylmaleimides. Russ. J. Org. Chem. 2011, 47, 547–555. [Google Scholar] [CrossRef]
- Shintani, R.; Fu, G.C. A new copper-catalyzed [3+2] cycloaddition: Enantioselective coupling of terminal alkynes with azomethine imines to generate five-membered nitrogen heterocycles. J. Am. Chem. Soc. 2003, 125, 10778–10779. [Google Scholar] [CrossRef]
- Suarez, A.; Downey, C.W.; Fu, G.C. Kinetic resolutions of azomethine imines via copper-catalyzed [3+2] cycloadditions. J. Am. Chem. Soc. 2005, 127, 11244–11245. [Google Scholar] [CrossRef]
- Sibi, M.P.; Rane, D.; Stanley, L.M.; Soeta, T. Copper(II)-Catalyzed Exo and enantioselective cycloadditions of azomethine imines. Org. Lett. 2008, 10, 2971–2974. [Google Scholar] [CrossRef]
- Hashimoto, T.; Maeda, Y.; Omote, M.; Nakatsu, H.; Maruoka, K. Catalytic enantioselective 1,3-Dipolar cycloaddition of C,N-Cyclic azomethine imines with α,β-Unsaturated aldehydes. J. Am. Chem. Soc. 2010, 132, 4076–4077. [Google Scholar] [CrossRef]
- Suga, H.; Funyu, A.; Kakehi, A. Highly enantioselective and diastereoselective 1,3-Dipolar cycloaddition reactions between azomethine imines and 3-Acryloyl-2-oxazolidinone catalyzed by Binaphthyldiimine-Ni(II) complexes. Org. Lett. 2007, 9, 97–100. [Google Scholar] [CrossRef]
- Chen, W.; Du, W.; Duan, Y.-Z.; Wu, Y.; Yang, S.-Y.; Chen, Y.-C. Enantioselective 1,3-Dipolar cycloaddition of cyclic enones catalyzed by multifunctional primary amines: Beneficial effects of hydrogen bonding. Angew. Chem. Int. Ed. 2007, 46, 7667–7670. [Google Scholar] [CrossRef]
- Chen, W.; Yuan, X.-H.; Li, R.; Du, W.; Wu, Y.; Ding, L.-S.; Chen, Y.-C. Organocatalytic and stereoselective [3+2] cycloadditions of azomethine imines with α,β-Unsaturated aldehydes. Adv. Synth. Catal. 2006, 348, 1818–1822. [Google Scholar] [CrossRef]
- Arai, T.; Mishiro, A.; Yokoyama, N.; Suzuki, K.; Sato, H. Chiral Bis(imidazolidine)pyridine-Cu(OTf)2: Catalytic asymmetric endo-selective [3+2] cycloaddition of imino esters with nitroalkenes. J. Am. Chem. Soc. 2010, 132, 5338–5339. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Arai, T.; Ogino, Y. Chiral Bis(Imidazolidine)Pyridine-Cu Complex-Catalyzed Enantioselective [3+2]-Cycloaddition of Azomethine Imines with Propiolates. Molecules 2012, 17, 6170-6178. https://doi.org/10.3390/molecules17056170
Arai T, Ogino Y. Chiral Bis(Imidazolidine)Pyridine-Cu Complex-Catalyzed Enantioselective [3+2]-Cycloaddition of Azomethine Imines with Propiolates. Molecules. 2012; 17(5):6170-6178. https://doi.org/10.3390/molecules17056170
Chicago/Turabian StyleArai, Takayoshi, and Yuta Ogino. 2012. "Chiral Bis(Imidazolidine)Pyridine-Cu Complex-Catalyzed Enantioselective [3+2]-Cycloaddition of Azomethine Imines with Propiolates" Molecules 17, no. 5: 6170-6178. https://doi.org/10.3390/molecules17056170