Embryonic Stem Cell Markers
Abstract
:1. Introduction

2. Cell Surface Markers
| SSEAs markers | Characteristics | Classification | References |
|---|---|---|---|
| SSEA-1 (CD15/Lewis x) | Murine embryos, mouse ES cells, mouse and human germ cells, embryonal carcinoma (EC) cells | Carbohydrate-associated molecules | [4,5,6,7,8,9,10,11] |
| SSEA-3 | Primate ES cells, human embryonic germ cells, human ES cells, embryonal carcinoma (EC) cells | Carbohydrate-associated molecules | |
| SSEA-4 | Primate ES cells, human embryonic germ cells, human ES cells, embryonal carcinoma (EC) cells | Carbohydrate-associated molecules | |
| CD markers | |||
| CD324 (E-Cadherin) | Human ES cells, mouse ES cells, embryonal carcinoma (EC) cells | Surface marker (Binding to integrin alphaE/beta7, homotypic interactions mediate cell adhesion) | [12,13,14,15,16,17,18,19,20,21] |
| CD90 (Thy-1) | Human ES cells, mouse ES cells, hematopoietic stem cells, embryonal carcinoma (EC) cells | Surface marker (hematopoietic stem cell and neuron differentiation, T activation) | |
| CD117 (c-KIT, SCFR) | Human ES cells, mouse ES cells, hematopoietic stem progenitors, neural crest-derived melanocytes, primordial germ cells, embryonal carcinoma (EC) cells | Surface marker (Stem Cell Factor receptor) | |
| CD326 | Human ES cells, mouse ES cells, embryonal carcinoma (EC) cells | Surface marker (function as growth factor receptor or adhesion molecule) | |
| CD9 (MRP1, TM4SF DRAP-27, p24) | Human ES cells, mouse ES cells | Surface marker (cell adhesion, migration, T co-stimulation) | |
| CD29 (β1 integrin) | Human ES cells, mouse ES cells | Surface marker | |
| CD24 (HAS) | Human ES cells, mouse ES cells | Surface marker (T co-stimulation, CD62P receptor) | |
| CD59 (Protectin) | Human ES cells, mouse ES cells | Surface marker (binds complement C8 and C9, blocks membrane attack complex assembly) | |
| CD133 | Human ES cells, mouse ES cells, embryonal carcinoma (EC) cells, Hematopoietic stem cells | Surface marker | |
| CD31 (PECAM-1) | Human ES cells, mouse ES cells | Surface marker (CD38 receptor, signaling, platelet-endoth adhesion) | |
| CD49f (Integrin α6/CD29) | Human ES cells, mouse ES cells | Membrane receptors | [22,23,24,25,26,27,28,29,30,31] |
| Markers | |||
| TRA-1-60 | Human ES cells, teratocarcinoma, embryonic germ cells, embryonal carcinoma (EC) cells | Surface antigen | [8,32,33,34,35] |
| TRA-1-81 | Human ES cells, teratocarcinoma, embryonic germ cells, embryonal carcinoma (EC) cells | Surface antigen | |
| Frizzled5 | Human ES cells, mouse ES cells | Seven transmembrane-spanning G-protein-coupled receptor | [36,37,38,39,40] |
| Stem cell factor (SCF or c-Kit ligand) | ES cells, mouse ES cells, Hematopoietic stem cells, Mesenchymal stem cells, embryonal carcinoma (EC) cells | Cytokine, exist both as a transmembrane protein and a soluble protein | [41,42] |
| Cripto (TDGF-1) | Mouse ES cells, human ES cells, cardiomyocyte, embryonal carcinoma (EC) cells | Receptor for the TGF- β signaling pathway | [43,44] |
2.1. Stage Specific Embryonic Antigens (SSEA)
2.2. Cluster of Differentiation (CD) Antigens
2.2.1. Integrins
2.3. TRA-1-60 and TRA-1-81
2.4. Frizzled (Fzd)
2.5. Stem Cell Factor (SCF or c-Kit Ligand)
2.6. Cripto (TDGF-1)
3. Transcription Factors
| CORE Nuclear transcription factors | Characteristics | Classification | References |
|---|---|---|---|
| Oct-3/4 (Pou5f1) | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells | POU family Transcription factors | [52,53] |
| Sox2 | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells, neural stem (NS) cells | POU family binder Transcription factors | [54,55] |
| KLF4 | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells | Zinc-finger Transcription factors | [56] |
| Nanog | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells | Transcription factors | [57,58,59] |
| Markers | |||
| Rex1 (Zfp42) | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells | Zinc-finger Transcription factor | [60,61,62] |
| UTF1 | Mouse human ES cells, germ line tissues in mouse and human, embryonal carcinoma (EC) cells | Transcriptional coactivator | [63,64] |
| ZFX | Murine ES cells, human ES cells, hematopoietic stem cells, embryonal carcinoma (EC) cells | X-linked zinc finger protein; Probable transcriptional activators | [65,66] |
| TBN | Mouse, human inner cell mass | New class of proteins with an important function in development | [67] |
| FoxD3 | Murine ES cells, human ES cells, embryonal carcinoma (EC) cells | Forkhead Box family, transcriptional regulator | [68,69,70] |
| HMGA2 | Mouse ES cells, human ES cells | Architectural transcription factors | [71,72,73,74] |
| NAC1 | Mouse ES cells, human ES cells | The POZ/BTB domain family, nuclear factor | [75,76,77] |
| GCNF (NR6A1) | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells | Nuclear receptor gene superfamily, nuclear receptor | [78,79] |
| Stat3 | Murine ES cells, Human ES cells, embryonal carcinoma (EC) cells | Transcription factor | [80,81] |
| LEF1, TCF3 | Mouse ES cells, Human ES cells, embryonal carcinoma (EC) cells | (HMG) DNA binding protein family, Transcription factor | [82] |
| Sall4 | Murine ES cells, Human ES cells, embryonal carcinoma (EC) cells | Zinc finger transcription factor | [83,84] |
| Fbxo15 | Mouse ES cells, early embryos, and testis tissue, embryonal carcinoma (EC) cells | F-box protein family, target of Oct3/4 | [85] |
| ECAT genes | |||
| ECAT11 (FLJ10884/ L1TD1) | Human ES cells, embryonal carcinoma (EC) cells | Downstream target of Nanog | [86] |
| Ecat1 | Mouse oocytes, embryonal carcinoma (EC) cells | KH domain containing RNA binding protein | [87] |
| ECAT9 (Gdf3) | Human ES cells, embryonal carcinoma (EC) cells | TGFβ superfamily, BMP inhibitor | [88] |
| Dppa genes | Oct4-related genes | ||
| Dppa5 (ESG1) | Mouse ES cells, Human ES cells, embryonal carcinoma (EC) cells | K homology RNA-binding (KH) domain | [89,90] |
| Dppa4 | Mouse ES cells, Human ES cells, embryonal carcinoma (EC) cells | Nuclear factor | [91] |
| Dppa2 (ECSA) | Mouse ES cells, Human ES cells, embryonal carcinoma (EC) cells | DNA-binding protein | [92,93] |
| Dppa3 (Stella) | Mouse ES cells, human ES cells, embryonal carcinoma (EC) cells, primordial germ cells, oocytes, preimplantation embryos | Maternal factor | [94,95] |
3.1. CORE Nuclear Transcription Factors
3.1.1. Octamer-binding Protein 4 (Oct4)
3.1.3. Krupple-like Factor (Klf) Family
3.1.4. Nanog
3.2. Reduced Expression 1 (Rex1 or Zfp-42)
3.3. Undifferentiated Embryonic Cell Transcription Factor (UTF1)
3.4. X-linked Zinc Finger Protein (ZFX)
3.5. Taube Nuss (Tbn)
3.6. Forkhead Box D3 (FoxD3)
3.7. HMGA2
3.8. Nucleus Accumbens-1 (NAC1)
3.9. Germ Cell Nuclear Factor (GCNF)
3.10. Stat3
3.11. LEF1 and TCF
3.12. SALL Family
3.13. F-box 15 (FBXO15)
3.14. ESC Associated Transcript (ECAT) Genes
3.15. Developmental Pluripotency-associated (DPPA) Genes
5. Enzymatic Markers
6. Other Markers
6.1. Lectins
6.2. Peptides Specific for ES Cells
| Mouse ES cells [191] | Macaca ES cells RS366.4 [192] | Human embryonal carcinoma (EC) cells [189] | Human embryonic stem cell line H9 [189] |
|---|---|---|---|
| GTYNLPNPPPPL | APWHLSSQYSRT | LTTAPKLPKVTR | APWHLSSQYSRT |
| KHMHWHPPALNT | GYPHPWTLWHLN | TVKHRPDALHPQ | DLNYFTLSSKRE |
| SAHGTSTGVPWP | LDVRPWYVTPLP | QLGTQPNSRTYA | HGEVPRFHAVHL |
| VPTATLMGASAR | WAPEKDYMQLMK | SRYITTMNTEQV | NRQSNWPIHKTI |
| WAETWPLAQRPP | TPLINMNALTVT | VTSRTIIPQGSA | QLSEECSYLISRP |
| LSTHTTESRSMV | TVKHRPDALHPQ | QLTKNVPTYKSS | |
| SGHQLLLNKMPN | FAKSPDVSLNPS | SNPQPYTILPPV | |
| THAAHMGYPSWW | SPLITSTLIPQR | ||
| LLADTTHHRPWT | SPNQPYTILPPV | ||
| TALATSSTYDPH | |||
| TPLTLRTQTLTQ | |||
| TTKQPHFHQKTL | |||
| TTLVSTGQRTHP |
7. Markers Overlapping with Tumor Stem Cells
| Overlapping with ESCs | Characteristics | Classification | References |
|---|---|---|---|
| SSEA-1 (CD15/ Lewis x) | Brain tumor stem cells | Surface marker | [240] |
| SSEA-3 | Breast cancer stem cells, breast Cancer | Surface marker | [202] |
| SSEA-4 | Breast Cancer, epithelial ovarian carcinoma | Surface marker | [241] |
| TRA-1-60 | Germ cell tumor | Surface marker | [32,34] |
| CD133 | Pancreatic exocrine cancer; colon cancer; glioma; | Surface marker | [204,242,243,244,245] |
| CD29 | Mouse mammary cells | Surface marker | [246] |
| CD24 | Breast tumor, tumor invasion, prostate cancer | Surface marker | [217,218,247] |
| CD90 | Hepatocellular carcinoma cell lines | Surface marker | [248] |
| CD9 (DRAP-27, MRP-1, p24) | Small cell lung cancer | Tetraspanin superfamily | [209,210,211] |
| CD59 | Lung cancer, breast cancer | Membrane attack complex inhibition factor | [213,214,215] |
| CD326 (EpCAM) | Hepatocellular carcinoma | Wnt-B-catenin signaling target gene | [249] |
| Nanog | Brain and other kinds of cancer stem cells | Transcription factors | [194] |
| SOX2 | Breast cancer stem cells, breast tumor | Transcription factors | [221] |
| OCT4 | Bladder cancer, Lung Cancer stem cells | Transcription factors | [220,250] |
| KLF4 | Breast cancer stem cells | Transcription factors | [222,251] |
| Rex1 (Zfp42) | Prostate Cancer, Renal cell carcinoma | Zinc-finger protein-42 | [223,224] |
| UTF-1 | Testicular germ cell tumours | Undifferentiated embryonic cell transcription factor 1 | [225] |
| ZFX | Gastric cancer | Zinc-finger protein family | [226] |
| LEF1 | Prostate cancer, lymphoblastic leukemia | Lymphoid enhancer-binding factor | [230,231,252] |
| SALL4 | Breast cancer, leukemogenesis, testicular germ cell tumors | Transcription factor | [253,254,255] |
| ECAT9 (Gdf3) | Melanoma, Breast Carcinoma, Seminoma, germ cell tumors | TGFβ superfamily, BMP inhibitor | [216,256,257] |
| DPPA2 (ECSA) | Lung Cancer | DNA-binding protein | [229] |
| HMGA2 | Pancreatic adenocarcinoma, bladder cancer | Architectural transcription factors | [227,228] |
| NAC1 | Breast, renal cell, and hepatocellular carcinoma | Nuclear factor | [126,127] |
| Cripto | Broad range of tumors | Extra-cellular plasma membrane growth factor | [14,17] |
| Stat3 | A number of human tumor | Transcription factor | [138,139,258] |
| Tumor stem cell markers | |||
| ALDH1 | Lung tumor | Surface marker | [232] |
| Musashi-1 | Endometrium tumor stem cells | Neural stem cell regulatory protein | [233] |
| LgR5 | Esophageal adenocarcinomas | G-protein coupled receptor | [234] |
| DCAMKL-1 | Intestinal neoplasia; adenoma stem cells | Related to β-catenin | [236] |
| TIM3 | Myeloid leukemia (AML) stem cell | Surface marker | [237] |
| Brca1 | Mammary tumor | Human caretaker gene | [238] |
| SDF-1, CXCR4 | Homing of stem cells and metastasis of cancer cells | Cell factor | [239] |
| PSCA | Prostate cancer | Cell surface antigen | [235] |
| CD96 | leukemic stem cells | Surface marker | [206] |
| CD44 | Breast tumor, tumor invasion, prostate cancer | Surface marker | [217,218,247] |
| CD45 | Hepatocellular carcinoma cell lines | Surface marker | [248] |
8. Conclusions and Future Prospects
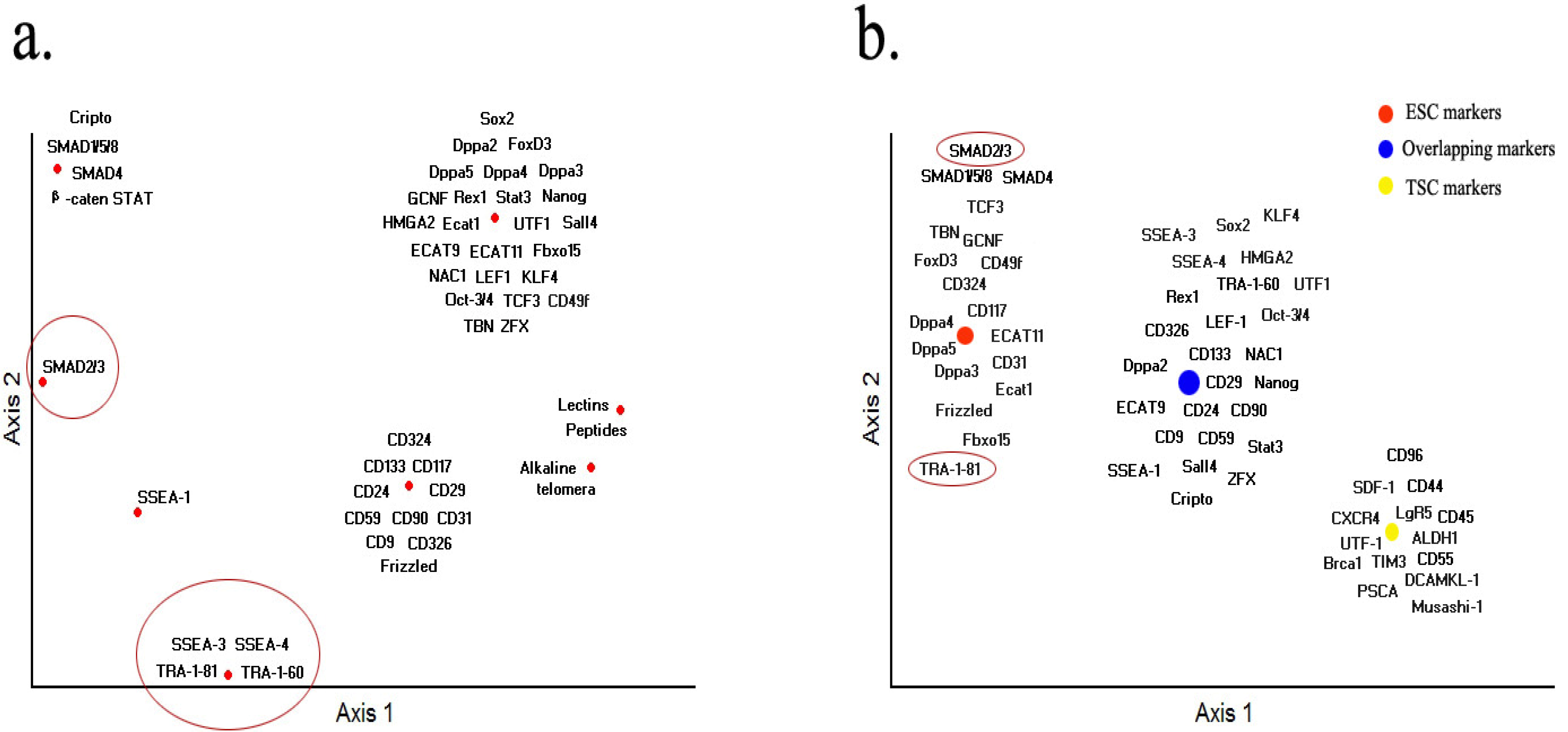
Acknowledgments
References
- Evans, M.J.; Kaufman, M.H. Establishment in culture of pluripotential cells from mouse embryos. Nature 1981, 292, 154–156. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic stem cell lines derived from human blastocysts. Science 1998, 282, 1145–1147. [Google Scholar]
- Prowse, A.B.; McQuade, L.R.; Bryant, K.J.; Marcal, H.; Gray, P.P. Identification of potential pluripotency determinants for human embryonic stem cells following proteomic analysis of human and mouse fibroblast conditioned media. J. Proteome Res. 2007, 6, 3796–3807. [Google Scholar] [CrossRef]
- Scholer, H.R.; Hatzopoulos, A.K.; Balling, R.; Suzuki, N.; Gruss, P. A family of octamer-specific proteins present during mouse embryogenesis—Evidence for germline-specific expression of an oct factor. EMBO J. 1989, 8, 2543–2550. [Google Scholar]
- Shamblott, M.J.; Axelman, J.; Wang, S.; Bugg, E.M.; Littlefield, J.W.; Donovan, P.J.; Blumenthal, P.D.; Huggins, G.R.; Gearhart, J.D. Derivation of pluripotent stem cells from cultured human primordial germ cells. Proc. Natl. Acad. Sci. USA 1998, 95, 13726–13731. [Google Scholar]
- Fox, N.; Damjanov, I.; Martinez-Hernandez, A.; Knowles, B.B.; Solter, D. Immunohistochemical localization of the early embryonic antigen (ssea-1) in postimplantation mouse embryos and fetal and adult tissues. Dev. Biol. 1981, 83, 391–398. [Google Scholar] [CrossRef]
- Fox, N.; Shevinsky, L.; Knowles, B.B.; Solter, D.; Dawjanov, I. Distribution of murine stage-specific embryonic antigens in the kidneys of three rodent species. Exp. Cell Res. 1982, 140, 331–339. [Google Scholar] [CrossRef]
- Henderson, J.K.; Draper, J.S.; Baillie, H.S.; Fishel, S.; Thomson, J.A.; Moore, H.; Andrews, P.W. Preimplantation human embryos and embryonic stem cells show comparable expression of stage-specific embryonic antigens. Stem Cells 2002, 20, 329–337. [Google Scholar] [CrossRef]
- Kannagi, R.; Cochran, N.A.; Ishigami, F.; Hakomori, S.; Andrews, P.W.; Knowles, B.B.; Solter, D. Stage-specific embryonic antigens (ssea-3 and -4) are epitopes of a unique globo-series ganglioside isolated from human teratocarcinoma cells. EMBO J. 1983, 2, 2355–2361. [Google Scholar]
- Knowles, B.B.; Aden, D.P.; Solter, D. Monoclonal antibody detecting a stage-specific embryonic antigen (ssea-1) on preimplantation mouse embryos and teratocarcinoma cells. Curr. Top. Microbiol. Immunol. 1978, 81, 51–53. [Google Scholar]
- Shevinsky, L.H.; Knowles, B.B.; Damjanov, I.; Solter, D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expressed on mouse embryos and human teratocarcinoma cells. Cell 1982, 30, 697–705. [Google Scholar] [CrossRef]
- Adewumi, O.; Aflatoonian, B.; Ahrlund-Richter, L.; Amit, M.; Andrews, P.W.; Beighton, G.; Bello, P.A.; Benvenisty, N.; Berry, L.S.; Bevan, S.; et al. Characterization of human embryonic stem cell lines by the international stem cell initiative. Nat. Biotechnol. 2007, 25, 803–816. [Google Scholar] [CrossRef]
- Assou, S.; Le Carrour, T.; Tondeur, S.; Strom, S.; Gabelle, A.; Marty, S.; Nadal, L.; Pantesco, V.; Reme, T.; Hugnot, J.P.; et al. A meta-analysis of human embryonic stem cells transcriptome integrated into a web-based expression atlas. Stem Cells 2007, 25, 961–973. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Miura, T.; Brandenberger, R.; Mejido, J.; Luo, Y.Q.; Yang, A.X.; Joshi, B.H.; Ginis, I.; Thies, R.S.; Amit, M.; et al. Gene expression in human embryonic stem cell lines: Unique molecular signature. Blood 2004, 103, 2956–2964. [Google Scholar] [CrossRef]
- Draper, J.S.; Pigott, C.; Thomson, J.A.; Andrews, P.W. Surface antigens of human embryonic stem cells: Changes upon differentiation in culture. J. Anat. 2002, 200, 249–258. [Google Scholar] [CrossRef]
- Lian, Q.Z.; Lye, E.; Yeo, K.S.; Tan, E.K.W.; Salto-Tellez, M.; Liu, T.M.; Palanisamy, N.; El Oakley, R.M.; Lee, E.H.; Lim, B.; et al. Derivation of clinically compliant mscs from cd105+, cd24-differentiated human escs. Stem Cells 2007, 25, 425–436. [Google Scholar] [CrossRef]
- Puri, R.K.; Bhattacharya, B.; Miura, T.; Mejido, J.; Luo, Y.Q.; Yang, A.X.; Joshi, B.H.; Irene, G.; Rao, M. Microrray analysis of gene expression identities unique molecular signature in human embryonic stem cell lines. FASEB J. 2004, 18, A1121. [Google Scholar]
- Skottman, H.; Mikkola, M.; Lundin, K.; Olsson, C.; Stromberg, A.M.; Tuuri, T.; Otonkoski, T.; Hovatta, O.; Lahesmaa, R. Gene expression signatures of seven individual human embryonic stem cell lines. Stem Cells 2005, 23, 1343–1356. [Google Scholar] [CrossRef]
- Sundberg, M.; Jansson, L.; Ketolainen, J.; Pihlajamaki, H.; Suuronen, R.; Skottman, H.; Inzunza, J.; Hovatta, O.; Narkilahti, S. Cd marker expression profiles of human embryonic stem cells and their neural derivatives, determined using flow-cytometric analysis, reveal a novel cd marker for exclusion of pluripotent stem cells. Stem Cell Res. 2009, 2, 113–124. [Google Scholar] [CrossRef]
- Xu, C.H.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef]
- Yin, A.H.; Miraglia, S.; Zanjani, E.D.; AlmeidaPorada, G.; Ogawa, M.; Leary, A.G.; Olweus, J.; Kearney, J.; Buck, D.W. Ac133, a novel marker for human hematopoietic stem and progenitor cells. Blood 1997, 90, 5002–5012. [Google Scholar]
- Aumailley, M.; Timpl, R.; Sonnenberg, A. Antibody to integrin alpha 6 subunit specifically inhibits cell-binding to laminin fragment 8. Exp. Cell Res. 1990, 188, 55–60. [Google Scholar] [CrossRef]
- Chute, J.P. Stem cell homing. Curr. Opin. Hematol. 2006, 13, 399–406. [Google Scholar] [CrossRef]
- Fassler, R.; Pfaff, M.; Murphy, J.; Noegel, A.A.; Johansson, S.; Timpl, R.; Albrecht, R. Lack of beta 1 integrin gene in embryonic stem cells affects morphology, adhesion, and migration but not integration into the inner cell mass of blastocysts. J. Cell Biol. 1995, 128, 979–988. [Google Scholar] [CrossRef]
- Hall, D.E.; Reichardt, L.F.; Crowley, E.; Holley, B.; Moezzi, H.; Sonnenberg, A.; Damsky, C.H. The alpha 1/beta 1 and alpha 6/beta 1 integrin heterodimers mediate cell attachment to distinct sites on laminin. J. Cell Biol. 1990, 110, 2175–2184. [Google Scholar]
- Harris, E.S.; McIntyre, T.M.; Prescott, S.M.; Zimmerman, G.A. The leukocyte integrins. J. Biol. Chem. 2000, 275, 23409–23412. [Google Scholar]
- Lee, S.T.; Yun, J.I.; Jo, Y.S.; Mochizuki, M.; van der Vlies, A.J.; Kontos, S.; Ihm, J.E.; Lim, J.M.; Hubbell, J.A. Engineering integrin signaling for promoting embryonic stem cell self-renewal in a precisely defined niche. Biomaterials 2010, 31, 1219–1226. [Google Scholar]
- Qian, H.; Georges-Labouesse, E.; Nystrom, A.; Domogatskaya, A.; Tryggvason, K.; Jacobsen, S.E.; Ekblom, M. Distinct roles of integrins alpha6 and alpha4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood 2007, 110, 2399–2407. [Google Scholar] [CrossRef]
- Rabinovitz, I.; Nagle, R.B.; Cress, A.E. Integrin alpha-6 expression in human prostate carcinoma-cells is associated with a migratory and invasive phenotype in-vitro and in-vivo. Clin. Exp. Metastasis 1995, 13, 481–491. [Google Scholar] [CrossRef]
- Ruoslahti, E.; Pierschbacher, M.D. New perspectives in cell adhesion: Rgd and integrins. Science 1987, 238, 491–497. [Google Scholar]
- Watt, F.M.; Hogan, B.L. Out of eden: Stem cells and their niches. Science 2000, 287, 1427–1430. [Google Scholar] [CrossRef]
- Andrews, P.W.; Banting, G.; Damjanov, I.; Arnaud, D.; Avner, P. Three monoclonal antibodies defining distinct differentiation antigens associated with different high molecular weight polypeptides on the surface of human embryonal carcinoma cells. Hybridoma 1984, 3, 347–361. [Google Scholar] [CrossRef]
- Draper, J.S.; Pigott, C.; Thomson, J.A.; Andrews, P.W. Surface antigens of human embryonic stem cells: Changes upon differentiation in culture. J. Anat. 2002, 200, 249–258. [Google Scholar] [CrossRef]
- Giwercman, A.; Andrews, P.W.; Jorgensen, N.; Muller, J.; Graem, N.; Skakkebaek, N.E. Immunohistochemical expression of embryonal marker tra-1-60 in carcinoma in situ and germ cell tumors of the testis. Cancer 1993, 72, 1308–1314. [Google Scholar] [CrossRef]
- Schopperle, W.M.; DeWolf, W.C. The tra-1-60 and tra-1-81 human pluripotent stem cell markers are expressed on podocalyxin in embryonal carcinoma. Stem Cells 2007, 25, 723–730. [Google Scholar] [CrossRef]
- Barker, N.; Clevers, H. Catenins, wnt signaling and cancer. Bioessays 2000, 22, 961–965. [Google Scholar] [CrossRef]
- Katoh, Y.; Katoh, M. Conserved pou-binding site linked to sp1-binding site within fzd5 promoter: Transcriptional mechanisms of fzd5 in undifferentiated human es cells, fetal liver/spleen, adult colon, pancreatic islet, and diffuse-type gastric cancer. Int. J. Oncol. 2007, 30, 751–755. [Google Scholar]
- Layden, B.T.; Newman, M.; Chen, F.; Fisher, A.; Lowe, W.L. G protein coupled receptors in embryonic stem cells: A role for gs-alpha signaling. PLoS One 2010, 5, e9105. [Google Scholar]
- Okoye, U.C.; Malbon, C.C.; Wang, H.Y. Wnt and frizzled rna expression in human mesenchymal and embryonic (h7) stem cells. J. Mol. Signal. 2008, 3, 16. [Google Scholar] [CrossRef]
- Polakis, P. Wnt signaling and cancer. Genes Dev. 2000, 14, 1837–1851. [Google Scholar]
- Geissler, E.N.; Liao, M.; Brook, J.D.; Martin, F.H.; Zsebo, K.M.; Housman, D.E.; Galli, S.J. Stem cell factor (scf), a novel hematopoietic growth factor and ligand for c-kit tyrosine kinase receptor, maps on human chromosome 12 between 12q14.3 and 12qter. Somat. Cell Mol. Genet. 1991, 17, 207–214. [Google Scholar] [CrossRef]
- Bashamboo, A.; Taylor, A.H.; Samuel, K.; Panthier, J.J.; Whetton, A.D.; Forrester, L.M. The survival of differentiating embryonic stem cells is dependent on the scf-kit pathway. J. Cell Sci. 2006, 119, 3039–3046. [Google Scholar] [CrossRef]
- Lonardo, E.; Parish, C.L.; Ponticelli, S.; Marasco, D.; Ribeiro, D.; Ruvo, M.; De Falco, S.; Arenas, E.; Minchiotti, G. A small synthetic cripto blocking peptide improves neural induction, dopaminergic differentiation, and functional integration of mouse embryonic stem cells in a rat model of parkinson's disease. Stem Cells 2010, 28, 1326–1337. [Google Scholar] [CrossRef]
- Strohmeyer, T.; Reese, D.; Press, M.; Ackermann, R.; Hartmann, M.; Slamon, D. Expression of the c-kit proto-oncogene and its ligand stem cell factor (scf) in normal and malignant human testicular tissue. J. Urol. 1995, 153, 511–515. [Google Scholar] [CrossRef]
- Solter, D.; Knowles, B.B. Monoclonal antibody defining a stage-specific mouse embryonic antigen (ssea-1). Proc. Natl. Acad. Sci. USA 1978, 75, 5565–5569. [Google Scholar] [CrossRef]
- Disatnik, M.H.; Rando, T.A. Integrin-mediated muscle cell spreading. The role of protein kinase c in outside-in and inside-out signaling and evidence of integrin cross-talk. J. Biol. Chem. 1999, 274, 32486–32492. [Google Scholar] [CrossRef]
- Sonnenberg, A.; Linders, C.J.; Modderman, P.W.; Damsky, C.H.; Aumailley, M.; Timpl, R. Integrin recognition of different cell-binding fragments of laminin (p1, e3, e8) and evidence that alpha 6 beta 1 but not alpha 6 beta 4 functions as a major receptor for fragment 8e. J. Cell Biol. 1990, 110, 2145–2155. [Google Scholar] [CrossRef]
- Malbon, C.C. Frizzleds: New members of the superfamily of g-protein-coupled receptors. Front. Biosci. 2004, 9, 1048–1058. [Google Scholar] [CrossRef]
- Hassan, S.; Kinoshita, Y.; Kawanami, C.; Kishi, K.; Matsushima, Y.; Ohashi, A.; Funasaka, Y.; Okada, A.; Maekawa, T.; He-Yao, W.; et al. Expression of protooncogene c-kit and its ligand stem cell factor (scf) in gastric carcinoma cell lines. Dig. Dis. Sci. 1998, 43, 8–14. [Google Scholar] [CrossRef]
- Gray, P.C.; Shani, G.; Aung, K.; Kelber, J.; Vale, W. Cripto binds transforming growth factor beta (tgf-beta) and inhibits tgf-beta signaling. Mol. Cell. Biol. 2006, 26, 9268–9278. [Google Scholar] [CrossRef]
- Calvanese, L.; Saporito, A.; Marasco, D.; D'Auria, G.; Minchiotti, G.; Pedone, C.; Paolillo, L.; Falcigno, L.; Ruvo, M. Solution structure of mouse cripto cfc domain and its inactive variant trp107ala. J. Med. Chem. 2006, 49, 7054–7062. [Google Scholar] [CrossRef]
- Pesce, M.; Scholer, H.R. Oct-4: Control of totipotency and germline determination. Molecular Reprod. Dev. 2000, 55, 452–457. [Google Scholar] [CrossRef]
- Pesce, M.; Scholer, H.R. Oct-4: Gatekeeper in the beginnings of mammalian development. Stem Cells 2001, 19, 271–278. [Google Scholar] [CrossRef]
- Botquin, V.; Hess, H.; Fuhrmann, G.; Anastassiadis, C.; Gross, M.K.; Vriend, G.; Scholer, H.R. New pou dimer configuration mediates antagonistic control of an osteopontin preimplantation enhancer by oct-4 and sox-2. Genes Dev. 1998, 12, 2073–2090. [Google Scholar] [CrossRef]
- Boyer, L.A.; Lee, T.I.; Cole, M.F.; Johnstone, S.E.; Levine, S.S.; Zucker, J.R.; Guenther, M.G.; Kumar, R.M.; Murray, H.L.; Jenner, R.G.; et al. Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 2005, 122, 947–956. [Google Scholar] [CrossRef]
- Jiang, J.M.; Chan, Y.S.; Loh, Y.H.; Cai, J.; Tong, G.Q.; Lim, C.A.; Robson, P.; Zhong, S.; Ng, H.H. A core klf circuitry regulates self-renewal of embryonic stem cells. Nat. Cell Biol. 2008, 10, 353–360. [Google Scholar] [CrossRef]
- Chambers, I.; Colby, D.; Robertson, M.; Nichols, J.; Lee, S.; Tweedie, S.; Smith, A. Functional expression cloning of nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 2003, 113, 643–655. [Google Scholar] [CrossRef]
- Hatano, S.; Tada, M.; Kimura, H.; Yamaguchi, S.; Kono, T.; Nakano, T.; Suemori, H.; Nakatsuji, N.; Tada, T. Pluripotential competence of cells associated with nanog activity. Mech. Dev. 2005, 122, 67–79. [Google Scholar] [CrossRef]
- Mitsui, K.; Tokuzawa, Y.; Itoh, H.; Segawa, K.; Murakami, M.; Takahashi, K.; Maruyama, M.; Maeda, M.; Yamanaka, S. The homeoprotein nanog is required for maintenance of pluripotency in mouse epiblast and es cells. Cell 2003, 113, 631–642. [Google Scholar] [CrossRef]
- Gordon, S.; Akopyan, G.; Garban, H.; Bonavida, B. Transcription factor yy1: Structure, function, and therapeutic implications in cancer biology. Oncogene 2006, 25, 1125–1142. [Google Scholar] [CrossRef]
- Koestenbauer, S.; Zech, N.H.; Juch, H.; Vanderzwalmen, P.; Schoonjans, L.; Dohr, G. Embryonic stem cells: Similarities and differences between human and murine embryonic stem cells. Am. J. Reprod. Immunol. 2006, 55, 169–180. [Google Scholar] [CrossRef]
- Rogers, M.B.; Hosler, B.A.; Gudas, L.J. Specific expression of a retinoic acid-regulated, zinc-finger gene, rex-1, in preimplantation embryos, trophoblast and spermatocytes. Development 1991, 113, 815–824. [Google Scholar]
- Kooistra, S.M.; Thummer, R.P.; Eggen, B.J. Characterization of human utf1, a chromatin-associated protein with repressor activity expressed in pluripotent cells. Stem Cell Res. 2009, 2, 211–218. [Google Scholar] [CrossRef]
- van den Boom, V.; Kooistra, S.M.; Boesjes, M.; Geverts, B.; Houtsmuller, A.B.; Monzen, K.; Komuro, I.; Essers, J.; Drenth-Diephuis, L.J.; Eggen, B.J. Utf1 is a chromatin-associated protein involved in es cell differentiation. J. Cell Biol. 2007, 178, 913–924. [Google Scholar] [CrossRef]
- Galan-Caridad, J.M.; Harel, S.; Arenzana, T.L.; Hou, Z.E.; Doetsch, F.K.; Mirny, L.A.; Reizis, B. Zfx controls the self-renewal of embryonic and hematopoietic stem cells. Cell 2007, 129, 345–357. [Google Scholar] [CrossRef]
- Kopito, R.R.; Lee, B.S.; Simmons, D.M.; Lindsey, A.E.; Morgans, C.W.; Schneider, K. Regulation of intracellular ph by a neuronal homolog of the erythrocyte anion-exchanger. Cell 1989, 59, 927–937. [Google Scholar] [CrossRef]
- Voss, A.K.; Thomas, T.; Petrou, P.; Anastassiadis, K.; Scholer, H.; Gruss, P. Taube nuss is a novel gene essential for the survival of pluripotent cells of early mouse embryos. Development 2000, 127, 5449–5461. [Google Scholar]
- Liu, Y.; Labosky, P.A. Regulation of embryonic stem cell self-renewal and pluripotency by foxd3. Stem Cells 2008, 26, 2475–2484. [Google Scholar] [CrossRef]
- Momma, T.; Hanna, L.A.; Clegg, M.S.; Keen, C.L. Zinc influences the in vitro development of peri-implantation mouse embryos. FASEB J. 2002, 16, A652. [Google Scholar]
- Sutton, J.; Costa, R.; Klug, M.; Field, L.; Xu, D.W.; Largaespada, D.A.; Fletcher, C.F.; Jenkins, N.A.; Copeland, N.G.; Klemsz, M.; et al. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J. Biol. Chem. 1996, 271, 23126–23133. [Google Scholar]
- Li, O.; Vasudevan, D.; Davey, C.A.; Droge, P. High-level expression of DNA architectural factor hmga2 and its association with nucleosomes in human embryonic stem cells. Genesis 2006, 44, 523–529. [Google Scholar] [CrossRef]
- Monzen, K.; Ito, Y.; Naito, A.T.; Kasai, H.; Hiroi, Y.; Hayashi, D.; Shiojima, I.; Yamazaki, T.; Miyazono, K.; Asashima, M.; et al. A crucial role of a high mobility group protein hmga2 in cardiogenesis. Nat. Cell Biol. 2008, 10, 567–574. [Google Scholar] [CrossRef]
- Nishino, J.; Kim, I.; Chada, K.; Morrison, S.J. Hmga2 promotes neural stem cell self-renewal in young but not old mice by reducing p16ink4a and p19arf expression. Cell 2008, 135, 227–239. [Google Scholar] [CrossRef]
- Pfannkuche, K.; Summer, H.; Li, O.; Hescheler, J.; Droge, P. The high mobility group protein hmga2: A co-regulator of chromatin structure and pluripotency in stem cells? Stem Cell Rev. 2009, 5, 224–230. [Google Scholar] [CrossRef]
- Kalivas, P.W.; Duffy, P.; Mackler, S.A. Interrupted expression of nac-1 augments the behavioral responses to cocaine. Synapse 1999, 33, 153–159. [Google Scholar] [CrossRef]
- Kim, J.; Chu, J.; Shen, X.; Wang, J.; Orkin, S.H. An extended transcriptional network for pluripotency of embryonic stem cells. Cell 2008, 132, 1049–1061. [Google Scholar] [CrossRef]
- Mackler, S.A.; Korutla, L.; Cha, X.Y.; Koebbe, M.J.; Fournier, K.M.; Bowers, M.S.; Kalivas, P.W. Nac-1 is a brain poz/btb protein that can prevent cocaine-induced sensitization in the rat. J. Neurosci. 2000, 20, 6210–6217. [Google Scholar]
- Lan, Z.J.; Xu, X.; Chung, A.C.; Cooney, A.J. Extra-germ cell expression of mouse nuclear receptor subfamily 6, group a, member 1 (nr6a1). Biol. Reprod. 2009, 80, 905–912. [Google Scholar] [CrossRef]
- Lei, W.; Hirose, T.; Zhang, L.X.; Adachi, H.; Spinella, M.J.; Dmitrovsky, E.; Jetten, A.M. Cloning of the human orphan receptor germ cell nuclear factor/retinoid receptor-related testis-associated receptor and its differential regulation during embryonal carcinoma cell differentiation. J. Mol. Endocrinol. 1997, 18, 167–176. [Google Scholar] [CrossRef]
- Heim, M.H. The jak-stat pathway: Cytokine signalling from the receptor to the nucleus. J. Recept. Signal Transduct. Res. 1999, 19, 75–120. [Google Scholar] [CrossRef]
- Takada, I.; Mihara, M.; Suzawa, M.; Ohtake, F.; Kobayashi, S.; Igarashi, M.; Youn, M.Y.; Takeyama, K.; Nakamura, T.; Mezaki, Y.; et al. A histone lysine methyltransferase activated by non-canonical wnt signalling suppresses ppar-gamma transactivation. Nat. Cell Biol. 2007, 9, 1273–1285. [Google Scholar] [CrossRef]
- Schilham, M.W.; Clevers, H. Hmg box containing transcription factors in lymphocyte differentiation. Semin. Immunol. 1998, 10, 127–132. [Google Scholar] [CrossRef]
- Kohlhase, J.; Pasche, B.; Burfeind, P.; Wischermann, A.; Reichenbach, H.; Froster, U.; Engel, W. Mutations in the sall1 putative transcription factor gene cause townes-brocks syndrome. Eur. J. Hum. Genet. 1998, 6, 33–33. [Google Scholar]
- Zhang, J.; Tam, W.L.; Tong, G.Q.; Wu, Q.; Chan, H.Y.; Soh, B.S.; Lou, Y.; Yang, J.; Ma, Y.; Chai, L.; et al. Sall4 modulates embryonic stem cell pluripotency and early embryonic development by the transcriptional regulation of pou5f1. Nat. Cell Biol. 2006, 8, 1114–1123. [Google Scholar] [CrossRef]
- Tokuzawa, Y.; Kaiho, E.; Maruyama, M.; Takahashi, K.; Mitsui, K.; Maeda, M.; Niwa, H.; Yamanaka, S. Fbx15 is a novel target of oct3/4 but is dispensable for embryonic stem cell self-renewal and mouse development. Mol. Cell. Biol. 2003, 23, 2699–2708. [Google Scholar] [CrossRef]
- Wong, R.C.B.; Ibrahim, A.; Fong, H.; Thompson, N.; Lock, L.F.; Donovan, P.J. L1td1 is a marker for undifferentiated human embryonic stem cells. PLoS One 2011, 6, e19355. [Google Scholar]
- Pierre, A.; Gautier, M.; Callebaut, I.; Bontoux, M.; Jeanpierre, E.; Pontarotti, P.; Monget, P. Atypical structure and phylogenomic evolution of the new eutherian oocyte-and embryo-expressed khdc1/dppa5/ecat1/ooep gene family. Genomics 2007, 90, 583–594. [Google Scholar]
- Levine, A.J.; Brivanlou, A.H. Gdf3, a bmp inhibitor, regulates cell fate in stem cells and early embryos. Development 2006, 133, 209–216. [Google Scholar]
- Tanaka, T.S.; de Silanes, I.L.; Sharova, L.V.; Akutsu, H.; Yoshikawa, T.; Amano, H.; Yamanaka, S.; Gorospe, M.; Ko, M.S.H. Esg1, expressed exclusively in preimplantation embryos, germline, and embryonic stem cells, is a putative rna-binding protein with broad rna targets. Dev. Growth Differ. 2006, 48, 381–390. [Google Scholar] [CrossRef]
- Western, P.; Maldonado-Saldivia, J.; Van den Bergen, J.; Hajkova, P.; Saitou, M.; Barton, S.; Surani, M.A. Analysis of esg1 expression in pluripotent cells and the germline reveals similarities with oct4 and sox2 and differences between human pluripotent cell lines. Stem Cells 2005, 23, 1436–1442. [Google Scholar] [CrossRef]
- Masaki, H.; Nishida, T.; Kitajima, S.; Asahina, K.; Teraoka, H. Developmental pluripotency-associated 4 (dppa4) localized in active chromatin inhibits mouse embryonic stem cell differentiation into a primitive ectoderm lineage. J. Biol. Chem. 2007, 282, 33034–33042. [Google Scholar]
- Du, J.; Chen, T.J.; Zou, X.; Xiong, B.; Lu, G.X. Dppa2 knockdown-induced differentiation and repressed proliferation of mouse embryonic stem cells. J. Biochem. 2010, 147, 265–271. [Google Scholar] [CrossRef]
- Maldonado-Saldivia, J.; Van den Bergen, J.; Krouskos, M.; Gilchrist, M.; Lee, C.; Li, R.; Sinclair, A.H.; Surani, M.A.; Western, P.S. Dppa2 and dppa4 are closely linked sap motif genes restricted to pluripotent cells and the germ line. Stem Cells 2007, 25, 19–28. [Google Scholar] [CrossRef]
- Bortvin, A.; Goodheart, M.; Liao, M.; Page, D.C. Dppa3 / pgc7 / stella is a maternal factor and is not required for germ cell specification in mice. BMC Dev. Biol. 2004, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Bowles, J.; Teasdale, R.P.; James, K.; Koopman, P. Dppa3 is a marker of pluripotency and has a human homologue that is expressed in germ cell tumours. Cytogenet. Genome Res. 2003, 101, 261–265. [Google Scholar] [CrossRef]
- Takahashi, K.; Yamanaka, S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 2006, 126, 663–676. [Google Scholar] [CrossRef] [Green Version]
- Okita, K.; Ichisaka, T.; Yamanaka, S. Generation of germline-competent induced pluripotent stem cells. Nature 2007, 448, 313–317. [Google Scholar]
- Nakagawa, M.; Koyanagi, M.; Tanabe, K.; Takahashi, K.; Ichisaka, T.; Aoi, T.; Okita, K.; Mochiduki, Y.; Takizawa, N.; Yamanaka, S. Generation of induced pluripotent stem cells without myc from mouse and human fibroblasts. Nat. Biotechnol. 2008, 26, 101–106. [Google Scholar] [CrossRef]
- Wernig, M.; Meissner, A.; Cassady, J.P.; Jaenisch, R. C-myc is dispensable for direct reprogramming of mouse fibroblasts. Cell Stem Cell 2008, 2, 10–12. [Google Scholar] [CrossRef]
- Kim, J.B.; Zaehres, H.; Wu, G.M.; Gentile, L.; Ko, K.; Sebastiano, V.; Arauzo-Bravo, M.J.; Ruau, D.; Han, D.W.; Zenke, M.; et al. Pluripotent stem cells induced from adult neural stem cells by reprogramming with two factors. Nature 2008, 454, 646–650. [Google Scholar]
- Kim, J.B.; Sebastiano, V.; Wu, G.M.; Arauzo-Bravo, M.J.; Sasse, P.; Gentile, L.; Ko, K.; Ruau, D.; Ehrich, M.; van den Boom, D.; et al. Oct4-induced pluripotency in adult neural stem cells. Cell 2009, 136, 411–419. [Google Scholar] [CrossRef]
- Kim, J.B.; Greber, B.; Arauzo-Bravo, M.J.; Meyer, J.; Park, K.I.; Zaehres, H.; Scholer, H.R. Direct reprogramming of human neural stem cells by oct4. Nature 2009, 461, 649–653. [Google Scholar]
- Pan, G.J.; Chang, Z.Y.; Scholer, H.R.; Pei, D.Q. Stem cell pluripotency and transcription factor oct4. Cell Res. 2002, 12, 321–329. [Google Scholar] [CrossRef]
- Schoorlemmer, J.; Kruijer, W. Octamer-dependent regulation of the kfgf gene in embryonal carcinoma and embryonic stem-cells. Mech. Dev. 1991, 36, 75–86. [Google Scholar] [CrossRef]
- Yu, J.; Vodyanik, M.A.; Smuga-Otto, K.; Antosiewicz-Bourget, J.; Frane, J.L.; Tian, S.; Nie, J.; Jonsdottir, G.A.; Ruotti, V.; Stewart, R.; et al. Induced pluripotent stem cell lines derived from human somatic cells. Science 2007, 318, 1917–1920. [Google Scholar]
- Ghaleb, A.M.; Nandan, M.O.; Chanchevalap, S.; Dalton, W.B.; Hisamuddin, I.M.; Yang, V.W. Kruppel-like factors 4 and 5: The yin and yang regulators of cellular proliferation. Cell Res. 2005, 15, 92–96. [Google Scholar] [CrossRef]
- Parisi, S.; Passaro, F.; Aloia, L.; Manabe, I.; Nagai, R.; Pastore, L.; Russo, T. Klf5 is involved in self-renewal of mouse embryonic stem cells. J. Cell Sci. 2008, 121, 2629–2634. [Google Scholar] [CrossRef]
- Masui, S.; Ohtsuka, S.; Yagi, R.; Takahashi, K.; Ko, M.S.H.; Niwa, H. Rex1/zfp42 is dispensable for pluripotency in mouse es cells. BMC Dev. Biol. 2008, 8, 45. [Google Scholar] [CrossRef]
- Scotland, K.B.; Chen, S.M.; Sylvester, R.; Gudas, L.J. Analysis of rex1 (zfp42) function in embryonic stem cell differentiation. Dev. Dyn. 2009, 238, 1863–1877. [Google Scholar] [CrossRef]
- Nishimoto, M.; Fukushima, A.; Okuda, A.; Muramatsu, M. The gene for the embryonic stem cell coactivator utf1 carries a regulatory element which selectively interacts with a complex composed of oct-3/4 and sox-2. Mol. Cell. Biol. 1999, 19, 5453–5465. [Google Scholar]
- Rossant, J. Stem cells from the mammalian blastocyst. Stem Cells 2001, 19, 477–482. [Google Scholar] [CrossRef]
- Boiani, M.; Eckardt, S.; Scholer, H.R.; McLaughlin, K.J. Oct4 distribution and level in mouse clones: Consequences for pluripotency. Genes Dev. 2002, 16, 1209–1219. [Google Scholar] [CrossRef]
- Zhao, Y.; Yin, X.; Qin, H.; Zhu, F.; Liu, H.; Yang, W.; Zhang, Q.; Xiang, C.; Hou, P.; Song, Z.; et al. Two supporting factors greatly improve the efficiency of human ipsc generation. Cell Stem Cell 2008, 3, 475–479. [Google Scholar] [CrossRef]
- Okuda, A.; Fukushima, A.; Nishimoto, M.; Orimo, A.; Yamagishi, T.; Nabeshima, Y.; Kuro-o, M.; Nabeshima, Y.; Boon, K.; Keaveney, M.; et al. Utf1, a novel transcriptional coactivator expressed in pluripotent embryonic stem cells and extra-embryonic cells. EMBO J. 1998, 17, 2019–2032. [Google Scholar] [CrossRef]
- Schneider-Gadicke, A.; Beer-Romero, P.; Brown, L.G.; Mardon, G.; Luoh, S.W.; Page, D.C. Putative transcription activator with alternative isoforms encoded by human zfx gene. Nature 1989, 342, 708–711. [Google Scholar]
- Arenzana, T.L.; Smith-Raska, M.R.; Reizis, B. Transcription factor zfx controls bcr-induced proliferation and survival of b lymphocytes. Blood 2009, 113, 5857–5867. [Google Scholar] [CrossRef]
- Tompers, D.M.; Foreman, R.K.; Wang, Q.H.; Kumanova, M.; Labosky, P.A. Foxd3 is required in the trophoblast progenitor cell lineage of the mouse embryo. Dev. Biol. 2005, 285, 126–137. [Google Scholar] [CrossRef]
- Teng, L.; Mundell, N.A.; Frist, A.Y.; Wang, Q.H.; Labosky, P.A. Requirement for foxd3 in the maintenance of neural crest progenitors. Development 2008, 135, 1615–1624. [Google Scholar] [CrossRef]
- Cleynen, I.; Van de Ven, W.J. The hmga proteins: A myriad of functions (review). Int. J. Oncol. 2008, 32, 289–305. [Google Scholar]
- Fusco, A.; Fedele, M. Roles of hmga proteins in cancer. Nat. Rev. Cancer 2007, 7, 899–910. [Google Scholar] [CrossRef]
- Rawlinson, N.J.; West, W.W.; Nelson, M.; Bridge, J.A. Aggressive angiomyxoma with t(12;21) and hmga2 rearrangement: Report of a case and review of the literature. Cancer Genet. Cytogenet. 2008, 181, 119–124. [Google Scholar] [CrossRef]
- Wei, J.J.; Wu, J.; Luan, C.; Yeldandi, A.; Lee, P.; Keh, P.; Liu, J. Hmga2: A potential biomarker complement to p53 for detection of early-stage high-grade papillary serous carcinoma in fallopian tubes. Am. J. Surg. Pathol. 2010, 34, 18–26. [Google Scholar] [CrossRef]
- Mahajan, A.; Liu, Z.; Gellert, L.; Zou, X.; Yang, G.; Lee, P.; Yang, X.; Wei, J.J. Hmga2: A biomarker significantly overexpressed in high-grade ovarian serous carcinoma. Mod. Pathol. 2010, 23, 673–681. [Google Scholar] [CrossRef]
- Korutla, L.; Wang, P.J.; Mackler, S.A. The poz/btb protein nac1 interacts with two different histone deacetylases in neuronal-like cultures. J. Neurochem. 2005, 94, 786–793. [Google Scholar] [CrossRef]
- Korutla, L.; Wang, P.; Jackson, T.G.; Mackler, S.A. Nac1, a poz/btb protein that functions as a corepressor. Neurochem. Int. 2009, 54, 245–252. [Google Scholar] [CrossRef]
- Ishibashi, M.; Nakayama, K.; Yeasmin, S.; Katagiri, A.; Iida, K.; Nakayama, N.; Fukumoto, M.; Miyazaki, K. A btb/poz gene, nac-1, a tumor recurrence-associated gene, as a potential target for taxol resistance in ovarian cancer. Clin. Cancer Res. 2008, 14, 3149–3155. [Google Scholar] [CrossRef]
- Yeasmin, S.; Nakayama, K.; Ishibashi, M.; Katagiri, A.; Iida, K.; Purwana, I.N.; Nakayama, N.; Miyazaki, K. Expression of the bric-a-brac tramtrack broad complex protein nac-1 in cervical carcinomas seems to correlate with poorer prognosis. Clin. Cancer Res. 2008, 14, 1686–1691. [Google Scholar] [CrossRef]
- Jinawath, N.; Vasoontara, C.; Yap, K.L.; Thiaville, M.M.; Nakayama, K.; Wang, T.L.; Shih, I.M. Nac-1, a potential stem cell pluripotency factor, contributes to paclitaxel resistance in ovarian cancer through inactivating gadd45 pathway. Oncogene 2009, 28, 1941–1948. [Google Scholar] [CrossRef]
- Chung, A.C.; Cooney, A.J. Germ cell nuclear factor. Int. J. Biochem. Cell Biol. 2001, 33, 1141–1146. [Google Scholar] [CrossRef]
- Chung, A.C.; Katz, D.; Pereira, F.A.; Jackson, K.J.; DeMayo, F.J.; Cooney, A.J.; O'Malley, B.W. Loss of orphan receptor germ cell nuclear factor function results in ectopic development of the tail bud and a novel posterior truncation. Mol. Cell Biol. 2001, 21, 663–677. [Google Scholar] [CrossRef]
- Akamatsu, W.; DeVeale, B.; Okano, H.; Cooney, A.J.; van der Kooy, D. Suppression of oct4 by germ cell nuclear factor restricts pluripotency and promotes neural stem cell development in the early neural lineage. J. Neurosci. 2009, 29, 2113–2124. [Google Scholar] [CrossRef]
- Hummelke, G.C.; Cooney, A.J. Reciprocal regulation of the mouse protamine genes by the orphan nuclear receptor germ cell nuclear factor and cremtau. Mol. Reprod. Dev. 2004, 68, 394–407. [Google Scholar] [CrossRef]
- Lan, Z.J.; Chung, A.C.; Xu, X.; DeMayo, F.J.; Cooney, A.J. The embryonic function of germ cell nuclear factor is dependent on the DNA binding domain. J. Biol. Chem. 2002, 277, 50660–50667. [Google Scholar]
- Takeda, K.; Noguchi, K.; Shi, W.; Tanaka, T.; Matsumoto, M.; Yoshida, N.; Kishimoto, T.; Akira, S. Targeted disruption of the mouse stat3 gene leads to early embryonic lethality. Proc. Natl. Acad. Sci. USA 1997, 94, 3801–3804. [Google Scholar]
- Niwa, H.; Ogawa, K.; Shimosato, D.; Adachi, K. A parallel circuit of lif signalling pathways maintains pluripotency of mouse es cells. Nature 2009, 460, 118–122. [Google Scholar]
- Burdon, T.; Smith, A.; Savatier, P. Signalling, cell cycle and pluripotency in embryonic stem cells. Trends Cell Biol. 2002, 12, 432–438. [Google Scholar] [CrossRef]
- Matsuda, T.; Nakamura, T.; Nakao, K.; Arai, T.; Katsuki, M.; Heike, T.; Yokota, T. Stat3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J. 1999, 18, 4261–4269. [Google Scholar] [CrossRef]
- Catlett-Falcone, R.; Landowski, T.H.; Oshiro, M.M.; Turkson, J.; Levitzki, A.; Savino, R.; Ciliberto, G.; Moscinski, L.; Fernandez-Luna, J.L.; Nunez, G.; et al. Constitutive activation of stat3 signaling confers resistance to apoptosis in human u266 myeloma cells. Immunity 1999, 10, 105–115. [Google Scholar] [CrossRef]
- Garcia, R.; Jove, R. Activation of stat transcription factors in oncogenic tyrosine kinase signaling. J. Biomed. Sci. 1998, 5, 79–85. [Google Scholar] [CrossRef]
- Bromberg, J.F.; Wrzeszczynska, M.H.; Devgan, G.; Zhao, Y.; Pestell, R.G.; Albanese, C.; Darnell, J.E., Jr. Stat3 as an oncogene. Cell 1999, 98, 295–303. [Google Scholar] [CrossRef]
- Darnell, J.E., Jr.; Kerr, I.M.; Stark, G.R. Jak-stat pathways and transcriptional activation in response to ifns and other extracellular signaling proteins. Science 1994, 264, 1415–1421. [Google Scholar]
- Ihle, J.N. Cytokine receptor signalling. Nature 1995, 377, 591–594. [Google Scholar] [CrossRef]
- Wen, Z.; Zhong, Z.; Darnell, J.E., Jr. Maximal activation of transcription by stat1 and stat3 requires both tyrosine and serine phosphorylation. Cell 1995, 82, 241–250. [Google Scholar] [CrossRef]
- Yokogami, K.; Wakisaka, S.; Avruch, J.; Reeves, S.A. Serine phosphorylation and maximal activation of stat3 during cntf signaling is mediated by the rapamycin target mtor. Curr. Biol. 2000, 10, 47–50. [Google Scholar] [CrossRef]
- Biethahn, S.; Alves, F.; Wilde, S.; Hiddemann, W.; Spiekermann, K. Expression of granulocyte colony-stimulating factor- and granulocyte-macrophage colony-stimulating factor-associated signal transduction proteins of the jak/stat pathway in normal granulopoiesis and in blast cells of acute myelogenous leukemia. Exp. Hematol. 1999, 27, 885–894. [Google Scholar] [CrossRef]
- Waterman, M.L. Lymphoid enhancer factor/t cell factor expression in colorectal cancer. Cancer Metastasis Rev. 2004, 23, 41–52. [Google Scholar] [CrossRef]
- Schilham, M.W.; Wilson, A.; Moerer, P.; Benaissa-Trouw, B.J.; Cumano, A.; Clevers, H.C. Critical involvement of tcf-1 in expansion of thymocytes. J. Immunol. 1998, 161, 3984–3991. [Google Scholar]
- Reya, T.; Clevers, H. Wnt signalling in stem cells and cancer. Nature 2005, 434, 843–850. [Google Scholar] [CrossRef]
- Nguyen, H.; Rendl, M.; Fuchs, E. Tcf3 governs stem cell features and represses cell fate determination in skin. Cell 2006, 127, 171–183. [Google Scholar] [CrossRef]
- Cole, M.F.; Johnstone, S.E.; Newman, J.J.; Kagey, M.H.; Young, R.A. Tcf3 is an integral component of the core regulatory circuitry of embryonic stem cells. Genes Dev. 2008, 22, 746–755. [Google Scholar] [CrossRef]
- Kohlhase, J.; Wischermann, A.; Reichenbach, H.; Froster, U.; Engel, W. Mutations in the sall1 putative transcription factor gene cause townes-brocks syndrome. Nat. Genet. 1998, 18, 81–83. [Google Scholar] [CrossRef]
- Yuri, S.; Fujimura, S.; Nimura, K.; Takeda, N.; Toyooka, Y.; Fujimura, Y.; Aburatani, H.; Ura, K.; Koseki, H.; Niwa, H.; et al. Sall4 is essential for stabilization, but not for pluripotency, of embryonic stem cells by repressing aberrant trophectoderm gene expression. Stem Cells 2009, 27, 796–805. [Google Scholar] [CrossRef]
- Rao, S.; Zhen, S.; Roumiantsev, S.; McDonald, L.T.; Yuan, G.C.; Orkin, S.H. Differential roles of sall4 isoforms in embryonic stem cell pluripotency. Mol. Cell. Biol. 2010, 30, 5364–5380. [Google Scholar] [CrossRef]
- Yang, J.; Gao, C.; Chai, L.; Ma, Y. A novel sall4/oct4 transcriptional feedback network for pluripotency of embryonic stem cells. PLoS One 2010, 5, e10766. [Google Scholar]
- Takahashi, K.; Mitsui, K.; Yamanaka, S. Role of eras in promoting tumour-like properties in mouse embryonic stem cells. Nature 2003, 423, 541–545. [Google Scholar] [CrossRef]
- Niwa, H.; Burdon, T.; Chambers, I.; Smith, A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of stat3. Genes Dev. 1998, 12, 2048–2060. [Google Scholar] [CrossRef]
- Ying, Q.L.; Nichols, J.; Chambers, I.; Smith, A. Bmp induction of id proteins suppresses differentiation and sustains embryonic stem cell self-renewal in collaboration with stat3. Cell 2003, 115, 281–292. [Google Scholar] [CrossRef]
- Sato, N.; Meijer, L.; Skaltsounis, L.; Greengard, P.; Brivanlou, A.H. Maintenance of pluripotency in human and mouse embryonic stem cells through activation of wnt signaling by a pharmacological gsk-3-specific inhibitor. Nat. Med. 2004, 10, 55–63. [Google Scholar] [CrossRef]
- Boiani, M.; Scholer, H.R. Regulatory networks in embryo-derived pluripotent stem cells. Nat. Rev. Mol. Cell Biol. 2005, 6, 872–884. [Google Scholar] [CrossRef]
- Chambers, I.; Smith, A. Self-renewal of teratocarcinoma and embryonic stem cells. Oncogene 2004, 23, 7150–7160. [Google Scholar] [CrossRef] [Green Version]
- Niwa, H.; Burdon, T.; Chambers, I.; Smith, A. Self-renewal of pluripotent embryonic stem cells is mediated via activation of stat3. Genes Dev. 1998, 12, 2048–2060. [Google Scholar] [CrossRef]
- Daheron, L.; Opitz, S.L.; Zaehres, H.; Lensch, W.M.; Andrews, P.W.; Itskovitz-Eldor, J.; Daley, G.Q. Lif/stat3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2007, 25, 3273–3273. [Google Scholar] [CrossRef]
- Daheron, L.; Opitz, S.L.; Zaehres, H.; Lensch, W.M.; Andrews, P.W.; Itskovitz-Eldor, J.; Daley, G.Q. Lif/stat3 signaling fails to maintain self-renewal of human embryonic stem cells. Stem Cells 2004, 22, 770–778. [Google Scholar] [CrossRef]
- Humphrey, R.K.; Beattie, G.M.; Lopez, A.D.; Bucay, N.; King, C.C.; Firpo, M.T.; Rose-John, S.; Hayek, A. Maintenance of pluripotency in human embryonic stem cells is stat3 independent. Stem Cells 2004, 22, 522–530. [Google Scholar] [CrossRef]
- Robertson, E.J.; Brennan, J.; Lu, C.; Trembley, K.D.; Bikoff, E.K.; Norris, D.P. Tgf beta signaling pathways controlling polarity of the early mouse embryo. Dev. Biol. 2000, 222, 223–223. [Google Scholar] [CrossRef]
- Massague, J.; Chen, Y.G. Controlling tgf-beta signaling. Genes Dev. 2000, 14, 627–644. [Google Scholar]
- Mishra, L.; Derynck, R.; Mishra, B. Transforming growth factor-beta signaling in stem cells and cancer. Science 2005, 310, 68–71. [Google Scholar] [CrossRef]
- Watabe, T.; Miyazono, K. Roles of tgf-beta family signaling in stem cell renewal and differentiation. Cell Res. 2009, 19, 103–115. [Google Scholar] [CrossRef]
- Gerami-Naini, B.; Dovzhenko, O.V.; Durning, M.; Wegner, F.H.; Thomson, J.A.; Golos, T.G. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology 2004, 145, 1517–1524. [Google Scholar]
- Xu, R.H.; Chen, X.; Li, D.S.; Li, R.; Addicks, G.C.; Glennon, C.; Zwaka, T.P.; Thomson, J.A. Bmp4 initiates human embryonic stem cell differentiation to trophoblast. Nat. Biotechnol. 2002, 20, 1261–1264. [Google Scholar] [CrossRef]
- Datto, M.; Wang, X.F. The smads: Transcriptional regulation and mouse models. Cytokine Growth Factor Rev. 2000, 11, 37–48. [Google Scholar] [CrossRef]
- Feng, X.H.; Derynck, R. Specificity and versatility in tgf-beta signaling through smads. Annu. Rev. Cell Dev. Biol. 2005, 21, 659–693. [Google Scholar] [CrossRef]
- Massague, J.; Seoane, J.; Wotton, D. Smad transcription factors. Genes Dev. 2005, 19, 2783–2810. [Google Scholar] [CrossRef]
- ten Dijke, P.; Hill, C.S. New insights into tgf-beta-smad signalling. Trends Biochem. Sci. 2004, 29, 265–273. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/beta-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef]
- Abu-Remaileh, M.; Gerson, A.; Farago, M.; Nathan, G.; Alkalay, I.; Rousso, S.Z.; Gur, M.; Fainsod, A.; Bergman, Y. Oct-3/4 regulates stem cell identity and cell fate decisions by modulating wnt/beta-catenin signalling. EMBO J. 2010, 29, 3236–3248. [Google Scholar] [CrossRef]
- Miyabayashi, T.; Teo, J.L.; Yamamoto, M.; McMillan, M.; Nguyen, C.; Kahn, M. Wnt/beta-catenin/cbp signaling maintains long-term murine embryonic stem cell pluripotency. Proc. Natl. Acad. Sci. USA 2007, 104, 5668–5673. [Google Scholar]
- Takao, Y.; Yokota, T.; Koide, H. Beta-catenin up-regulates nanog expression through interaction with oct-3/4 in embryonic stem cells. Biochem. Biophys. Res. Commun. 2007, 353, 699–705. [Google Scholar] [CrossRef]
- Vallier, L.; Alexander, M.; Pedersen, R.A. Activin/nodal and fgf pathways cooperate to maintain pluripotency of human embryonic stem cells. J. Cell Sci. 2005, 118, 4495–4509. [Google Scholar] [CrossRef]
- Ogawa, K.; Saito, A.; Matsui, H.; Suzuki, H.; Ohtsuka, S.; Shimosato, D.; Morishita, Y.; Watabe, T.; Niwa, H.; Miyazono, K. Activin-nodal signaling is involved in propagation of mouse embryonic stem cells. J. Cell Sci. 2007, 120, 55–65. [Google Scholar]
- Albelda, S.M.; Buck, C.A. Integrins and other cell-adhesion molecules. FASEB J. 1990, 4, 2868–2880. [Google Scholar]
- Adi, M.M.; Chisholm, D.M.; Waterhouse, J.P. Histochemical-study of lectin-binding in the human fetal minor salivary-glands. J. Oral Pathol. Med. 1995, 24, 130–135. [Google Scholar] [CrossRef]
- Chapman, S.A.; Bonshek, R.E.; Stoddart, R.W.; Jones, C.J.P.; Mackenzie, K.R.; Odonaghue, E.; Mcleod, D. Glycoconjugates of the human trabecular meshwork—A lectin histochemical-study. Histochem. J. 1995, 27, 869–881. [Google Scholar]
- Fang, Y.Q.; Welsch, U. A histochemical-study of the distribution of lectin-binding sites in the developing oocytes of the lancelet branchiostoma-belcheri. Cell Tissue Res. 1995, 280, 427–434. [Google Scholar] [CrossRef]
- Takagi, Y.; Talbot, N.C.; Rexroad, C.E.; Pursel, V.G. Identification of pig primordial germ cells by immunocytochemistry and lectin binding. Mol. Reprod. Dev. 1997, 46, 567–580. [Google Scholar] [CrossRef]
- Mandai, M.; Ikeda, H.; Jin, Z.B.; Iseki, K.; Ishigami, C.; Takahashi, M. Use of lectins to enrich mouse es-derived retinal progenitor cells for the purpose of transplantation therapy. Cell Transpl. 2010, 19, 9–19. [Google Scholar] [CrossRef]
- Venable, A.; Mitalipova, M.; Lyons, I.; Jones, K.; Shin, S.J.; Pierce, M.; Stice, S. Lectin binding profiles of ssea-4 enriched, pluripotent human embryonic stem cell surfaces. BMC Dev. Biol. 2005, 5, 15. [Google Scholar] [CrossRef] [Green Version]
- Wearne, K.A.; Winter, H.C.; O'Shea, K.; Goldstein, I.J. Use of lectins for probing differentiated human embryonic stem cells for carbohydrates. Glycobiology 2006, 16, 981–990. [Google Scholar] [CrossRef]
- Derda, R.; Musah, S.; Orner, B.P.; Klim, J.R.; Li, L.Y.; Kiessling, L.L. High-throughput discovery of synthetic surfaces that support proliferation of pluripotent cells. J. Am. Chem. Soc. 2010, 132, 1289–1295. [Google Scholar]
- Smith, G.P. Filamentous fusion phage: Novel expression vectors that display cloned antigens on the virion surface. Science 1985, 228, 1315–1317. [Google Scholar]
- Zhao, S.J.; Zhao, W.X.; Ma, L. Novel peptide ligands that bind specifically to mouse embryonic stem cells. Peptides 2010, 31, 2027–2034. [Google Scholar] [CrossRef]
- Lu, S.; Xu, X.; Zhao, W.X.; Wu, W.W.; Yuan, H.; Shen, H.B.; Zhou, C.H.; Li, S.; Ma, L. Targeting of embryonic stem cells by peptide-conjugated quantum dots. PLoS One 2010, 5, e12075. [Google Scholar]
- Hombach-Klonisch, S.; Panigrahi, S.; Rashedi, I.; Seifert, A.; Alberti, E.; Pocar, P.; Kurpisz, M.; Schulze-Osthoff, K.; Mackiewicz, A.; Los, M. Adult stem cells and their trans-differentiation potential-perspectives and therapeutic applications. J. Mol. Med. 2008, 86, 1301–1314. [Google Scholar] [CrossRef]
- Field, M.; Alvarez, A.; Bushnev, S.; Sugaya, K. Embryonic stem cell markers distinguishing cancer stem cells from normal human neuronal stem cell populations in malignant glioma patients. Clin. Neurosurg. 2010, 57, 151–159. [Google Scholar]
- Visvader, J.E.; Lindeman, G.J. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat. Rev. Cancer 2008, 8, 755–768. [Google Scholar] [CrossRef]
- Friedman, G.K.; Gillespie, G.Y. Cancer stem cells and pediatric solid tumors. Cancers (Basel) 2011, 3, 298–318. [Google Scholar]
- Anderson, E.C.; Hessman, C.; Levin, T.G.; Monroe, M.M.; Wong, M.H. The role of colorectal cancer stem cells in metastatic disease and therapeutic response. Cancers (Basel) 2011, 3, 319–339. [Google Scholar]
- Mitra, M.; Kandalam, M.; Harilal, A.; Verma, R.S.; Krishnan, U.M.; Swaminathan, S.; Krishnakumar, S. Epcam is a putative stem marker in retinoblastoma and an effective target for t-cell-mediated immunotherapy. Mol. Vis. 2012, 18, 290–308. [Google Scholar]
- Galizia, G.; Gemei, M.; Del Vecchio, L.; Zamboli, A.; Di Noto, R.; Mirabelli, P.; Salvatore, F.; Castellano, P.; Orditura, M.; De Vita, F.; et al. Combined cd133/cd44 expression as a prognostic indicator of disease-free survival in patients with colorectal cancer. Arch. Surg. 2012, 147, 18–24. [Google Scholar] [CrossRef]
- Piscuoglio, S.; Lehmann, F.S.; Zlobec, I.; Tornillo, L.; Dietmaier, W.; Hartmann, A.; Wunsch, P.H.; Sessa, F.; Rummele, P.; Baumhoer, D.; et al. Effect of epcam, cd44, cd133 and cd166 expression on patient survival in tumours of the ampulla of vater. J. Clin. Pathol. 2012, 65, 140–145. [Google Scholar] [CrossRef]
- Mao, X.G.; Zhang, X.; Xue, X.Y.; Guo, G.; Wang, P.; Zhang, W.; Fei, Z.; Zhen, H.N.; You, S.W.; Yang, H. Brain tumor stem-like cells identified by neural stem cell marker cd15. Transl. Oncol. 2009, 2, 247–257. [Google Scholar]
- Chang, W.W.; Lee, C.H.; Lee, P.; Lin, J.; Hsu, C.W.; Hung, J.T.; Lin, J.J.; Yu, J.C.; Shao, L.E.; Yu, J.; et al. Expression of globo h and ssea3 in breast cancer stem cells and the involvement of fucosyl transferases 1 and 2 in globo h synthesis. Proc. Natl. Acad. Sci. USA 2008, 105, 11667–11672. [Google Scholar]
- Kamijo, T. Role of stemness-related molecules in neuroblastoma. Pediatr. Res. 2012, 71, 511–515. [Google Scholar] [CrossRef]
- Immervoll, H.; Hoem, D.; Sakariassen, P.O.; Steffensen, O.J.; Molven, A. Expression of the "stem cell marker" cd133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer 2008, 8, 48. [Google Scholar] [CrossRef]
- Horst, D.; Scheel, S.K.; Liebmann, S.; Neumann, J.; Maatz, S.; Kirchner, T.; Jung, A. The cancer stem cell marker cd133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J. Pathol. 2009, 219, 427–434. [Google Scholar] [CrossRef]
- Hosen, N.; Park, C.Y.; Tatsumi, N.; Oji, Y.; Sugiyama, H.; Gramatzki, M.; Krensky, A.M.; Weissman, I.L. Cd96 is a leukemic stem cell-specific marker in human acute myeloid leukemia. Proc. Natl. Acad. Sci. USA 2007, 104, 11008–11013. [Google Scholar]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.K.; Lam, C.T.; Poon, R.T.P.; Fan, S.T. Significance of cd90(+) cancer stem cells in human liver cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef]
- Patriarca, C.; Macchi, R.M.; Marschner, A.K.; Mellstedt, H. Epithelial cell adhesion molecule expression (cd326) in cancer: A short review. Cancer Treat. Rev. 2012, 38, 68–75. [Google Scholar] [CrossRef]
- Kohmo, S.; Kijima, T.; Otani, Y.; Mori, M.; Minami, T.; Takahashi, R.; Nagatomo, I.; Takeda, Y.; Kida, H.; Goya, S.; et al. Cell surface tetraspanin cd9 mediates chemoresistance in small cell lung cancer. Cancer Res. 2010, 70, 8025–8035. [Google Scholar]
- Setoguchi, T.; Kikuchi, H.; Yamamoto, M.; Baba, M.; Ohta, M.; Kamiya, K.; Tanaka, T.; Baba, S.; Goto-Inoue, N.; Setou, M.; et al. Microarray analysis identifies versican and cd9 as potent prognostic markers in gastric gastrointestinal stromal tumors. Cancer Sci. 2011, 102, 883–889. [Google Scholar] [CrossRef]
- Yamazaki, H.; Xu, C.W.; Naito, M.; Nishida, H.; Okamoto, T.; Ghani, F.I.; Iwata, S.; Inukai, T.; Sugita, K.; Morimoto, C. Regulation of cancer stem cell properties by cd9 in human b-acute lymphoblastic leukemia. Biochem. Biophys. Res. Commun. 2011, 409, 14–21. [Google Scholar] [CrossRef]
- Ikeda, J.; Morii, E.; Liu, Y.; Qiu, Y.; Nakamichi, N.; Jokoji, R.; Miyoshi, Y.; Noguchi, S.; Aozasa, K. Prognostic significance of cd55 expression in breast cancer. Clin. Cancer Res. 2008, 14, 4780–4786. [Google Scholar] [CrossRef]
- Zhao, W.P.; Zhu, B.; Duan, Y.Z.; Chen, Z.T. Neutralization of complement regulatory proteins cd55 and cd59 augments therapeutic effect of herceptin against lung carcinoma cells. Oncol. Rep. 2009, 21, 1405–1411. [Google Scholar]
- Li, B.; Chu, X.; Gao, M.; Xu, Y. The effects of cd59 gene as a target gene on breast cancer cells. Cell. Immunol. 2011, 272, 61–70. [Google Scholar] [CrossRef]
- Cui, W.; Zhao, Y.; Shan, C.; Kong, G.; Hu, N.; Zhang, Y.; Zhang, S.; Zhang, W.; Zhang, X.; Ye, L. Hbxip upregulates cd46, cd55 and cd59 through erk1/2/nf-kappab signaling to protect breast cancer cells from complement attack. FEBS Lett. 2012, 586, 766–771. [Google Scholar] [CrossRef]
- Ehira, N.; Oshiumi, H.; Matsumoto, M.; Kondo, T.; Asaka, M.; Seya, T. An embryo-specific expressing tgf-beta family protein, growth-differentiation factor 3 (gdf3), augments progression of b16 melanom. J. Exp. Clin. Cancer Res. 2010, 29, 135. [Google Scholar] [CrossRef]
- Ponti, D.; Costa, A.; Zaffaroni, N.; Pratesi, G.; Petrangolini, G.; Coradini, D.; Pilotti, S.; Pierotti, M.A.; Daidone, M.G. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 2005, 65, 5506–5511. [Google Scholar]
- Ouhtit, A.; Abd Elmageed, Z.Y.; Abdraboh, M.E.; Lioe, T.F.; Raj, M.H. In vivo evidence for the role of cd44s in promoting breast cancer metastasis to the liver. Am. J. Pathol. 2007, 171, 2033–2039. [Google Scholar] [CrossRef]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified cd44(+) prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef]
- Atlasi, Y.; Mowla, S.J.; Ziaee, S.A.; Bahrami, A.R. Oct-4, an embryonic stem cell marker, is highly expressed in bladder cancer. Int. J. Cancer 2007, 120, 1598–1602. [Google Scholar] [CrossRef]
- Leis, O.; Eguiara, A.; Lopez-Arribillaga, E.; Alberdi, M.J.; Hernandez-Garcia, S.; Elorriaga, K.; Pandiella, A.; Rezola, R.; Martin, A.G. Sox2 expression in breast tumours and activation in breast cancer stem cells. Oncogene 2012, 31, 1354–1365. [Google Scholar] [CrossRef]
- Yu, F.; Li, J.; Chen, H.; Fu, J.; Ray, S.; Huang, S.; Zheng, H.; Ai, W. Kruppel-like factor 4 (klf4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene 2011, 30, 2161–2172. [Google Scholar] [CrossRef]
- Lee, M.Y.; Lu, A.; Gudas, L.J. Transcriptional regulation of rex1 (zfp42) in normal prostate epithelial cells and prostate cancer cells. J. Cell. Physiol. 2010, 224, 17–27. [Google Scholar]
- Raman, J.D.; Mongan, N.P.; Liu, L.; Tickoo, S.K.; Nanus, D.M.; Scherr, D.S.; Gudas, L.J. Decreased expression of the human stem cell marker, rex-1 (zfp-42), in renal cell carcinoma. Carcinogenesis 2006, 27, 499–507. [Google Scholar]
- Kristensen, D.M.; Nielsen, J.E.; Skakkebaek, N.E.; Graem, N.; Jacobsen, G.K.; Rajpert-De Meyts, E.; Leffers, H. Presumed pluripotency markers utf-1 and rex-1 are expressed in human adult testes and germ cell neoplasms. Hum. Reprod. 2008, 23, 775–782. [Google Scholar] [CrossRef]
- Nikpour, P.; Emadi-Baygi, M.; Mohammad-Hashem, F.; Maracy, M.R.; Haghjooy-Javanmard, S. Differential expression of zfx gene in gastric cancer. J. Biosci. 2012, 37, 85–90. [Google Scholar] [CrossRef]
- Piscuoglio, S.; Zlobec, I.; Pallante, P.; Sepe, R.; Esposito, F.; Zimmermann, A.; Diamantis, I.; Terracciano, L.; Fusco, A.; Karamitopoulou, E. Hmga1 and hmga2 protein expression correlates with advanced tumour grade and lymph node metastasis in pancreatic adenocarcinoma. Histopathology 2012, 60, 397–404. [Google Scholar] [CrossRef]
- Yang, G.L.; Zhang, L.H.; Bo, J.J.; Hou, K.L.; Cai, X.; Chen, Y.Y.; Li, H.; Liu, D.M.; Huang, Y.R. Overexpression of hmga2 in bladder cancer and its association with clinicopathologic features and prognosis hmga2 as a prognostic marker of bladder cancer. Eur. J. Surg. Oncol. 2011, 37, 265–271. [Google Scholar] [CrossRef]
- John, T.; Caballero, O.L.; Svobodova, S.J.; Kong, A.; Chua, R.; Browning, J.; Fortunato, S.; Deb, S.; Hsu, M.; Gedye, C.A.; et al. Ecsa/dppa2 is an embryo-cancer antigen that is coexpressed with cancer-testis antigens in non-small cell lung cancer. Clin. Cancer Res. 2008, 14, 3291–3298. [Google Scholar]
- Niemann, C.; Owens, D.M.; Schettina, P.; Watt, F.M. Dual role of inactivating lef1 mutations in epidermis: Tumor promotion and specification of tumor type. Cancer Res. 2007, 67, 2916–2921. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.; Zhang, M.; Melamed, J.; Liu, X.; Reiter, R.; Wei, J.; Peng, Y.; Zou, X.; Pellicer, A.; et al. Lef1 in androgen-independent prostate cancer: Regulation of androgen receptor expression, prostate cancer growth, and invasion. Cancer Res. 2009, 69, 3332–3338. [Google Scholar]
- Jiang, F.; Qiu, Q.; Khanna, A.; Todd, N.W.; Deepak, J.; Xing, L.X.; Wang, H.J.; Liu, Z.Q.; Su, Y.; Stass, S.A.; et al. Aldehyde dehydrogenase 1 is a tumor stem cell-associated marker in lung cancer. Mol. Cancer Res. 2009, 7, 330–338. [Google Scholar] [CrossRef]
- Gotte, M.; Wolf, M.; Staebler, A.; Buchweitz, O.; Kelsch, R.; Schuring, A.N.; Kiesel, L. Increased expression of the adult stem cell marker musashi-1 in endometriosis and endometrial carcinoma. J. Pathol. 2008, 215, 317–329. [Google Scholar] [CrossRef]
- von Rahden, B.H.A.; Kircher, S.; Lazariotou, M.; Reiber, C.; Stuermer, L.; Otto, C.; Germer, C.T.; Grimm, M. Lgr5 expression and cancer stem cell hypothesis: Clue to define the true origin of esophageal adenocarcinomas with and without barrett's esophagus? J. Exp. Clin. Cancer Res. 2011, 30, 23. [Google Scholar] [CrossRef]
- Reiter, R.E.; Gu, Z.N.; Watabe, T.; Thomas, G.; Szigeti, K.; Davis, E.; Wahl, M.; Nisitani, S.; Yamashiro, J.; Le Beau, M.M.; et al. Prostate stem cell antigen: A cell surface marker overexpressed in prostate cancer. Proc. Natl. Acad. Sci. USA 1998, 95, 1735–1740. [Google Scholar]
- May, R.; Riehl, T.E.; Hunt, C.; Sureban, S.M.; Anant, S.; Houchen, C.W. Identification of a novel putative gastrointestinal stem cell and adenoma stem cell marker, doublecortin and cam kinase-like-1, following radiation injury and in adenomatous polyposis coli/multiple intestinal neoplasia mice. Stem Cells 2008, 26, 630–637. [Google Scholar] [CrossRef]
- Jan, M.; Chao, M.P.; Cha, A.C.; Alizadeh, A.A.; Gentles, A.J.; Weissman, I.L.; Majeti, R. Prospective separation of normal and leukemic stem cells based on differential expression of tim3, a human acute myeloid leukemia stem cell marker. Proc. Natl. Acad. Sci. USA 2011, 108, 5009–5014. [Google Scholar]
- Wright, M.H.; Calcagno, A.M.; Salcido, C.D.; Carlson, M.D.; Ambudkar, S.V.; Varticovski, L. Brca1 breast tumors contain distinct cd44+/cd24- and cd133+ cells with cancer stem cell characteristics. Breast Cancer Res. 2008, 10, R10. [Google Scholar] [CrossRef]
- Miki, J.; Furusato, B.; Li, H.; Gu, Y.; Takahashi, H.; Egawa, S.; Sesterhenn, I.A.; McLeod, D.G.; Srivastava, S.; Rhim, J.S. Identification of putative stem cell markers, cd133 and cxcr4, in htert-immortalized primary nonmalignant and malignant tumor-derived human prostate epithelial cell lines and in prostate cancer specimens. Cancer Res. 2007, 67, 3153–3161. [Google Scholar] [CrossRef]
- Mao, X.G.; Zhang, X.; Xue, X.Y.; Guo, G.; Wang, P.; Zhang, W.; Fei, Z.; Zhen, H.N.; You, S.W.; Yang, H. Brain tumor stem-like cells identified by neural stem cell marker cd15. Transl. Oncol. 2009, 2, 247–257. [Google Scholar]
- Ye, F.; Li, Y.; Hu, Y.; Zhou, C.; Chen, H. Stage-specific embryonic antigen 4 expression in epithelial ovarian carcinoma. Int. J. Gynecol. Cancer 2010, 20, 958–964. [Google Scholar] [CrossRef]
- Hagiwara, S.; Kudo, M.; Ueshima, K.; Chung, H.; Yamaguchi, M.; Takita, M.; Haji, S.; Kimura, M.; Arao, T.; Nishio, K.; et al. The cancer stem cell marker cd133 is a predictor of the effectiveness of s1+ pegylated interferon alpha-2b therapy against advanced hepatocellular carcinoma. J. Gastroenterol. 2011, 46, 212–221. [Google Scholar] [CrossRef]
- Horst, D.; Scheel, S.K.; Liebmann, S.; Neumann, J.; Maatz, S.; Kirchner, T.; Jung, A. The cancer stem cell marker cd133 has high prognostic impact but unknown functional relevance for the metastasis of human colon cancer. J. Pathol. 2009, 219, 427–434. [Google Scholar] [CrossRef]
- Yang, J.P.; Liu, Y.; Zhong, W.; Yu, D.; Wen, L.J.; Jin, C.S. Chemoresistance of cd133+ cancer stem cells in laryngeal carcinoma. Chin. Med. J. (Engl) 2011, 124, 1055–1060. [Google Scholar]
- Zeppernick, F.; Ahmadi, R.; Campos, B.; Dictus, C.; Helmke, B.M.; Becker, N.; Lichter, P.; Unterberg, A.; Radlwimmer, B.; Herold-Mende, C.C. Stem cell marker cd133 affects clinical outcome in glioma patients. Clin. Cancer Res. 2008, 14, 123–129. [Google Scholar]
- Shackleton, M.; Vaillant, F.; Simpson, K.J.; Stingl, J.; Smyth, G.K.; Asselin-Labat, M.L.; Wu, L.; Lindeman, G.J.; Visvader, J.E. Generation of a functional mammary gland from a single stem cell. Nature 2006, 439, 84–88. [Google Scholar]
- Patrawala, L.; Calhoun, T.; Schneider-Broussard, R.; Li, H.; Bhatia, B.; Tang, S.; Reilly, J.G.; Chandra, D.; Zhou, J.; Claypool, K.; et al. Highly purified cd44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene 2006, 25, 1696–1708. [Google Scholar] [CrossRef]
- Yang, Z.F.; Ho, D.W.; Ng, M.N.; Lau, C.K.; Yu, W.C.; Ngai, P.; Chu, P.W.; Lam, C.T.; Poon, R.T.; Fan, S.T. Significance of cd90+ cancer stem cells in human liver cancer. Cancer Cell 2008, 13, 153–166. [Google Scholar] [CrossRef]
- Yamashita, T.; Budhu, A.; Forgues, M.; Wang, X.W. Activation of hepatic stem cell marker epcam by wnt-beta-catenin signaling in hepatocellular carcinoma. Cancer Res. 2007, 67, 10831–10839. [Google Scholar] [CrossRef]
- Chen, Y.C.; Hsu, H.S.; Chen, Y.W.; Tsai, T.H.; How, C.K.; Wang, C.Y.; Hung, S.C.; Chang, Y.L.; Tsai, M.L.; Lee, Y.Y.; et al. Oct-4 expression maintained cancer stem-like properties in lung cancer-derived cd133-positive cells. PLoS One 2008, 3, e2637. [Google Scholar]
- Moon, J.S.; Kim, H.E.; Koh, E.; Park, S.H.; Jin, W.J.; Park, B.W.; Park, S.W.; Kim, K.S. Kruppel-like factor 4 (klf4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (pfkp) in breast cancer. J. Biol. Chem. 2011, 286, 23808–23816. [Google Scholar]
- Gutierrez, A.; Sanda, T.; Ma, W.; Zhang, J.; Grebliunaite, R.; Dahlberg, S.; Neuberg, D.; Protopopov, A.; Winter, S.S.; Larson, R.S.; et al. Inactivation of lef1 in t-cell acute lymphoblastic leukemia. Blood 2010, 115, 2845–2851. [Google Scholar] [CrossRef]
- Cao, D.; Li, J.; Guo, C.C.; Allan, R.W.; Humphrey, P.A. Sall4 is a novel diagnostic marker for testicular germ cell tumors. Am. J. Surg. Pathol. 2009, 33, 1065–1077. [Google Scholar] [CrossRef]
- Cao, D.; Humphrey, P.A.; Allan, R.W. Sall4 is a novel sensitive and specific marker for metastatic germ cell tumors, with particular utility in detection of metastatic yolk sac tumors. Cancer 2009, 115, 2640–2651. [Google Scholar] [CrossRef]
- Cao, D.; Guo, S.; Allan, R.W.; Molberg, K.H.; Peng, Y. Sall4 is a novel sensitive and specific marker of ovarian primitive germ cell tumors and is particularly useful in distinguishing yolk sac tumor from clear cell carcinoma. Am. J. Surg. Pathol. 2009, 33, 894–904. [Google Scholar] [CrossRef]
- Ezeh, U.I.; Turek, P.J.; Reijo, R.A.; Clark, A.T. Human embryonic stem cell genes oct4, nanog, stellar, and gdf3 are expressed in both seminoma and breast carcinoma. Cancer 2005, 104, 2255–2265. [Google Scholar] [CrossRef]
- Gopalan, A.; Dhall, D.; Olgac, S.; Fine, S.W.; Korkola, J.E.; Houldsworth, J.; Chaganti, R.S.; Bosl, G.J.; Reuter, V.E.; Tickoo, S.K. Testicular mixed germ cell tumors: A morphological and immunohistochemical study using stem cell markers, oct3/4, sox2 and gdf3, with emphasis on morphologically difficult-to-classify areas. Mod. Pathol. 2009, 22, 1066–1074. [Google Scholar] [CrossRef]
- Catlett-Falcone, R.; Dalton, W.S.; Jove, R. Stat proteins as novel targets for cancer therapy. Signal transducer an activator of transcription. Curr. Opin. Oncol. 1999, 11, 490–496. [Google Scholar] [CrossRef]
- Tian, F.; DaCosta Byfield, S.; Parks, W.T.; Yoo, S.; Felici, A.; Tang, B.; Piek, E.; Wakefield, L.M.; Roberts, A.B. Reduction in smad2/3 signaling enhances tumorigenesis but suppresses metastasis of breast cancer cell lines. Cancer Res. 2003, 63, 8284–8292. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zhao, W.; Ji, X.; Zhang, F.; Li, L.; Ma, L. Embryonic Stem Cell Markers. Molecules 2012, 17, 6196-6236. https://doi.org/10.3390/molecules17066196
Zhao W, Ji X, Zhang F, Li L, Ma L. Embryonic Stem Cell Markers. Molecules. 2012; 17(6):6196-6236. https://doi.org/10.3390/molecules17066196
Chicago/Turabian StyleZhao, Wenxiu, Xiang Ji, Fangfang Zhang, Liang Li, and Lan Ma. 2012. "Embryonic Stem Cell Markers" Molecules 17, no. 6: 6196-6236. https://doi.org/10.3390/molecules17066196




