Phenolics and Flavonoids Compounds, Phenylanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae)
Abstract
:1. Introduction
2. Results and Discussion
2.1. HPLC Analysis of Phenolics
| CO2 levels (µmol·mol−1) | Varieties | Gallic acid * | Pyragallol | Caffeic acid |
|---|---|---|---|---|
| Alata | 448.12 ± 2.44 d | 810.03 ± 2.44 | 47.83 ± 3.22 f | |
| 400 | Pumila | 215.48 ± 4.32 g | ND | 43.92 ± 2.11 f |
| Lanceolata | 406.03 ± 3.22 f | ND | 115.21 ± 1.14 e | |
| Alata | 837.434 ± 0.87 b | ND | 215.51 ± 2.54 c | |
| 800 | Pumila | 282.17 ± 0.43 g | ND | 177.35 ± 2.56 d |
| Lanceolata | 474.33 ± 3.67 c | ND | ND | |
| Alata | 948.28 ± 6.77 a | ND | 543.88 ± 3.44 a | |
| 1200 | Pumila | 435.69 ± 9.87 e | ND | 237.86 ± 5.66 b |
| Lanceolata | 935.91 ± 4.34 a | ND | ND |

| Phenolic compounds | Var. alata | Var. pumila | Var. lanceolata | |||
|---|---|---|---|---|---|---|
| 800 (µmol·mol−1) | 1,200 (µmol·mol−1) | 800 (µmol·mol−1) | 1,200 (µmol·mol−1) | 800 (µmol·mol−1) | 1,200 (µmol·mol−1) | |
| Gallic acid | +86% | +111% | +30% | +101% | +16.4% | +130% |
| Pyragallol | −100% | −100% | 0% | 0% | 0% | 0% |
| Caffeic acid | +338% | +1010% | +298% | +433% | −100% | −100% |
2.2. HPLC Analysis of Flavonoids
| CO2 levels (µmol·mol−1) | Varieties | Flavonoid content (µg·g−1 dry weight) | ||||
|---|---|---|---|---|---|---|
| Kaempferol | Quercetin * | Myricetin | Rutin | Naringenin | ||
| Alata | 186.71 ± 0.34 b | 57.61 ± 1.22 g | 87.81 ± 0.34 c | ND | 139.20 ± 2.56 c | |
| 400 | Pumila | 221.91 ± 0.21 a | 105.66 ± 2.11 f | 30.41 ± 2.33 e | 24.51 ± 0.45 c | 80.44 ± 0.98 d |
| Lanceolata | 162.71 ± 0.31 c | 56.90 ± 2.34 g | 27.45 ± 3.11 f | ND | 87.11 ± 1.78 e | |
| Alata | ND | 160.88 ± 3.44 c | 273.84 ± 7.44 b | ND | ND | |
| 800 | Pumila | ND | 117.42 ± 4.11 e | ND | 41.8 ± 3.22 b | 619.59 ± 9.78 b |
| Lanceolata | ND | 103.13 ± 2.78 f | 49.73 ± 0.54 d | ND | ND | |
| Alata | ND | 183.32 ± 5.43 b | 287.77 ± 0.21 a | ND | ND | |
| 1200 | Pumila | ND | 127.52 ± 0.45 d | ND | 87.45 ± 2.54 a | 947.85 ± 9.76 a |
| Lanceolata | ND | 205.91 ± 0.21 a | 85.76 ± 1.45 c | ND | ND | |

| Flavonoid compounds | Var alata | Var pumila | Var lanceolata | |||
|---|---|---|---|---|---|---|
| 800 (µmol·mol−1) | 1,200 (µmol·mol−1) | 800 (µmol·mol−1) | 1,200 (µmol·mol−1) | 800 (µmol·mol−1) | 1,200 (µmol·mol−1) | |
| Kaempferol | −100% | −100% | −100% | −100% | −100% | −100% |
| Quercetin | +179% | +221% | +10% | +20% | +81% | +260% |
| Myricetin | +210% | +226% | −100% | −100% | +75% | +203% |
| Rutin | 0% | 0% | +70% | +262% | 0% | 0% |
| Naringenin | −100% | −100% | +683% | +1100% | −100% | −100% |
2.3. Phenylalanine Ammonia Lyase (PAL) Activity

2.4. Radical Scavenging Activity (DPPH)

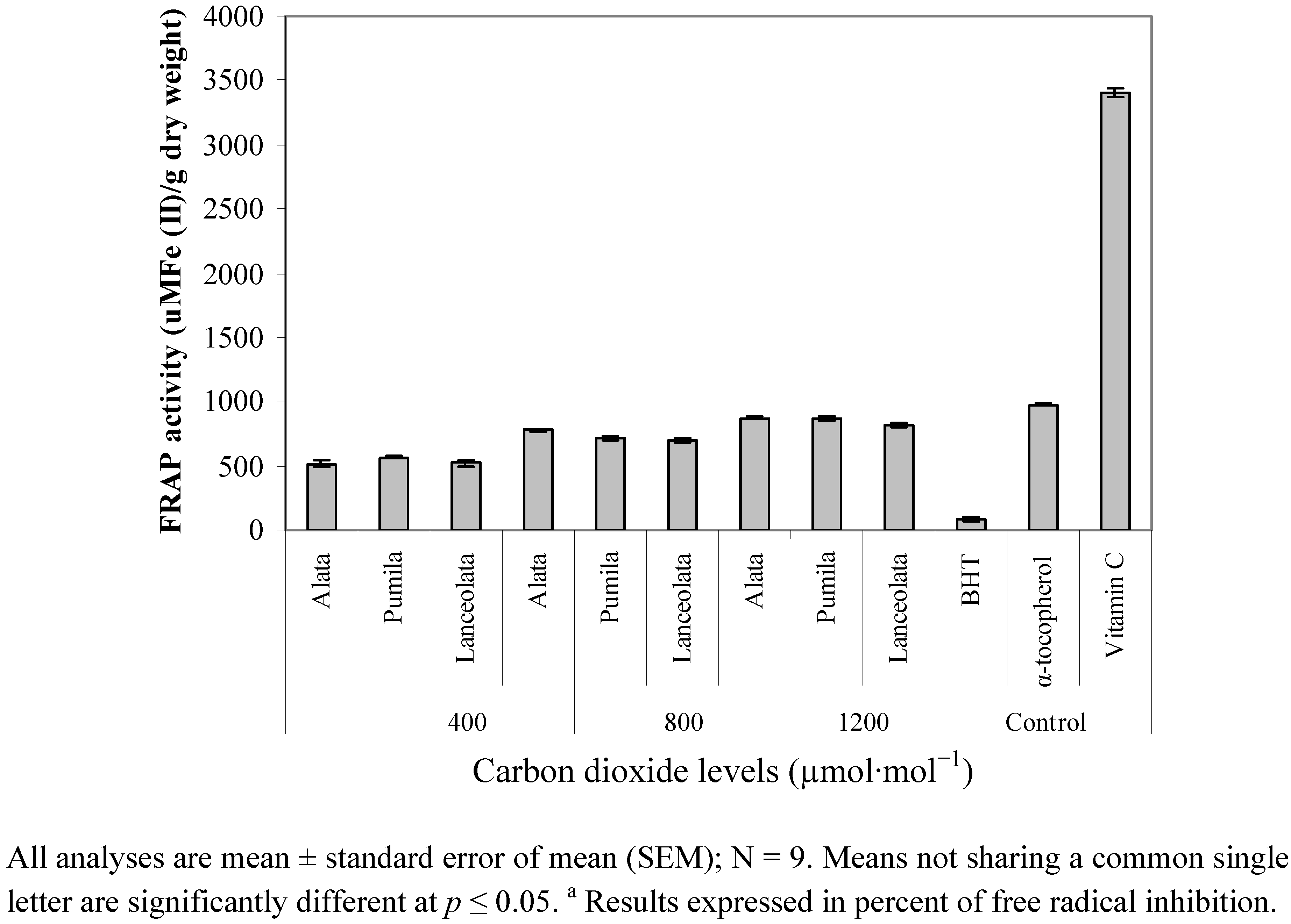
2.5. Reducing Ability (FRAP)
3. Experimental
3.1. Experimental Location, Plant Materials and CO2 Treatments
3.2. Preparation of Extracts for RP-HPLC
3.3. Analyses of Phenolic and Flavonoid Compounds by RP-HPLC
3.4. Phenylalanine-Ammonia-Lyase (PAL)
3.5. DPPH Radical Scavenging Assay

3.6. Reducing Ability (FRAP Assay)
3.7. Statistical Analysis
4. Conclusions
Acknowledgements
References and Notes
- Taylor, J.A.; Llyold, J. Sources and sinks of atmospheric CO2. Aust. J. Bot. 1992, 40, 401–418. [Google Scholar]
- Porter, M.A.; Grodzinski, B. CO2 enrichment of protected crops. Hort. Res. 1985, 7, 345–398. [Google Scholar]
- Brevoort, P. The blooming United State botanical market: A new overview. Herbalgram 1998, 44, 33–46. [Google Scholar]
- Mark, S.J.; Jackson, S.B. Growth responses of Quercus petraea, Fraxinus excelsior and Pinus sylvestris to elevated carbon dioxide, ozone and water supply. New Phytol. 2000, 146, 437–451. [Google Scholar] [CrossRef]
- Byers, T.; Guerrero, N. Epidemilogic evidence for vitamin C and vitamin E in cancer prevention. Am. J. Clin. Nutr. 1995, 62, 1385–1392. [Google Scholar]
- Heijnen, C.G.; Haenen, G.R.; Vanacker, F.A.; Vijgh, W.J.; Bast, A. Flavonoids as peroxynitrite scavengers:the role of the hydroxyl groups. Toxicol. In Vitro 2001, 15, 3–6. [Google Scholar] [CrossRef]
- Chun, O.K.; Kim, D.O.; Lee, C.Y. Superoxide radical scavenging activity of the major polyphenols in fresh plums. J. Agric. Food Chem. 2003, 51, 8067–8072. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Lim, Y.Y.; Wong, L.F.; Lianto, F.S.; Wong, S.K.; Lim, K.K.; Joe, C.E.; Lim, T.Y. Antioxidant and tyrosinase inhibition properties of leaves and rhizomes of ginger species. Food Chem. 2008, 109, 477–483. [Google Scholar] [CrossRef]
- Ho, C.T.; Lee, C.Y.; Hungan, M.T. Phenolics compounds in food and their effects on health (analysis, occurance, and chemistry). J. Am. Chem. Soc. 1992, 43, 2–19. [Google Scholar]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research science. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Booker, F.L. Influence of carbon dioxide enrichment, ozone and nitrogen fertilization on cotton (Gossypium hirsutum L.) leaf and root composition. Plant Cell Environ. 2000, 23, 573–583. [Google Scholar] [CrossRef]
- Polle, A.; Eiblmeier, M.; Sheppard, L.; Murray, M. Responses of antioxidative enzymes to elevated CO2 in leaves of beech (Fagus sylatica L.) seedlings grown under a range of nutrient regimes. Plant Cell Environ. 1997, 20, 1317–1321. [Google Scholar]
- Schwanz, P.; Polle, A. Antioxidative systems, pigment and protein contents in leaves of adult Mediterranean oak species (Quercus pubescens and Quercus. ilex) with lifetime exposure to elevated CO2. New Phytol. 1998, 140, 411–423. [Google Scholar] [CrossRef]
- Pérez-López, U.; Robredo, A.; Lacuesta, M.; Sgherri, C.; Muñoz-Rueda, A.; Navari-Izzo, F.; Mena-Petite, A. The oxidative stress caused by salinity in two barley cultivars is mitigated by elevated CO2. Physiol. Plantarum 2009, 135, 29–42. [Google Scholar] [CrossRef]
- Mattson, W.J.; Julkunen-Tiitto, R.; Herms, D.A. CO2 enrichment and carbon partitioning to phenolics: Do plant responses accord better with the protein competition or the growth-differentiation balance models? Oikos 2005, 111, 337–347. [Google Scholar]
- Bryant, J.P.; Chapin, F.S.; Klein, D.R. Carbon nutrient balance of boreal plants in relation to vertebrate herbivory. Oikos 1983, 40, 357–368. [Google Scholar] [CrossRef]
- Herms, D.A.; Matson, W.J. The dilemma of plants: To grow or defend. Quart. Rev. Biol. 1992, 67, 283–335. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Rahmat, A.; Abdul Rahman, Z. The relationship between phenolics and flavonoids production with total non structural carbohydrate and photosynthetic rate in Labisia pumila Benth. under high CO2 and nitrogen fertilization. Molecules 2011, 16, 162–174. [Google Scholar]
- Ibrahim, M.H.; Hawa, Z.E.J. Carbon dioxide fertilization enhanced antioxidant compounds in Malaysian Kacip Fatimah (Labisia pumila Blume). Molecules 2011, 16, 6068–6081. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Elevated carbon dioxide increases contents of flavonoids and phenolic compounds, and antioxidant activities in Malaysian young ginger (Zingiber officinale Roscoe.) varieties. Molecules 2010, 15, 7907–7922. [Google Scholar] [CrossRef]
- Chaturvedi, A.K.; Vashistha, R.J.; Rawat, N. Effect of CO2 Enrichment on photosynthetic behavior of Podophyllum Hexandrum Royle, an endangered medicinal herb. J. Am. Sci. 2009, 5, 113–118. [Google Scholar]
- Stuhlfauth, T.; Fock, H.P. Effect of whole season CO2 enrichment on the cultivation of a medicinal plant, Digitalis lanata. J. Agron. Crop Sci. 1990, 164, 168–173. [Google Scholar] [CrossRef]
- Schonhof, I.; Klaring, H.P.; Krumbein, A.; Schreiner, M. Interaction between atmospheric CO2 and glucosinolates in broccoli. J. Chem. Ecol. 2007, 33, 105–114. [Google Scholar]
- Save, R. Effects of atmospheric carbon dioxide fertilization on biomass and secondary metabolites of some plant species with pharmacological interest under greenhouse conditions. Afinidad 2007, 64, 237–241. [Google Scholar]
- Ibrahim, M.H.; Jaafar, H.Z.E. The relationship of nitrogen and C/N ratio with secondary metabolites levels and antioxidant activities in three varieties of malaysian kacip fatimah (Labisia pumila Blume). Molecules 2011, 16, 5514–5526. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.; Rahmat, A.; Rahman, Z.A. Effects of nitrogen fertilization on synthesis of primary and secondary metabolites in three varieties of kacip fatimah (Labisia Pumila Blume). Int. J. Mol. Sci. 2011, 12, 5238–5254. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z. Enhancement of leaf gas exchange and primary metabolites under carbon dioxide enrichment up-regulates the production of secondary metabolites in Labisia pumila seedlings. Molecules 2011, 16, 3761–3777. [Google Scholar] [CrossRef]
- Karimi, E.; Jaafar, H.Z.; Ahmad, S. Phytochemical analysis and antimicrobial activities of methanolic extracts of leaf, stem and root from different varieties of Labisa pumila Benth. Molecules 2011, 16, 4438–4450. [Google Scholar] [CrossRef]
- Lindroth, R.L.; Kinney, K.K.; Platz, C.L. Responses of diciduous trees to elevated CO2: Productivity, phytochemistry, and insect performance. Ecology 1993, 74, 763–777. [Google Scholar] [CrossRef]
- Vurro, E.; Bruni, R.; Bianchi, A.; Sanità, L. Elevated atmospheric CO2 decreases oxidative stress and increases essential oil yield in leaves of Thymus vulgaris grown in a mini FACE system. Environ. Exp. Bot. 2009, 65, 99–106. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Photosynthetic capacity, photochemical efficiency and chlorophyll content of three varieties of Labisia pumila Benth. Exposed to open field and greenhouse growing conditions. Acta Physiol. Plant. 2011, 33, 2179–2185. [Google Scholar] [CrossRef]
- Felgines, C.; Texier, O.; Morand, C.; Manach, C.; Scalbert, A.; Régerat, F.; Rémésy, C. Bioavailability of the flavanone naringenin and its glycosides in rats. Am. J. Physiol. Gastrointest. L. 2000, 279, G1148–G1154. [Google Scholar]
- Ho, P.C.; Saville, D.J.; Coville, P.F.; Wanwimoluk, S. Content of CYP3A4 inhibitors, naringin, naringenin and bergapten in grapefruit and grapefruit juice products tomato. Pharm. Acta Helv. 2000, 74, 379–385. [Google Scholar] [CrossRef]
- Wang, Y.S.H.; Bunce, A.J.; Maas, L.J. Elevated carbon dioxide increases contents of antioxidant compounds in field-grown strawberries. J. Agric. Food Chem. 2003, 51, 4315–4320. [Google Scholar] [CrossRef]
- Malikov, V.M.; Yuledashev, M.P. Phenolic compounds of plants of the Scutellaria L. genus: Distribution, structure, and properties. Chem. Nat. Compd. 2002, 38, 358–406. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E. Involvement of carbohydrate, protein and phenylanine ammonia lyase on up-regulation of production of secondary metabolites (total phenolics and flavonoid) in Labisia pumila (Blume) Fern-Vill (Kacip Fatimah) under high CO2 and different nitrogen levels. Molecules 2011, 16, 4172–4190. [Google Scholar] [CrossRef]
- Hilal, M.; Parrado, M.E.; Rosa, M.; Gallardo, M.; Orce, L.; Massa, E.D. Epidermal lignin deposition in quinoa cotyledons in response to UV-B radiation. Photochem. Photobiol. 2004, 79, 205–210. [Google Scholar] [CrossRef]
- Dennis, D.T.; Blakeley, S.D. Carbohydrate Metabolism. In Biochemistry and Molecular Biology of Plants; Buchanan, B.B., Gruisem, W., Jones, R.L., Eds.; American Society of Plant Physiologists: Rockville, MD, USA, 2000; pp. 630–675. [Google Scholar]
- Johnson, R.H.; Lincoln, D.E. Sagebrush carbon allocation patterns and grasshopper nutrition: The influence of CO2 enrichment and soil mineral limitation. Oecologica 1991, 87, 127–134. [Google Scholar] [CrossRef]
- Chabot, S.; Belrhlid, R.; Chenevert, R.; Pichie, Y. Hyphal growth promotion in vitro of 27 mycorrhizal fungus, Gigaspora margarita Becker & Hall, by the activity of structurally specific 28 flavonoid compounds under CO2 enriched condition. New Phytol. 1992, 122, 461–467. [Google Scholar] [CrossRef]
- Estiarte, M.; Penuelas, J.; Kimball, B.A.; Hendrix, D.L.; Pinter, P.J.; Wall, G.W.; Lam-orte, R.L.; Hunsaker, D.J. Free-air CO2 enrichment of wheat: Leaf flavonoid concentration throughout the growth cycle. Physiol. Plant. 1999, 105, 423–433. [Google Scholar] [CrossRef]
- Schwanz, P.; Kimball, B.A.; Idso, S.B.; Hendrix, D.L.; Polle, A. Antioxidants in sun and shade leaves of sour orange trees (Citrus aurantium) after long-term acclimation to elevated CO2. J. Exp. Bot. 1996, 47, 1941–1950. [Google Scholar] [CrossRef]
- Herrmann, K.M.; Weaver, L.M. The shikimate pathway. Ann. Rev. Plant Phys. Plant Mol. Biol. 1999, 50, 473–503. [Google Scholar] [CrossRef]
- Penuelas, J.; Estiarte, M. Can elevated CO2 affect secondary metabolism and ecosystem function? Trees 1998, 13, 20–24. [Google Scholar]
- Muzika, R.M. Terpenes and phenolics in response to nitrogen fertilization: A test of the carbon/nutrient balance hypothesis. Chemoecology 1993, 4, 3–7. [Google Scholar] [CrossRef]
- Fajer, E.D.; Bowers, M.D.; Bazaaz, F.A. The effects of nutrients and enriched CO2 environment on the production of carbon based allelochemicals in Plantago: A test of the carbon/nutrient balance hypothesis. Am. Nat. 1992, 140, 707–723. [Google Scholar]
- Meyer, S.; Cerovic, Z.G.; Goulas, Y.; Montpied, P.; Demotes, S.; Bidel, L.P.R.; Moya, I.; Dreyer, E. Relationship between assessed polyphenols and chlorophyll contents and leaf mass per area ratio in woody plants. Plant Cell Environ. 2006, 29, 1338–1348. [Google Scholar] [CrossRef]
- Winger, A.; Purdy, S.; Maclean, A.; Pourtau, N. The role of sugars in integrating environmental signals during the regulation of leaf senescence. New Phytol. 2006, 161, 781–789. [Google Scholar]
- Matros, A.; Amme, S.; Kettig, B.; Buck, S.G.H.; Sonnewald, U.Y.; Mock, H.P. Growth at elevated CO2 concentrations leads to modified profiles of secondary metabolites in tobacco cv. SamsumNN and to increased resistance against infection with potato virus Y. Plant Cell Environ. 2006, 29, 126–137. [Google Scholar] [CrossRef]
- Hartley, S.E.; Jones, C.G.; Couper, G.C.; Jones, T.H. Biosynthesis of plant phenolics compounds in elevated atmospheric CO2. Glob. Change Biol. 2000, 6, 497–506. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.W.; Kanner, J.; German, J.B. Interfacial phenomena in the evaluation of antioxidants: Bulk oils versus emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.F. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Haniff, M.H.; Raffi, M.Y. Changes in growth and photosynthetic patterns of oil palm seedling exposed to short term CO2 enrichment in a closed top chamber. Acta Physiol. Plant. 2010, 32, 305–313. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Hawa, Z.E.J. Primary, secondary metabolites, H2O2, maliondialdehyde and photosynthetic responses of Orthosiphon stimaneus Benth. to different irradiance levels. Molecules 2012, 17, 1159–1176. [Google Scholar] [CrossRef]
- Crozier, A.; Lean, M.E.J.; McDonald, M.S.; Black, C. Quantitative analysis of the flavonoid content of commercial tomatoes, onions, lettuce, and celery. J. Agric. Food Chem. 1997, 45, 590–595. [Google Scholar] [CrossRef]
- Martinez-Tellez, M.A.; Lafuente, M.T. Effects of high temperature conditioning on ethylene, phenylalanine ammonia lyase, peroxidase and polyphenol oxidase in flavedo of chilled “Fortune” mandarin fruit. J. Plant Physiol. 1997, 150, 674–678. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z.E.; Asmah, R.; Zaharah, A.R. Involvement of Nitrogen on Flavonoids, Glutathione, Anthocyanin, Ascorbic Acid and Antioxidant Activities of Malaysian Medicinal Plant Labisia pumila Blume (Kacip Fatima. Int. J. Mol. Sci. 2012, 13, 393–408. [Google Scholar]
- Ibrahim, M.H.; Hawa, Z.E.J. Reduced photoinhibition enhanced kacip fatimah (Labisia pumila Blume) secondary metabolites, phenyl alanine lyase and antioxidant activity. Int. J. Mol. Sci. 2012, 13, 5290–5306. [Google Scholar] [CrossRef]
- Ibrahim, M.H.; Jaafar, H.Z. Impact of elevated carbon dioxide on primary, secondary metabolites and antioxidant responses of Eleais guineensis Jacq. (Oil Palm) seedlings. Molecules 2012, 17, 5195–5211. [Google Scholar] [CrossRef]
- Sample Availability: Not available.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jaafar, H.Z.E.; Ibrahim, M.H.; Karimi, E. Phenolics and Flavonoids Compounds, Phenylanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae). Molecules 2012, 17, 6331-6347. https://doi.org/10.3390/molecules17066331
Jaafar HZE, Ibrahim MH, Karimi E. Phenolics and Flavonoids Compounds, Phenylanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae). Molecules. 2012; 17(6):6331-6347. https://doi.org/10.3390/molecules17066331
Chicago/Turabian StyleJaafar, Hawa Z.E., Mohd Hafiz Ibrahim, and Ehsan Karimi. 2012. "Phenolics and Flavonoids Compounds, Phenylanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae)" Molecules 17, no. 6: 6331-6347. https://doi.org/10.3390/molecules17066331
APA StyleJaafar, H. Z. E., Ibrahim, M. H., & Karimi, E. (2012). Phenolics and Flavonoids Compounds, Phenylanine Ammonia Lyase and Antioxidant Activity Responses to Elevated CO2 in Labisia pumila (Myrisinaceae). Molecules, 17(6), 6331-6347. https://doi.org/10.3390/molecules17066331




