Reversible Low-Light Induced Photoswitching of Crowned Spiropyran-DO3A Complexed with Gadolinium(III) Ions
Abstract
:1. Introduction

2. Results and Discussion
2.1. Spectroscopic Characteristics of Gd(III) Complexed and Non-Complexed Spiropyran-DO3A

2.2. Correlation between Rate Constants and Total Photon Emission Rate


2.3. Investigation of Increased Temperature on Back-Conversion and Repeated Switching Cycles

2.4. Relaxometric Properties

2.5. Illumination with Two Different LED’s
| LED emission max | Solvent | Δmax (nm) | Spiropyran-DO3A rate constant | Δmax (nm) | Spiropyran-DO3A-Gd rate constant |
|---|---|---|---|---|---|
| 465 nm | Water | 31 | 1.55 × 10−2 ± (2.38 × 10−3) s−1 | 5 | 1.59 × 10−2 ± (1.99 × 10−3) s−1 |
| EtOH | 53 | 2.29 × 10−2 ± (1.81 × 10−3) s−1 | 13 | 1.89 × 10−2 ± (3.51 × 10−4) s−1 | |
| 525 nm | Water | 29 | 1.29 × 10−3 ± (3.68 × 10−3) s−1 | 55 | 6.27 × 10−3 ± (4.76 × 10−4) s−1 |
| EtOH | 7 | 1.94 × 10−2 ± (7.02 × 10−4) s−1 | 47 | 8.31 × 10−3 ± (1.50 × 10−3) s−1 |
2.6. Overexpression of Gaussia Princeps Luciferase



3. Experimental
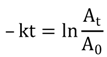


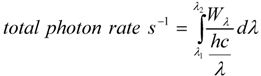
| Blue emitting LED | Green emitting LED | |||
|---|---|---|---|---|
| Current (mA) | Total radiant flux (μW) | Photon emission rate (photons·s−1) | Total radiant flux (μW) | Photon emission rate (photons·s−1) |
| 17.676 | 7536 | 1.7542 × 1016 | 2467.615 | 6.5372 × 1015 |
| 6.314 | 2927.58 | 6.8873 × 1015 | 1133.615 | 2.9919 × 1015 |
| 1.3978 | 588.72 | 1.3702 × 1015 | 298.405 | 7.9035 × 1014 |
| 0.6687 | 248.4 | 5.7821 × 1014 | 151.08 | 4.0141 × 1014 |
| 0.3031 | 82.884 | 1.9289 × 1014 | 66.3875 | 1.7577 × 1014 |
| 0.1443 | 26.334 | 6.1284 × 1013 | 29.29 | 7.7539 × 1013 |
| 0.0984 | 13.46 | 3.132 × 1013 | 18.93 | 5.0114 × 1013 |
| 0.0675 | 6.4596 | 1.5027 × 1013 | 12.15 | 3.2162 × 1013 |
| 0.0309 | 1.0182 | 2.37 × 1013 | 4.634 | 1.2267 × 1013 |
4. Conclusions
Acknowledgments
Conflict of Interest
- Sample Availability: Samples of the compounds spiropyran-DO3A and spiropyran-DO3A-Gd are available from the authors.
References and Notes
- Lukyanov, B.S.; Lukyanova, M.B. Spiropyrans: Synthesis, Properties, and Application. (Review). Chem. Heterocyc. Compd. 2005, 41, 281–311. [Google Scholar]
- Tu, C.Q.; Osborne, E.A.; Louie, A.Y. Synthesis and characterization of a redox- and light-sensitive MRI contrast agent. Tetrahedron 2009, 65, 1241–1246. [Google Scholar] [CrossRef]
- Garcia, A.A.; Cherian, S.; Park, J.; Gust, D.; Jahnke, F.; Rosario, R. Photon-controlled phase partitioning of spiropyrans. J. Phys. Chem. A 2000, 104, 6103–6107. [Google Scholar]
- Sumaru, K.; Satoh, T.; Takagi, T.; Takai, K.; Kanamori, T. Isomerization of spirobenzopyrans bearing electron-donating and electron-withdrawing groups in acidic aqueous solutions. Phys. Chem. Chem. Phys. 2011, 13, 7322–7329. [Google Scholar]
- Sunamoto, J.; Iwamoto, K.; Akutagawa, M.; Nagase, M.; Kondo, H. Rate Control by Restricting Mobility of Substrate in Specific Reaction Field-Negative Photochromism of Water-Soluble Spiropyran in Aot Reversed Micelles. J. Am. Chem. Soc. 1982, 104, 4904–4907. [Google Scholar]
- Salhin, A.M.A.; Tanaka, M.; Kamada, K.; Ando, H.; Ikeda, T.; Shibutani, Y.; Yajima, S.; Nakamura, M.; Kimura, K. Decisive factors in the photoisomerization behavior of crowned spirobenzopyrans: Metal ion interaction with crown ether and phenolate anion moieties. Eur. J. Org. Chem. 2002, 655–662. [Google Scholar]
- Fedorova, O.A.; Gromov, S.P.; Alfimov, M.V. Cation-dependent pericyclic reactions of crown-containing photochromic compounds. Russ. Chem. Bull. 2001, 50, 1970–1983. [Google Scholar] [CrossRef]
- Tu, C.Q.; Louie, A.Y. Photochromically-controlled, reversibly-activated MRI and optical contrast agent. Chem. Commun. 2007, 1331–1333. [Google Scholar]
- Stitzel, S.; Byrne, R.; Diamond, D. LED switching of spiropyran-doped polymer films. J. Mater. Sci. 2006, 41, 5841–5844. [Google Scholar] [CrossRef]
- Schaudel, B.; Guermeur, C.; Sanchez, C.; Nakatani, K.; Delaire, J.A. Spirooxazine- and spiropyran-doped hybrid organic-inorganic matrices with very fast photochromic responses. J. Mater. Chem. 1997, 7, 61–65. [Google Scholar] [CrossRef]
- Whelan, J.; Abdallah, D.; Wojtyk, J.; Buncel, E. Micro-environmental fine-tuning of electronic and kinetic properties of photochromic dyes. J. Mater. Chem. 2010, 20, 5727–5735. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Zhu, L.Y.; Han, J.J.; Wu, W.W.; Hurst, J.K.; Li, A.D.Q. Spiropyran-based photochromic polymer nanoparticles with optically switchable luminescence. J. Am. Chem. Soc. 2006, 128, 4303–4309. [Google Scholar]
- Li, Y.Y.; Fan, M.G.; Zhang, S.X.; Yao, J.N. Photochromism induced aggregate-monomer interconversion and fluorescence switch of porphyrin with spiropyran. J. Phys. Org. Chem. 2007, 20, 884–887. [Google Scholar]
- Giordano, L.; Macareno, J.; Song, L.; Jovin, T.M.; Irie, M.; Jares-Erijman, E.A. Fluorescence resonance energy transfer using spiropyran and diarylethene photochromic acceptors. Molecules 2000, 5, 591–593. [Google Scholar] [CrossRef]
- Tanaka, M.; Nakamura, M.; Salhin, M.A.A.; Ikeda, T.; Kamada, K.; Ando, H.; Shibutani, Y.; Kimura, K. Synthesis and photochromism of spirobenzopyran derivatives bearing an oxymethylcrown ether moiety: Metal ion-induced switching between positive and negative photochromisms. J. Org. Chem. 2001, 66, 1533–1537. [Google Scholar]
- Natali, M.; Aakeroy, C.; Desper, J.; Giordani, S. The role of metal ions and counterions in the switching behavior of a carboxylic acid functionalized spiropyran. Dalton T. 2010, 39, 8269–8277. [Google Scholar]
- Scarmagnani, S.; Walsh, Z.; Slater, C.; Alhashimy, N.; Paull, B.; Macka, M.; Diamond, D. Polystyrene bead-based system for optical sensing using spiropyran photoswitches. J. Mater. Chem. 2008, 18, 5063–5071. [Google Scholar] [CrossRef]
- Yoshida, J.; Watanuki, A.; Takano, H.; Kobayashi, H.; Ikeda, H.; Ogata, N. Optically-Controlled Photonic Switches Based on Spiropyran-Doped Marine-Biopolymer DNA-Lipid Complex Films. In Organic Photonic Materials and Devices VIII; Grote, J.G., Kajzar, F., Kim, N., Eds.; Proceedings of SPIE: San Jose, CA, USA, 2006. [Google Scholar]
- Bhawalkar, J.D.; Kumar, N.D.; Zhao, C.F.; Prasad, P.N. Two-photon photodynamic therapy. J. Clin. Laser Med. Surg. 1997, 15, 201–204. [Google Scholar]
- Wang, R.K.K.; Xu, X.Q.; Tuchin, V.V.; Elder, J.B. Concurrent enhancement of imaging depth and contrast for optical coherence tomography by hyperosmotic agents. J. Opt. Soc. Am. B 2001, 18, 948–953. [Google Scholar]
- Ipe, B.I.; Mahima, S.; Thomas, K.G. Light-induced modulation of self-assembly on spiropyran-capped gold nanoparticles: A potential system for the controlled release of amino acid derivatives. J. Am. Chem. Soc. 2003, 125, 7174–7175. [Google Scholar] [CrossRef]
- Zhu, M.Q.; Zhang, G.F.; Li, C.; Aldred, M.P.; Chang, E.; Drezek, R.A.; Li, A.D. Reversible Two-Photon Photoswitching and Two-Photon Imaging of Immunofunctionalized Nanoparticles Targeted to Cancer Cells. J. Am. Chem. Soc. 2011, 133, 365–372. [Google Scholar]
- Wilson, T.; Hastings, J.W. Bioluminescence. Annu. Rev. Cell. Dev. Biol. 1998, 14, 197–230. [Google Scholar] [CrossRef]
- Sadikot, R.T.; Blackwell, T.S. Bioluminescence imaging. Proc. Am. Thorac. Soc. 2005, 2, 511-512,537-540. [Google Scholar]
- Contag, C.H.; Jenkins, D.; Contag, P.R.; Negrin, R.S. Use of reporter genes for optical measurements of neoplastic disease in vivo. Neoplasia 2000, 2, 41–52. [Google Scholar] [CrossRef]
- Niwa, K.; Ichino, Y.; Kumata, S.; Nakajima, Y.; Hiraishi, Y.; Kato, D.; Viviani, V.R.; Ohmiya, Y. Quantum Yields and Kinetics of the Firefly Bioluminescence Reaction of Beetle Luciferases. Photochem. Photobiol. 2010, 86, 1046–1049. [Google Scholar] [CrossRef]
- Nakajima, Y.; Yamazaki, T.; Nishii, S.; Noguchi, T.; Hoshino, H.; Niwa, K.; Viviani, V.R.; Ohmiya, Y. Enhanced Beetle Luciferase for High-Resolution Bioluminescence Imaging. PLoS One 2010, 5, e10011. [Google Scholar]
- Verhaegen, M.; Christopoulos, T.K. Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal. Chem. 2002, 74, 4378–4385. [Google Scholar] [CrossRef]
- Venisnik, K.M.; Olafsen, T.; Gambhir, S.S.; Wu, A.M. Fusion of Gaussia luciferase to an engineered anti-carcinoembryonic antigen (CEA) antibody for in vivo optical imaging. Mol. Imaging. Biol. 2007, 9, 267–277. [Google Scholar] [CrossRef]
- Tannous, B.A.; Kim, D.E.; Fernandez, J.L.; Weissleder, R.; Breakefield, X.O. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol. Ther. 2005, 11, 435–443. [Google Scholar]
- Zhang, S.G.; Zhang, Q.H.; Ye, B.X.; Li, X.L.; Zhang, X.P.; Deng, Y.Q. Photochromism of Spiropyran in Ionic Liquids: Enhanced Fluorescence and Delayed Thermal Reversion. J. Phys. Chem. B 2009, 113, 6012–6019. [Google Scholar]
- Pimienta, V.; Lavabre, D.; Levy, G.; Samat, A.; Guglielmetti, R.; Micheau, J.C. Kinetic analysis of photochromic systems under continuous irradiation. Application to spiropyrans. J. Phys. Chem. 1996, 100, 4485–4490. [Google Scholar]
- Gorner, H. Photochromism of nitrospiropyrans: Effects of structure, solvent and temperature. Phys. Chem. Chem. Phys. 2001, 3, 416–423. [Google Scholar] [CrossRef]
- Diamond, D.; Radu, A.; Byrne, R.; Alhashimy, N.; Fusaro, M.; Scarmagnani, S. Spiropyran-based reversible, light-modulated sensing with reduced photofatigue. J. Photochem. Photobiol. A 2009, 206, 109–115. [Google Scholar] [CrossRef]
- Giordani, S.; Movia, D.; Prina-Mello, A.; Volkov, Y. Determination of Spiropyran Cytotoxicity by High Content Screening and Analysis for Safe Application in Bionanosensing. Chem. Res. Toxicol. 2010, 23, 1459–1466. [Google Scholar] [CrossRef]
- Kim, J.B.; Urban, K.; Cochran, E.; Lee, S.; Ang, A.; Rice, B.; Bata, A.; Campbell, K.; Coffee, R.; Gorodinsky, A.; et al. Non-invasive detection of a small number of bioluminescent cancer cells in vivo. PLoS One 2010, 5, e9364. [Google Scholar]
- Yanagihara, K.; Takigahira, M.; Takeshita, F.; Komatsu, T.; Nishio, K.; Hasegawa, F.; Ochiya, T. A photon counting technique for quantitatively evaluating progression of peritoneal tumor dissemination. Cancer Res. 2006, 66, 7532–7539. [Google Scholar]
- Welsh, J.P.; Patel, K.G.; Manthiram, K.; Swartz, J.R. Multiply mutated Gaussia luciferases provide prolonged and intense bioluminescence. Biochem. Biophys. Res. Commun. 2009, 389, 563–568. [Google Scholar] [CrossRef]
- Verhaegent, M.; Christopoulos, T.K. Recombinant Gaussia luciferase. Overexpression, purification, and analytical application of a bioluminescent reporter for DNA hybridization. Anal. Chem. 2002, 74, 4378–4385. [Google Scholar] [CrossRef]
- Goerke, A.R.; Loening, A.M.; Gambhir, S.S.; Swartz, J.R. Cell-free metabolic engineering promotes high-level production of bioactive Gaussia princeps luciferase. Metab. Eng. 2008, 10, 187–200. [Google Scholar] [CrossRef]
- Theodossiou, T.; Hothersall, J.S.; Woods, E.A.; Okkenhaug, K.; Jacobson, J.; MacRobert, A.J. Firefly luciferin-activated rose bengal: In vitro photodynamic therapy by intracellular chemiluminescence in transgenic NIH 3T3 cells. Cancer Res. 2003, 63, 1818–1821. [Google Scholar]
- Carpenter, S.; Fehr, M.J.; Kraus, G.A.; Petrich, J.W. Chemiluminescent activation of the antiviral activity of hypericin: a molecular flashlight. Proc. Natl. Acad. Sci. USA 1994, 91, 12273–12277. [Google Scholar]
- Yao, J.; Liu, Y.; Fan, M.; Zhang, S.; Sheng, X. Basic amino acid induced isomerization of a spiropyran: Towards visual recognition of basic amino acids in water. New J. Chem. 2007, 31, 1878–1881. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kruttwig, K.; Yankelevich, D.R.; Brueggemann, C.; Tu, C.; L’Etoile, N.; Knoesen, A.; Louie, A.Y. Reversible Low-Light Induced Photoswitching of Crowned Spiropyran-DO3A Complexed with Gadolinium(III) Ions. Molecules 2012, 17, 6605-6624. https://doi.org/10.3390/molecules17066605
Kruttwig K, Yankelevich DR, Brueggemann C, Tu C, L’Etoile N, Knoesen A, Louie AY. Reversible Low-Light Induced Photoswitching of Crowned Spiropyran-DO3A Complexed with Gadolinium(III) Ions. Molecules. 2012; 17(6):6605-6624. https://doi.org/10.3390/molecules17066605
Chicago/Turabian StyleKruttwig, Klaus, Diego R. Yankelevich, Chantal Brueggemann, Chuqiao Tu, Noelle L’Etoile, André Knoesen, and Angelique Y. Louie. 2012. "Reversible Low-Light Induced Photoswitching of Crowned Spiropyran-DO3A Complexed with Gadolinium(III) Ions" Molecules 17, no. 6: 6605-6624. https://doi.org/10.3390/molecules17066605
APA StyleKruttwig, K., Yankelevich, D. R., Brueggemann, C., Tu, C., L’Etoile, N., Knoesen, A., & Louie, A. Y. (2012). Reversible Low-Light Induced Photoswitching of Crowned Spiropyran-DO3A Complexed with Gadolinium(III) Ions. Molecules, 17(6), 6605-6624. https://doi.org/10.3390/molecules17066605





