The Potential Biotechnological Applications of the Exopolysaccharide Produced by the Halophilic Bacterium Halomonas almeriensis
Abstract
:1. Introduction
2. Results
2.1. EPS Production


| Salt concentration a (%, w/v) | Incubation time (h) b | Incubation temperature (°C) c | Shaking speed (rpm) d | Glucose concentration (%, w/v) e | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2.5 | 5 | 7.5 * | 10 | 24 | 48 | 72 | 96 | 120 * | 144 | 168 | 192 | 22 | 32 * | 42 | 0 | 100 * | 200 | 0 | 1 * | 2 | 5 | 7 | 10 | |
| EPS (g/100 mL) | 0 | 0.15 | 0.17 | 0.15 | 0.13 | 0.14 | 0.16 | 0.17 | 0.18 | 0.17 | 0.159 | 0.14 | 0.015 | 0.17 | 0.02 | 0.035 | 0.17 | 0.085 | 0.13 | 0.17 | 0.15 | 0.14 | 0 | 0 |
2.2. Chemical Composition and Molecular Mass

2.3. Rheological Behaviour of EPS Solutions
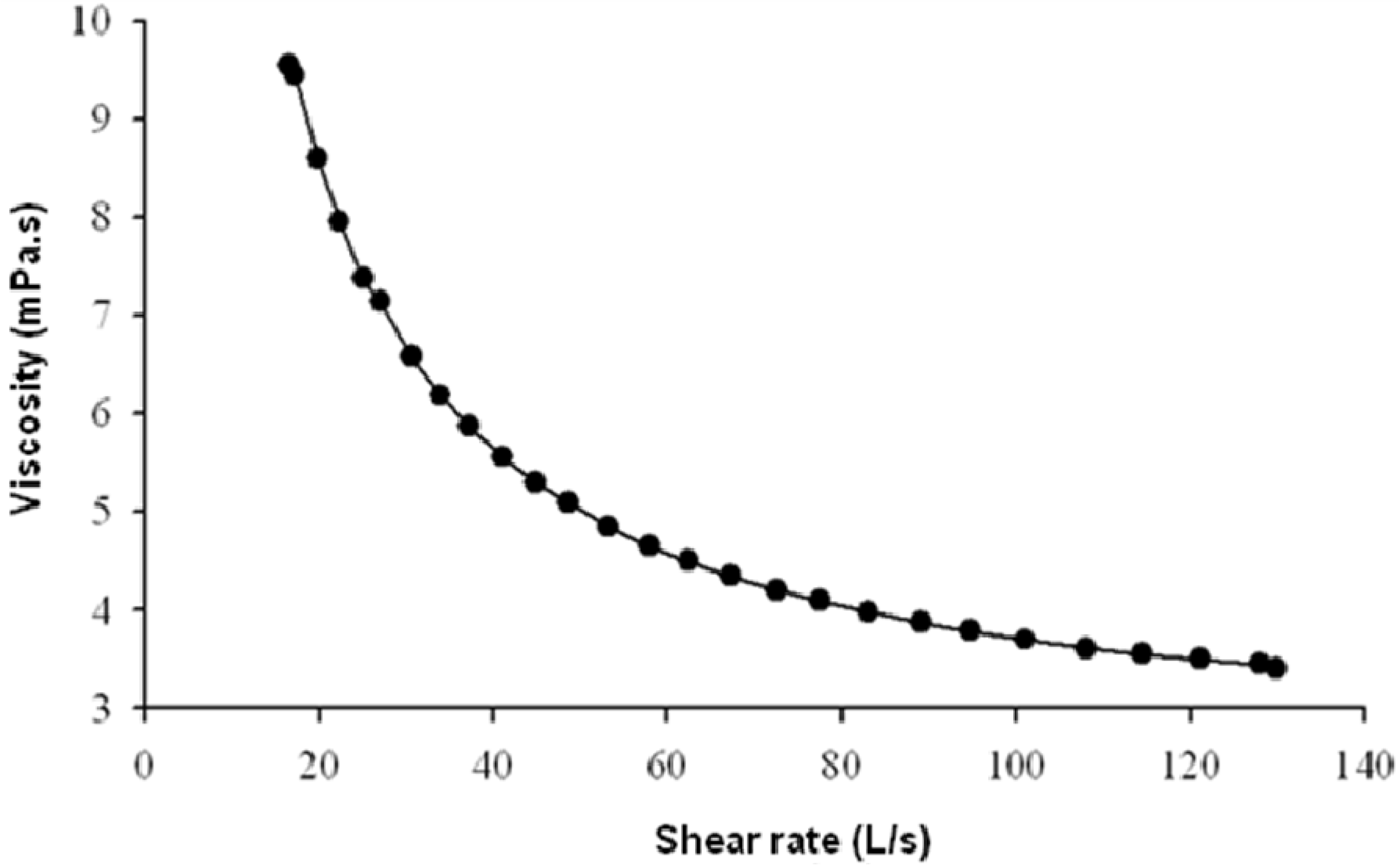
2.4. Emulsifying Activity
| EPS origin | Emulsifying activity (%) * | ||||||
|---|---|---|---|---|---|---|---|
| Sunflower oil | Mineral oil | Olive oil | Tetradecane | Octane | Kerosene | Isopropil Miristate | |
| H. almeriensis | 65 | 67.5 | 67.5 | 62.5 | 65 | 65 | 70 |
| Apo-EPS | 45 | 47.5 | 50 | 46.5 | 42.5 | 45 | 50 |
| Comparisons | |||||||
| Sugin 472 | 52.9 | 52.5 | 53.7 | 53.3 | 50 | 49.6 | 49.9 |
| Tween 20 | 62.5 | 57.5 | 60 | 65 | 62.5 | 62 | 67.5 |
| Tween 80 | 62 | 60 | 61.5 | 60 | 60 | 60 | 60 |
| Triton X-100 | 67.5 | 65 | 60 | 65 | 62.5 | 60 | 65.5 |
2.5. Heavy-Metal Uptake
3. Discussion
| Strain | Yield (g/100 mL) | Composition EPS (%, w/w) | MM (Daltons) | Monosaccharide (%, w/w) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Carbohy-drates | Proteins | Acetyl residues | Sulfate | Glu | Man | Rha | Gal | Ara | Xyl | Fuc | AGlu | AGal | ||||
| Halomonas | ||||||||||||||||
| H. almeriensis | M8T | 0.17 | 30.5 | 1.1 | 0.8 | 1.4 | 6.3 × 106 | 27.5 | 72 | 0.5 | ND * | ND | ND | ND | ND | ND |
| 1.5 × 104 | 30 | 70 | ND | ND | ND | ND | ND | ND | ND | |||||||
| H. maura [25] | S-30 | 0.38 | 65 | 2.5 | 0.18 | 6.5 | 4.7 × 106 | 29 | 35 | ND | 14 | ND | ND | ND | 22 | ND |
| H. eurihalina [27] | F2-7 | 0.14 | 37 | 7.5 | 0.5 | 11.2 | ND | 3.2 | 1 | 1.1 | ND | ND | ND | ND | ND | ND |
| H. ventosae [32] | Al-12T | 0.28 | 30.9 | 2.07 | 1.4 | 1.1 | 5.3 × 104 | 24 | 60 | ND | 12 | 2 | 4 | ND | ND | ND |
| Al-16 | 0.30 | 30.8 | 3.95 | 1.5 | 0.7 | 5.2 × 104 | 24 | 57 | ND | 12 | 3 | 4 | ND | ND | ND | |
| H. anticarinesis [32] | FP35T | 0.29 | 35.5 | 0.3 | 1.55 | 0.75 | 2 × 104 | 15 | 45 | 1.5 | ND | ND | ND | ND | ND | 37 |
| FP36 | 0.49 | 33.7 | 0.4 | 2.05 | 1.5 | 4.6 × 104 | 17 | 43 | 1.5 | ND | ND | 1.5 | ND | ND | 37.5 | |
| Idiomarina | ||||||||||||||||
| I. ramblicola [33] | R-22T | 0.15 | 56.5 | 0.8 | 1.15 | 0.5 | 5.5 × 105 | 2 | 68 | 7 | ND | ND | ND | ND | ND | ND |
| 2 × 104 | 19 | 54 | ND | ND | ND | ND | ND | ND | 26 | |||||||
| I. fontislapidosi [33] | F-23T | 0.14 | 50.85 | 0.8 | 1.85 | 0.65 | 1.5 × 106 | 28 | 46 | ND | 15 | 3 | 5 | 2 | ND | ND |
| 1.5 × 104 | 40 | 60 | ND | 20 | ND | ND | ND | ND | ND | |||||||
| Alteromonas hispanica [33] | F32T | 0.1 | 0.25 | 4.3 | 0.25 | 0.25 | 1.9 × 107 | 18 | 63 | ND | ND | ND | 12 | ND | ND | ND |
| Salipiger mucescens [34] | A3T | 0.16 | 53.15 | 1.65 | 0.9 | 0.95 | 2.5 × 105 | 20 | 34 | ND | 33 | ND | ND | 13 | ND | ND |
4. Experimental
4.1. Microorganism and Culture Media
4.2. EPS Production
4.3. Chemical Analysis and Determination of Molecular Mass
4.4. Rheological Analysis
4.5. Emulsifying Activity
4.6. Heavy-Metal Binding Capacity
4.7. Electron Microscopy
5. Conclusions
Acknowledgments
- Sample Availability: Samples of the EPS produced by Halomonas almeriensis M8T is available from the authors.
References and Notes
- Sutherland, I.W. Biotechnology of Microbial Exopolysaccharides; Cambridge University Press: New York, NY, USA, 1990. [Google Scholar]
- Sutherland, I.W. Biofilmexopolysaccharides: A strong and sticky framework. Microbiology 2001, 147, 3–9. [Google Scholar]
- Sutherland, I.W. Microbial polysaccharides from Gram-negative bacteria. Int. Dairy J. 2001, 11, 663–674. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Reis, M.A. Advances in bacterial exopolysaccharides: From production to biotechnological applications. Trends Biotechnol. 2011, 29, 388–398. [Google Scholar] [CrossRef]
- Wolfaardt, G.M.; Lawrence, J.R.; Korber, D.R. Functions of EPS. In Microbial Extracelullar Polymeric Substances; Wingender, J., Neu, T.R., Flemming, H.C., Eds.; Sringer-Verlag: Berlin, Germany, 1999; pp. 171–200. [Google Scholar]
- Sutherland, I.W. Structure-function relationships in microbial exopolysaccharides. Biotechnol. Adv. 1994, 12, 393–448. [Google Scholar] [CrossRef]
- Sutherland, I.W. Polysaccharides from Microorganisms, Plants and Animals. In Biopolymers, Polysaccharides I. Polysaccharides from Prokaryotes; Vandamme, E.J., De Baets, S., Steinbüchel, A., Eds.; Wiley: Weinheim, Germany, 2002; pp. 1–19. [Google Scholar]
- Quesada, E.; Valderrama, M.J.; Béjar, V.; Ventosa, A.; Gutierrez, M.C.; Ruiz-Berraquero, F.; Ramos-Cormenzana, A. Volcaniella eurihalina gen nov., sp. nov., a moderately halophilic nonmotile gram-negative rod. Int. J. Syst. Bacteriol. 1990, 40, 261–267. [Google Scholar] [CrossRef]
- Shea, C.; Nunley, J.W.; Williamson, J.C.; Smith-Somerville, H.E. Comparison of the adhesion properties of Deleya marina and the exopolysaccharide-defective mutant strain DMR. Appl. Environ. Microbiol. 1995, 57, 3107–3113. [Google Scholar]
- Bouchotroch, S.; Quesada, E.; del Moral, A.; Llamas, I.; Béjar, V. Halomonas maura sp. nov., a novel moderately halophilic, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2001, 51, 1625–1632. [Google Scholar] [CrossRef]
- Martínez-Cánovas, M.J.; Béjar, V.; Martínez-Checa, F.; Quesada, E. Halomonas anticariensis sp. nov., from Fuente de Piedra, a saline-wetland wildfowl reserve in Malaga, southern Spain. Int. J. Syst. Evol. Microbiol. 2004, 54, 1329–1332. [Google Scholar] [CrossRef]
- Martínez-Cánovas, M.J.; Quesada, E.; Llamas, I.; Béjar, V. Halomonas ventosae sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2004, 54, 1329–1332. [Google Scholar] [CrossRef]
- Martínez-Checa, F.; Béjar, V.; Martínez-Cánovas, M.J.; Llamas, I.; Quesada, E. Halomonas almeriensis sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium from Cabo de Gata, Almeria, south-east Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 2007–2011. [Google Scholar] [CrossRef]
- González-Domenech, C.M.; Béjar, V.; Martínez-Checa, F.; Quesada, E. Halomonas nitroreducens sp. nov., a novel nitrate- and nitrite-reducing species. Int. J. Syst. Evol. Microbiol. 2008, 58, 872–876. [Google Scholar] [CrossRef]
- González-Domenech, C.M.; Martínez-Checa, F.; Quesada, E.; Béjar, V. Halomonas cerina sp. nov., a moderately halophilic, denitrifying, exopolysaccharide-producing bacterium. Int. J. Syst. Evol. Microbiol. 2008, 58, 803–809. [Google Scholar] [CrossRef]
- González-Domenech, C.M.; Martínez-Checa, F.; Quesada, E.; Béjar, V. Halomonas fontilapidosi sp. nov., a moderately halophilic, denitrifying bacterium. Int. J. Syst. Evol. Microbiol. 2009, 59, 1290–1296. [Google Scholar] [CrossRef]
- Amjres, H.; Béjar, V.; Quesada, E.; Abrini, J.; Llamas, I. Halomonas rifensis sp. nov., an exopolysaccharide-producing, halophilic bacterium isolated from a solar saltern. Int. J. Syst. Evol. Microbiol. 2011, 61, 2600–2605. [Google Scholar] [CrossRef]
- Llamas, I.; Béjar, V.; Martínez-Checa, F.; Martínez-Cánovas, M.J.; Molina, I.; Quesada, E. Halomonas stenophila sp. nov., a halophilic bacterium that produces sulphate exopolysaccharides with biological activity. Int. J. Syst. Evol. Microbiol. 2011, 61, 2508–2514. [Google Scholar] [CrossRef]
- Martínez-Cánovas, M.J.; Béjar, V.; Martínez-Checa, F.; Paez, R.; Quesada, E. Idiomarina fontislapidosi sp. nov. and Idiomarina ramblicola sp. nov., isolated from inland hypersaline habitats in Spain. Int. J. Syst. Evol. Microbiol. 2004, 54, 1793–1797. [Google Scholar] [CrossRef]
- Martínez-Checa, F.; Béjar, V.; Llamas, I.; Del Moral, A.; Quesada, E. Alteromonas hispanica sp. nov., a polyunsaturated-fatty-acid-producing, halophilic bacterium isolated from Fuente de Piedra, southern Spain. Int. J. Syst. Evol. Microbiol. 2005, 55, 2385–2390. [Google Scholar] [CrossRef]
- Martínez-Cánovas, M.J.; Quesada, E.; Martínez-Checa, F.; Del Moral, A.; Béjar, V. Salipiger mucescens gen. nov., sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium isolated from hypersaline soil, belonging to the alpha-Proteobacteria. Int. J. Syst. Evol. Microbiol. 2004, 54, 1735–1740. [Google Scholar] [CrossRef]
- Martínez-Checa, F.; Quesada, E.; Martínez-Cánovas, M.J.; Llamas, I.; Béjar, V. Palleronia marisminoris gen. nov., sp. nov., a moderately halophilic, exopolysaccharide-producing bacterium belonging to the ‘Alphaproteobacteria’, isolated from a saline soil. Int. J. Syst. Evol. Microbiol. 2005, 55, 2525–2530. [Google Scholar] [CrossRef]
- Oren, A. Industrial and environmental applications of halophilic microorganisms. Environ. Technol. 2010, 31, 825–834. [Google Scholar] [CrossRef]
- Bouchotroch, S.; Quesada, E.; Izquierdo, I.; Rodríguez, M.; Béjar, V. Bacterial exopolysaccharides produced by new discovered bacteria belonging to the genus Halomonas isolated from hypersaline habitats in Morocco. J. Ind. Microbiol. Biotechnol. 2000, 24, 374–378. [Google Scholar] [CrossRef]
- Arias, S.; del Moral, A.; Ferrer, M.R.; Tallón, R.; Quesada, E.; Béjar, V. Mauran, an exopolysaccharide produced by the halophilic bacterium Halomonas maura, with a novel composition and interesting properties for biotechnology. Extremophiles 2003, 7, 319–326. [Google Scholar] [CrossRef]
- Quesada, E.; Béjar, V.; Ferrer, M.R.; Calvo, C.; Llamas, I.; Martínez-Checa, F.; Arias, S.; Ruiz-García, C.; Páez, R.; Martínez-Cánovas, M.J.; et al. Moderately Halophilic Exopolysaccharide-Producing Bacteria. In Halophilic Microorganisms; Ventosa, A., Ed.; Springer: Berlin, Germany, 2004. [Google Scholar]
- Quesada, E.; Béjar, V.; Calvo, C. Exopolysaccharide production by Volcaniella eurihalina. Experientia 1993, 49, 1037–1041. [Google Scholar] [CrossRef]
- Calvo, C.; Ferrer, M.R.; Martínez-Checa, F.; Béjar, V.; Quesada, E. Some rheological properties of the extracellular polysaccharide produced by Volcaniella eurihalina F2-7. Appl. Biochem. Biotechnol. 1995, 55, 45–54. [Google Scholar] [CrossRef]
- Béjar, V.; Calvo, C.; Moliz, J.; Diaz-Martínez, F.; Quesada, E. Effect of growth conditions on the rheologial properties and chemical composition of Volcaniella eurihalina exopolysaccharide. Appl. Biochem. Biotechnol. 1996, 59, 77–85. [Google Scholar] [CrossRef]
- Béjar, V.; Llamas, I.; Calvo, C.; Quesada, E. Characterization of exopolysaccharides produced by 19 halophilic strains of the species Halomonas eurihalina. J. Biotechnol. 1998, 61, 135–141. [Google Scholar]
- Calvo, C.; Martínez-Checa, F.; Mota, A.; Béjar, V.; Quesada, E. Effect of cations, pH and sulfate content on the viscosity and emulsifyng activity of the Halomonas eurihalina exopolysaccharide. J. Ind. Microbiol. Biotechnol. 1998, 20, 205–209. [Google Scholar] [CrossRef]
- Mata, J.A.; Béjar, V.; Llamas, I.; Arias, S.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E. Exopolysaccharides produced by the recently described bacteria Halomonas ventosae and Halomonas anticariensis. Res. Microbiol. 2006, 157, 827–835. [Google Scholar] [CrossRef]
- Mata, J.A.; Béjar, V.; Bressollier, P.; Tallon, R.; Urdaci, M.C.; Quesada, E.; Llamas, I. Characterization of exopolysaccharides produced by three moderately halophilic bacteria belonging to the family Alteromonadaceae. J. Appl. Microbiol. 2008, 105, 521–528. [Google Scholar] [CrossRef]
- Llamas, I.; Mata, J.A.; Tallon, R.; Bressollier, P.; Urdaci, M.C.; Quesada, E.; Béjar, V. Characterization of the exopolysaccharide produced by Salipiger mucosus A3, a halophilic species belonging to the Alphaproteobacteria, isolated on the Spanish Mediterranean seaboard. Mar. Drugs 2010, 8, 2240–2251. [Google Scholar] [CrossRef]
- Antón, J.; Meseguer, I.; Rodríguez-Valera, F. Production of an Extracellular Polysaccharide by Haloferax mediterranei. Appl. Environ. Microbiol. 1988, 54, 2381–2386. [Google Scholar]
- Parolis, H.; Parolis, L.A.; Boan, I.F.; Rodriguez-Valera, F.; Widmalm, G.; Manca, M.C.; Jansson, P.E.; Sutherland, I.W. The structure of the exopolysaccharide produced by the halophilic Archaeon Haloferax mediterranei strain R4 (ATCC 33500). Carbohydr. Res. 1996, 295, 147–156. [Google Scholar]
- Nicolaus, B.; Schiano, M.; Lama, L.; Poli, A.; Gambacorta, A. Polysaccharides from extremophilic microorganisms. Orig. Life Evol. Biosph. 2004, 34, 159–169. [Google Scholar] [CrossRef]
- Pham, P.L.; Dupont, I.; Roy, D.; Lapointe, G.; Cerning, J. Production of exopolysaccharide by Lactobacillus rhamnosus R and analysis of its enzymatic degradation during prolonged fermentation. Appl. Environ. Microbiol. 2000, 66, 2302–2310. [Google Scholar]
- Torino, M.I.; Mozzi, F.; Sesma, F.; Font de Valdez, G. Semidefined media for the exopolysaccharide (EPS) production by Lactobacillus helveticus ATCC 15807 and evaluation of the components interfering with the EPS quantification. Milchwissenschaft 2000, 55, 314–316. [Google Scholar]
- Degeest, B.; Janssens, B.; De Vuyst, L. Exopolysaccharide (EPS) biosynthesis by Lactobacillus sakei 0-1: Production kinetics, enzyme activities and EPS yields. J. Appl. Microbiol. 2001, 91, 470–477. [Google Scholar] [CrossRef]
- Tallon, R.; Bressollier, P.; Urdaci, M. Isolation and characterization of two exopolysaccharides produced by Lactobacillus plantarum EP56. Res. Microbiol. 2003, 154, 705–712. [Google Scholar] [CrossRef]
- Petronella, J.L.; Hugenholtz, J. Uncoupling of growth and exopolysaccharide production by Lactococcus lactis subsp. cremoris NIZO B40 and optimization of its synthesis. J. Biosci. Bioeng. 1999, 88, 178–182. [Google Scholar] [CrossRef]
- Cheirslip, B.; Shimizu, H.; Shioya, S. Modelling and optimization of environmental conditions for kefiran production by Lactobacillus kefiranofaciens. Appl. Microbiol. Biotechnol. 2001, 57, 639–643. [Google Scholar] [CrossRef]
- Gorret, A.U.; Maubois, N.; Engasser, J.L.; Ghoul, J.M. Study of the effects of temperature, pH and yeast extract on growth and exopolysaccharide production by Propionibacterium acidipropionici on milk microfiltrate using a response surface methodology. J. Appl. Microbiol. 2001, 90, 788–796. [Google Scholar] [CrossRef]
- Kojic, M.; Vujcic, M.; Banina, A.; Cocconcelli, P.; Cerning, J.; Topisirovic, L. Analysis of exopolysaccharide production by Lactobacillus casei CG11, isolated from cheese. Appl. Environ. Microbiol. 1992, 58, 4086–4088. [Google Scholar]
- Grobben, G.J.; Sikkema, J.; Smith, M.R.; de Bont, J.A.M. Production of extracellular polysaccharides by Lactobacillus delbrueckii NCFB 2772 grown in a chemically defined medium. J. Appl. Bacteriol. 1995, 79, 103–107. [Google Scholar] [CrossRef]
- Wu, X.Z.; Chen, D. Effects of sulfated polysaccharides on tumour biology. West Indian Med. J. 2006, 55, 270–273. [Google Scholar]
- Colliec, J.S.; Chevolot, L.; Helley, D.; Ratiskol, J.; Bros, A.; Sinquin, C.; Roger, O.; Fischer, A.M. Characterization, chemical modifications and in vitro anticoagulant properties of an exopolysaccharide produced by Alteromonas infernus. Biochim. Biophys. Acta 2001, 1528, 141–151. [Google Scholar]
- Ciancia, M.; Quintana, I.; Cerezo, A.S. Overview of anticoagulant activity of sulfated polysaccharides from seaweeds in relation to their structures, focusing on those of green seaweeds. Curr. Med. Chem. 2010, 17, 2503–2529. [Google Scholar] [CrossRef]
- Engelberg, H. Heparin, non-heparin glycosaminoglycans, and heparinoids: an overview of their application in atherosclerosis. Semin. Thromb. Hemost. 1991, 17, 5–8. [Google Scholar]
- Logeart, D.; Prigent-Richard, S.; Boisson-Vidal, C.; Chaubet, F.; Durand, P.; Jozefonvicz, J.; Letourneur, D. Fucans, sulfated polysaccharides extracted from brown seaweeds, inhibit vascular smooth muscle cell proliferation. II. Degradation and molecular weight effect. Eur. J. Cell Biol. 1997, 74, 385–390. [Google Scholar]
- Ganesan, P.; Matsubara, K.; Ohkubo, T.; Tanaka, Y.; Noda, K.; Sugawara, T.; Hirata, T. Anti-angiogenic effect of siphonaxanthin from green alga, Codium fragile. Phytomedicine 2010, 17, 1140–1144. [Google Scholar] [CrossRef] [Green Version]
- Parish, C.R.; Coombe, D.R.; Jakobsen, K.B.; Bennett, F.A.; Underwood, P.A. Evidence that sulphated polysaccharides inhibit tumour metastasis by blocking tumour-cell-derived heparanases. Int. J. Cancer 1987, 40, 511–518. [Google Scholar] [CrossRef]
- Matsui, M.S.; Muizzuddin, N.; Arad, S.; Marenus, K. Sulfated polysaccharides from red microalgae have antiinflammatory properties in vitro and in vivo. Appl. Biochem. Biotechnol. 2003, 104, 13–22. [Google Scholar] [CrossRef]
- Blondin, C.; Fischer, E.; Boisson-Vidal, C.; Kazatchkine, M.D.; Jozefonvicz, J. Inhibition of complement activation by natural sulfated polysaccharides (fucans) from brown seaweed. Mol. Immunol. 1994, 31, 247–253. [Google Scholar] [CrossRef]
- Ghosh, T.; Chattopadhyay, K.; Marschall, M.; Karmakar, P.; Mandal, P.; Ray, B. Focus on antivirally active sulfated polysaccharides: From structure-activity analysis to clinical evaluation. Glycobiology 2009, 19, 2–15. [Google Scholar]
- Pérez-Fernández, M.E.; Quesada, E.; Gálvez, J.; Ruíz, C. Effect of exopolysaccharide V2-7 isolated from Halomonas eurihalina on the proliferation in vitro of human peripheral blood lymphocites. Immunopharmacol. Immunotoxicol. 2000, 22, 131–141. [Google Scholar] [CrossRef]
- Ruiz-Ruiz, C.; Srivastava, G.K.; Carranza, D.; Mata, J.A.; Llamas, I.; Santamaria, M.; Quesada, E.; Molina, I.J. An exopolysaccharide produced by the novel halophilic bacterium Halomonas stenophila strain B100 selectively induces apoptosis in human T leukaemia cells. Appl. Microbiol. Biotechnol. 2011, 89, 345–355. [Google Scholar] [CrossRef]
- Nishimura-Uemura, J.; Kitazawa, H.; Kawai, Y.; Itoh, T.; Oda, M.; Saito, T. Functional alteration of murine macrophages stimulated with extracellular polysaccharides from Lactobacillus delbrueckii ssp. bulgaricus OLL1073R-1. Food Microbiol. 2003, 20, 267–273. [Google Scholar] [CrossRef]
- Ebina, T.; Ogata, N.; Murata, K. Antitumor effect of Lactobacillus bulgaricus 878R. Biotherapy 1995, 9, 65–70. [Google Scholar]
- Rosenberg, E.; Zuckerberg, A.; Rubinovitz, C.; Gutnick, D.L. Emulsifier of Arthrobacter RAG-1: Isolation and emulsifying properties. Appl. Environ. Microbiol. 1979, 37, 402–408. [Google Scholar]
- Navon-Venezia, S.; Banin, E.; Ron, E.Z.; Rosenberg, E. The bioemulsifieralasan: Role of protein in maintaining structure and activity. Appl. Microbiol. Biotechnol. 1998, 49, 382–384. [Google Scholar] [CrossRef]
- Toren, A.; Orr, E.; Paitan, Y.; Ron, E.Z.; Rosenberg, E. The active component of the bioemulsifier alasan from Acinetobacter radioresistant KA53 is an OmpA-like protein. J. Bacteriol. 2002, 184, 165–170. [Google Scholar] [CrossRef]
- Banat, I.M.; Makkar, R.S.; Cameotra, S.S. Potential commercial applications of microbial surfactants. Appl. Microbiol. Biotechnol. 2000, 53, 495–508. [Google Scholar] [CrossRef]
- Singh, P.; Cameotra, S.S. Potential applications of microbial surfactants in biomedical sciences. Trends Biotechnol. 2004, 22, 142–146. [Google Scholar] [CrossRef]
- Desai, J.D.; Banat, I.M. Microbial production of surfactants and their commercial potential. Microbiol. Mol. Biol. Rev. 1997, 61, 47–64. [Google Scholar]
- Iyer, A.; Mody, K.; Jha, B. Biosorption of heavy metals by a marine bacterium. Mar. Pollut. Bull. 2005, 50, 340–343. [Google Scholar] [CrossRef]
- Kim, S.Y.; Lee, H.W.; Hong, J.W.; Kang, Y.S.; Kim, J.D.; Chang, M.W.; Bae, S.K. Metal adsorption of the polysaccharide produced from Methylobacterium organophilum. Biotechnol. Lett. 1996, 18, 1161–1164. [Google Scholar] [CrossRef]
- Valls, M.; de Lorenzo, V. Exploiting the genetic and biochemical capacities of bacteria forthe remediation of heavy metal pollution. FEMS Microbiol. Rev. 2002, 26, 327–338. [Google Scholar]
- Moraine, R.A.; Rogovin, P. Kinetics of polysaccharide B-1459 fermentation. Biotech.Bioeng. 1966, 8, 511–524. [Google Scholar] [CrossRef]
- Rodríguez-Valera, F.; Ruíz-Berraquero, F.; Ramos-Comenzana, A. Characteristics of the heterotrophic bacterial populations in hypersaline environments of different salt concentrations. Microbiol. Ecol. 1981, 7, 235–243. [Google Scholar] [CrossRef]
- Bergmeyer, H.V.; Bent, E. Determination with glucose oxidase and peroxidase. In Method of Enzymatic Analysis; Bergmeyer, H.V., Ed.; Academic Press: New York, NY, USA, 1965; pp. 123–130. [Google Scholar]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- McComb, E.A.; McCready, R.M. Determination of acetyl in pectin and in acetylated carbohydrate polymers. Anal. Chem. 1957, 29, 819–821. [Google Scholar] [CrossRef]
- Sloneker, J.H.; Orentas, D.G. Quantitative determination of pyruvic acid. Nature 1962, 194, 478. [Google Scholar] [CrossRef]
- Johnson, A.R. Improved method of hexosamine determination. Anal. Biochem. 1971, 44, 628–635. [Google Scholar] [CrossRef]
- Chaplin, M.F. A rapid and sensitive method for the analysis of carbohydrate components in glycoproteins using gas-liquid chromatography. Anal. Biochem. 1982, 123, 336–341. [Google Scholar]
- Cooper, D.; Goldenberg, G. Surface active agents from two Bacillus species. Appl. Environ. Microbiol. 1987, 54, 224–229. [Google Scholar]
- Westphal, O.; Jann, K. Bacterial lipopolysaccharides: Extraction with phenol-water and further application of the procedure. In Carbohydrate Chemistry; Whistler, R.L., Ed.; Academic Press: New York, NY, 1965; pp. 83–91. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef]
- Geddie, J.L.; Sutherland, I.W. Uptake of metals by bacterial polysaccharides. J. Appl. Bacteriol. 1993, 74, 467–472. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Llamas, I.; Amjres, H.; Mata, J.A.; Quesada, E.; Béjar, V. The Potential Biotechnological Applications of the Exopolysaccharide Produced by the Halophilic Bacterium Halomonas almeriensis. Molecules 2012, 17, 7103-7120. https://doi.org/10.3390/molecules17067103
Llamas I, Amjres H, Mata JA, Quesada E, Béjar V. The Potential Biotechnological Applications of the Exopolysaccharide Produced by the Halophilic Bacterium Halomonas almeriensis. Molecules. 2012; 17(6):7103-7120. https://doi.org/10.3390/molecules17067103
Chicago/Turabian StyleLlamas, Inmaculada, Hakima Amjres, Juan Antonio Mata, Emilia Quesada, and Victoria Béjar. 2012. "The Potential Biotechnological Applications of the Exopolysaccharide Produced by the Halophilic Bacterium Halomonas almeriensis" Molecules 17, no. 6: 7103-7120. https://doi.org/10.3390/molecules17067103
APA StyleLlamas, I., Amjres, H., Mata, J. A., Quesada, E., & Béjar, V. (2012). The Potential Biotechnological Applications of the Exopolysaccharide Produced by the Halophilic Bacterium Halomonas almeriensis. Molecules, 17(6), 7103-7120. https://doi.org/10.3390/molecules17067103





