6-Shogaol-Rich Extract from Ginger Up-Regulates the Antioxidant Defense Systems in Cells and Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. 6-Shogaol Content of the Ginger Extract

2.2. Cell Viability in HepG2 Cells

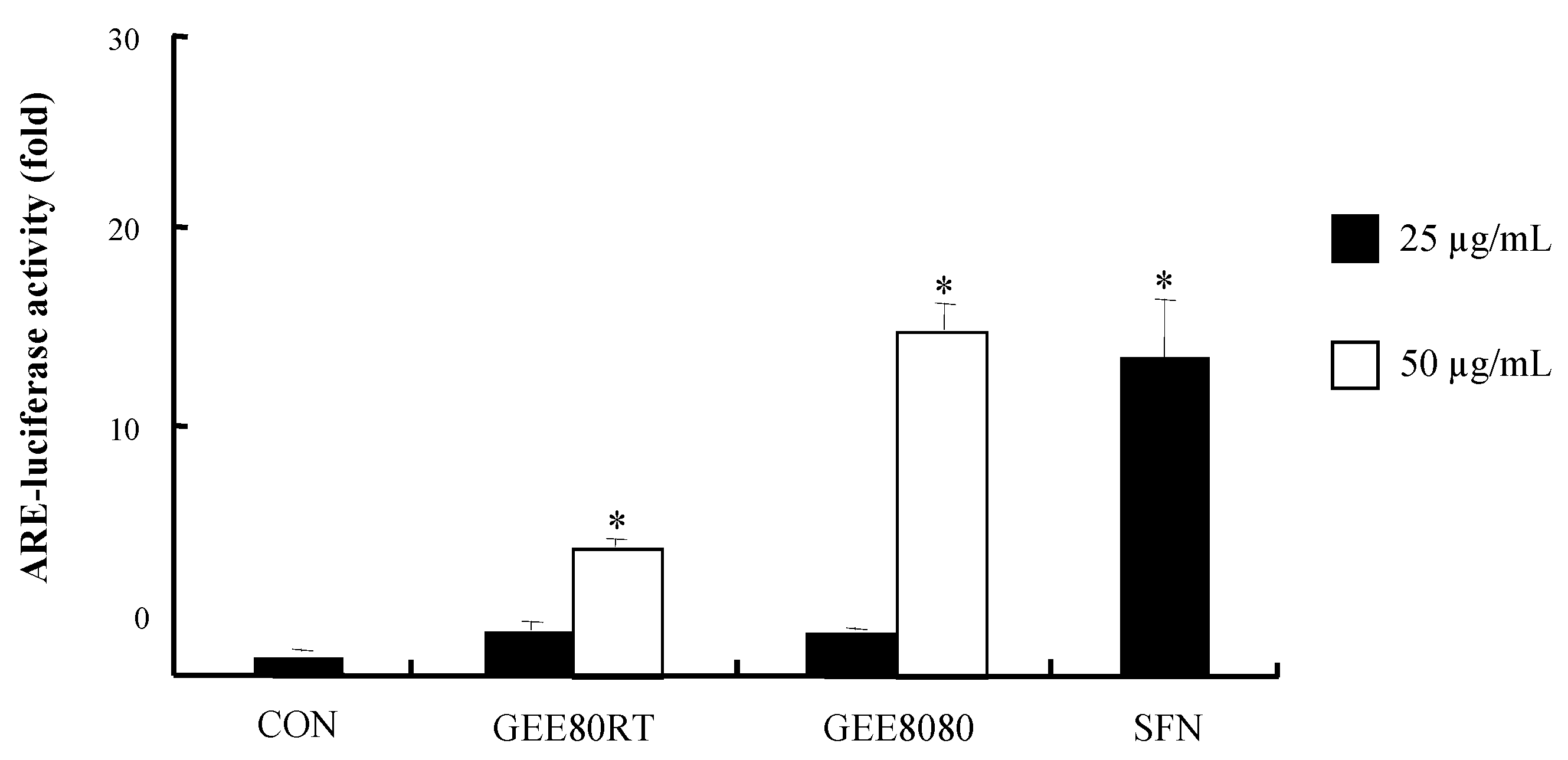
2.3. ARE-Luciferase Reporter Gene Activity in HepG2 Cells
2.4. Induction of Nrf2 and HO-1 Expression in HepG2 Cells

2.5. Phosphorylation of MAPKs and PI3K/Akt Pathway in HepG2 Cells

2.6. Serum AST and ALT Activity in DEN-Treated Mice

2.7. Hepatic TBARS Content in DEN-Treated Mice

2.8. Nrf2 and HO-1 Expression in the Liver of DEN-Induced Mice
2.9. Antioxidant Enzymes Activity and Expression in the Liver of DEN-Induced Mice


3. Experimental
3.1. Reagents
3.2. Preparation of Ginger Extracts
3.3. Cell Culture
3.4. Animals and Experimental Design
3.5. Cell Viability Assay in Cells
3.6. ARE-reporter Gene Activity Assay in Cells
3.7. Western Blot Analysis
3.8. Measurement of AST and ALT Activities in Serum of Mice
3.9. Measurement of Lipid Peroxidation
3.10. Assessment of SOD-like Activity in Liver Tissues
3.11. Assessment of GPx and CAT Activity in Liver Tissues
3.12. Graphs and Statistical Analysis
4. Conclusions
Acknowledgments
References
- Bak, M.J.; Jun, M.; Jeong, W.S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int. J. Mol. Sci. 2012, 13, 2314–2330. [Google Scholar] [CrossRef]
- Jeong, W.S.; Jun, M.; Kong, A.N. Nrf2: A potential molecular target for cancer chemoprevention by natural compounds. Antioxid. Redox Sign. 2006, 8, 99–106. [Google Scholar] [CrossRef]
- Rushmore, T.H.; Kong, A.N. Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr. Drug Metab. 2002, 3, 481–490. [Google Scholar] [CrossRef]
- Itoh, K.; Chiba, T.; Takahashi, S.; Ishii, T.; Igarashi, K.; Katoh, Y.; Oyake, T.; Hayashi, N.; Satoh, K.; Hatayama, I.; et al. An Nrf2/small Mafheterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem. Biophys. Res. Commun. 1997, 236, 313–322. [Google Scholar] [CrossRef]
- Kong, A.N.; Yu, R.; Hebbar, V.; Chen, C.; Owuor, E.; Hu, R.; Ee, R.; Mandlekar, S. Signal transduction events elicited by cancer prevention compounds. Mutat. Res. 2001, 480–481, 231–241. [Google Scholar] [CrossRef]
- Zipper, L.M.; Mulcahy, R.T. Inhibition of ERK and p38 MAP kinases inhibits binding of Nrf2 and induction of GCS genes. Biochem. Biophys. Res. Commun. 2000, 278, 484–492. [Google Scholar] [CrossRef]
- Afzal, M.; Al-Hadidi, D.; Menon, M.; Pesek, J.; Dhami, M.S. Ginger: An ethnomedical, chemical and pharmacological review. Drug Metabol. Drug Interact. 2001, 18, 159–190. [Google Scholar] [CrossRef]
- Thomson, M.; Al-Qattan, K.K.; Al-Sawan, S.M.; Alnaqeeb, M.A.; Khan, I.; Ali, M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot. Essent. Fatty Acids 2002, 67, 475–478. [Google Scholar] [CrossRef]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef]
- Chen, C.Y.; Cheng, K.C.; Chang, A.Y.; Lin, Y.T.; Hseu, Y.C.; Wang, H.M. 10-shogaol, an antioxidant from zingiberofficinale for skin cell proliferation and migration enhancer. Int. J. Mol. Sci. 2012, 13, 1762–1777. [Google Scholar]
- Suekawa, M.; Ishige, A.; Yuasa, K.; Sudo, K.; Aburada, M.; Hosoya, E. Pharmacological studies on ginger. I. Pharmacological actions of pungent constitutents, (6)-gingerol and (6)-shogaol. J. Pharmacobiodyn. 1984, 7, 836–848. [Google Scholar] [CrossRef]
- Koo, K.L.; Ammit, A.J.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb. Res. 2001, 103, 387–397. [Google Scholar] [CrossRef]
- Ippoushi, K.; Azuma, K.; Ito, H.; Horie, H.; Higashio, H. [6]-Gingerol inhibits nitric oxide synthesis in activated J774.1 mouse macrophages and prevents peroxynitrite-induced oxidation and nitration reactions. Life Sci. 2003, 73, 3427–3437. [Google Scholar] [CrossRef]
- Kundu, J.; Surh, Y.-J. Molecular basis of chemoprevention with dietary phytochemicals: Redox-regulated transcription factors as relevant targets. Phytochem. Rev. 2009, 8, 333–347. [Google Scholar] [CrossRef]
- Kim, E.C.; Min, J.K.; Kim, T.Y.; Lee, S.J.; Yang, H.O.; Han, S.; Kim, Y.M.; Kwon, Y.G. [6]-Gingerol, a pungent ingredient of ginger, inhibits angiogenesis in vitro and in vivo. Biochem. Biophys. Res. Commun. 2005, 335, 300–308. [Google Scholar] [CrossRef]
- Weng, C.J.; Wu, C.F.; Huang, H.W.; Ho, C.T.; Yen, G.C. Anti-invasion effects of 6-shogaol and 6-gingerol, two active components in ginger, on human hepatocarcinoma cells. Mol. Nutr. Food Res. 2010, 54, 1618–1627. [Google Scholar]
- Peng, F.; Tao, Q.; Wu, X.; Dou, H.; Spencer, S.; Mang, C.; Xu, L.; Sun, L.; Zhao, Y.; Li, H.; et al. Cytotoxic, cytoprotective and antioxidant effects of isolated phenolic compounds from fresh ginger. Fitoterapia 2012, 83, 568–585. [Google Scholar] [CrossRef]
- Wu, H.; Hsieh, M.C.; Lo, C.Y.; Liu, C.B.; Sang, S.; Ho, C.T.; Pan, M.H. 6-Shogaol is more effective than 6-gingerol and curcumin in inhibiting 12-O-tetradecanoylphorbol 13-acetate-induced tumor promotion in mice. Mol. Nutr. Food Res. 2010, 54, 1296–1306. [Google Scholar] [CrossRef]
- Dugasani, S.; Pichika, M.R.; Nadarajah, V.D.; Balijepalli, M.K.; Tandra, S.; Korlakunta, J.N. Comparative antioxidant and anti-inflammatory effects of [6]-gingerol, [8]-gingerol, [10]-gingerol and [6]-shogaol. J. Ethnopharmacol. 2010, 127, 515–520. [Google Scholar]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. Sci. 2001, 90, 1658–1664. [Google Scholar] [CrossRef]
- Zhang, Y.; Kensler, T.W.; Cho, C.G.; Posner, G.H.; Talalay, P. Anticarcinogenic activities of sulforaphane and structurally related synthetic norbornylisothiocyanates. Proc. Natl. Acad. Sci. USA 1994, 91, 3147–3150. [Google Scholar]
- Surh, Y.J. Cancer chemoprevention with dietary phytochemicals. Nat. Rev. Cancer 2003, 3, 768–780. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Loboda, A.; Jazwa, A.; Grochot-Przeczek, A.; Rutkowski, A.J.; Cisowski, J.; Agarwal, A.; Jozkowicz, A.; Dulak, J. Heme oxygenase-1 and the vascular bed: From molecular mechanisms to therapeutic opportunities. Antioxid. Redox Signal. 2008, 10, 1767–1812. [Google Scholar] [CrossRef]
- Hsu, C.L.; Wu, Y.L.; Tang, G.J.; Lee, T.S.; Kou, Y.R. Ginkgo biloba extract confers protection from cigarette smoke extract-induced apoptosis in human lung endothelial cells: Role of heme oxygenase-1. Pulm. Pharmacol. Ther. 2009, 22, 286–296. [Google Scholar] [CrossRef]
- Hu, R.; Xu, C.; Shen, G.; Jain, M.R.; Khor, T.O.; Gopalkrishnan, A.; Lin, W.; Reddy, B.; Chan, J.Y.; Kong, A.N. Identification of Nrf2-regulated genes induced by chemopreventiveisothiocyanate PEITC by oligonucleotide microarray. Life Sci. 2006, 79, 1944–1955. [Google Scholar] [CrossRef]
- Kweon, S.; Park, K.A.; Choi, H. Chemopreventive effect of garlic powder diet in diethylnitrosamine-induced rat hepatocarcinogenesis. Life Sci. 2003, 73, 2515–2526. [Google Scholar]
- Keum, Y.S.; Yu, S.; Chang, P.P.; Yuan, X.; Kim, J.H.; Xu, C.; Han, J.; Agarwal, A.; Kong, A.N. Mechanism of action of sulforaphane: Inhibition of p38 mitogen-activated protein kinaseisoforms contributing to the induction of antioxidant response element-mediated heme oxygenase-1 in human hepatoma HepG2 cells. Cancer Res. 2006, 66, 8804–8813. [Google Scholar]
- McNally, S.J.; Harrison, E.M.; Ross, J.A.; Garden, O.J.; Wigmore, S.J. Curcumin induces hemeoxygenase 1 through generation of reactive oxygen species, p38 activation and phosphatase inhibition. Int. J. Mol. Med. 2007, 19, 165–172. [Google Scholar]
- Gong, P.; Hu, B.; Cederbaum, A.I. Diallyl sulfide induces heme oxygenase-1 through MAPK pathway. Arch. Biochem. Biophys. 2004, 432, 252–260. [Google Scholar] [CrossRef]
- Jeong, W.S.; Keum, Y.S.; Chen, C.; Jain, M.R.; Shen, G.; Kim, J.H.; Li, W.; Kong, A.N. Differential expression and stability of endogenous nuclear factor E2-related factor 2 (Nrf2) by natural chemopreventive compounds in HepG2 human hepatoma cells. J. Biochem. Mol. Biol. 2005, 38, 167–176. [Google Scholar] [CrossRef]
- Bak, M.J.; Jun, M.; Jeong, W.S. Procyanidins from wild grape (Vitisamurensis) seeds regulate ARE-mediated enzyme expression via Nrf2 coupled with p38 and PI3K/Akt pathway in HepG2 cells. Int. J. Mol. Sci. 2012, 13, 801–818. [Google Scholar] [CrossRef]
- Yu, R.; Lei, W.; Mandlekar, S.; Weber, M.J.; Der, C.J.; Wu, J.; Kong, A.N. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J. Biol. Chem. 1999, 274, 27545–27552. [Google Scholar]
- Shen, G.; Hebbar, V.; Nair, S.; Xu, C.; Li, W.; Lin, W.; Keum, Y.S.; Han, J.; Gallo, M.A.; Kong, A.N. Regulation of Nrf2 transactivation domain activity. The differential effects of mitogen-activated protein kinase cascades and synergistic stimulatory effect of Raf and CREB-binding protein. J. Biol. Chem. 2004, 279, 23052–23060. [Google Scholar]
- Hayes, J.D.; Pulford, D.J. The glutathione S-transferase supergene family: Regulation of GST and the contribution of the isoenzymes to cancer chemoprotection and drug resistance. Crit. Rev. Biochem. Mol. Biol. 1995, 30, 445–600. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Choi, J.M.; Chung, Y.C.; Jeong, H.G. Protective mechanisms of anthocyanins from purple sweet potato against tert-butyl hydroperoxide-induced hepatotoxicity. Food Chem. Toxicol. 2011, 49, 2081–2089. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Jeong, H.G. Mechanism of phytoestrogenpuerarin-mediated cytoprotection followingoxidative injury: Estrogen receptor-dependent up-regulation of PI3K/Akt and HO-1. Toxicol. Appl. Pharmacol. 2008, 233, 371–381. [Google Scholar] [CrossRef]
- Kang, J.S.; Wanibuchi, H.; Morimura, K.; Gonzalez, F.J.; Fukushima, S. Role of CYP2E1 in diethylnitrosamine-induced hepatocarcinogenesis in vivo. Cancer Res. 2007, 67, 11141–11146. [Google Scholar] [CrossRef]
- Chuang, S.E.; Cheng, A.L.; Lin, J.K.; Kuo, M.L. Inhibition by curcumin of diethylnitrosamine-induced hepatic hyperplasia, inflammation, cellular gene products and cell-cycle-related proteins in rats. Food Chem. Toxicol. 2000, 38, 991–995. [Google Scholar] [CrossRef]
- Kohle, C.; Schwarz, M.; Bock, K.W. Promotion of hepatocarcinogenesis in humans and animal models. Arch. Toxicol. 2008, 82, 623–631. [Google Scholar]
- Naik, S.R.; Panda, V.S. Antioxidant and hepatoprotective effects of Ginkgo bilobaphytosomes in carbon tetrachloride-induced liver injury in rodents. Liver Int. 2007, 27, 393–399. [Google Scholar] [CrossRef]
- Kalantari, H.; Salehi, M. The protective effect of garlic oil on hepatotoxicity induced by acetaminophen in mice and comparison with N-acetylcysteine. Saudi. Med. J. 2001, 22, 1080–1084. [Google Scholar]
- Benzie, I.F. Lipid peroxidation: A review of causes, consequences, measurement and dietary influences. Int. J. Food Sci. Nutr. 1996, 47, 233–261. [Google Scholar] [CrossRef]
- Singh, B.N.; Singh, B.R.; Sarma, B.K.; Singh, H.B. Potential chemoprevention of N-nitrosodiethylamine-induced hepatocarcinogenesis by polyphenolics from Acacia nilotica bark. Chem. Biol. Interact. 2009, 181, 20–28. [Google Scholar]
- Dianzani, M.U. Lipid peroxidation and cancer: A critical reconsideration. Tumori 1989, 75, 351–357. [Google Scholar]
- Shaarawy, S.M.; Tohamy, A.A.; Elgendy, S.M.; Elmageed, Z.Y.; Bahnasy, A.; Mohamed, M.S.; Kandil, E.; Matrougui, K. Protective effects of garlic and silymarin on NDEA-induced rats hepatotoxicity. Int. J. Biol. Sci. 2009, 5, 549–557. [Google Scholar]
- Nguyen, T.; Nioi, P.; Pickett, C.B. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J. Biol. Chem. 2009, 284, 13291–13295. [Google Scholar] [CrossRef]
- Perrella, M.A.; Yet, S.F. Role of heme oxygenase-1 in cardiovascular function. Curr. Pharm. Des. 2003, 9, 2479–2487. [Google Scholar] [CrossRef]
- Prawan, A.; Kundu, J.K.; Surh, Y.J. Molecular basis of heme oxygenase-1 induction: Implications for chemoprevention and chemoprotection. Antioxid. Redox. Sign. 2005, 7, 1688–1703. [Google Scholar] [CrossRef]
- Sabina, E.P.; Pragasam, S.J.; Kumar, S.; Rasool, M. 6-gingerol, an active ingredient of ginger, protects acetaminophen-induced hepatotoxicity in mice. ZhongXi Yi Jie He Xue Bao 2011, 9, 1264–1269. [Google Scholar] [CrossRef]
- Banakar, M.C.; Paramasivan, S.K.; Chattopadhyay, M.B.; Datta, S.; Chakraborty, P.; Chatterjee, M.; Kannan, K.; Thygarajan, E. 1α,25-dihydroxyvitamin D3 prevents DNA damage and restores antioxidant enzymes in rat hepatocarcinogenesis induced by diethylnitrosamine and promoted by phenobarbital. World J. Gastroenterol. 2004, 10, 1268–1275. [Google Scholar]
- Anis, K.V.; Rajeshkumar, N.V.; Kuttan, R. Inhibition of chemical carcinogenesis by berberine in rats and mice. J. Pharm. Pharmacol. 2001, 53, 763–768. [Google Scholar]
- Gurski, R.R.; Schirmer, C.C.; Kruel, C.R.; Komlos, F.; Kruel, C.D.; Edelweiss, M.I. Induction of esophageal carcinogenesis by diethylnitrosamine and assessment of the promoting effect of ethanol and N-nitrosonornicotine: Experimental model in mice. Dis. Esophagus 1999, 12, 99–105. [Google Scholar] [CrossRef]
- Jin, Y.S.; Sa, J.H.; Shim, T.H.; Rhee, H.I.; Wang, M.H. Hepatoprotective and antioxidant effects of Morus bombycis Koidzumi on CCl4-induced liver damage. Biochem. Biophys. Res. Commun. 2005, 329, 991–995. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar]
- Liu, F.; Ooi, V.E.; Chang, S.T. Free radical scavenging activities of mushroom polysaccharide extracts. Life Sci. 1997, 60, 763–771. [Google Scholar] [CrossRef]
- Bogdanska, J.J.; Korneti, P.; Todorova, B. Erythrocyte superoxide dismutase, glutathione peroxidase and catalase activities in healthy male subjects in Republic of Macedonia. Bratisl. Lek. Listy 2003, 104, 108–114. [Google Scholar]
- Carrillo, M.C.; Kanai, S.; Nokubo, M.; Kitani, K. (-)Deprenyl induces activities of both superoxide dismutase and catalase but not of glutathione peroxidase in the striatum of young male rats. Life Sci. 1991, 48, 517–521. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the ginger extract GEE8080 are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Bak, M.-J.; Ok, S.; Jun, M.; Jeong, W.-S. 6-Shogaol-Rich Extract from Ginger Up-Regulates the Antioxidant Defense Systems in Cells and Mice. Molecules 2012, 17, 8037-8055. https://doi.org/10.3390/molecules17078037
Bak M-J, Ok S, Jun M, Jeong W-S. 6-Shogaol-Rich Extract from Ginger Up-Regulates the Antioxidant Defense Systems in Cells and Mice. Molecules. 2012; 17(7):8037-8055. https://doi.org/10.3390/molecules17078037
Chicago/Turabian StyleBak, Min-Ji, Seon Ok, Mira Jun, and Woo-Sik Jeong. 2012. "6-Shogaol-Rich Extract from Ginger Up-Regulates the Antioxidant Defense Systems in Cells and Mice" Molecules 17, no. 7: 8037-8055. https://doi.org/10.3390/molecules17078037
APA StyleBak, M.-J., Ok, S., Jun, M., & Jeong, W.-S. (2012). 6-Shogaol-Rich Extract from Ginger Up-Regulates the Antioxidant Defense Systems in Cells and Mice. Molecules, 17(7), 8037-8055. https://doi.org/10.3390/molecules17078037




