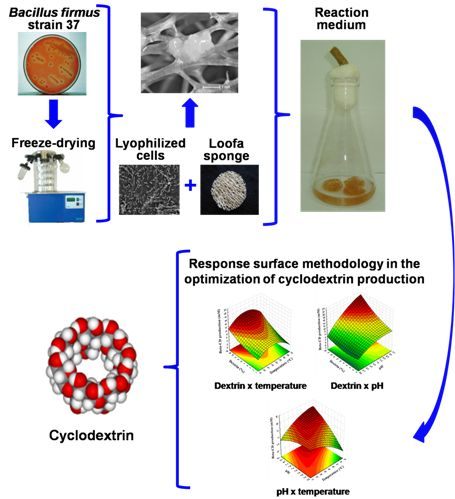
Preservation of Bacillus firmus Strain 37 and Optimization of Cyclodextrin Biosynthesis by Cells Immobilized on Loofa Sponge
Abstract
:1. Introduction
2. Results and Discussion
2.1. Immobilization of Bacillus firmus Strain 37 Cells on Loofa Sponge
2.2. Bacillus firmus Strain 37 Preservation by Lyophilization


2.3. Optimization of β-Cyclodextrin Production by Immobilized Cells on Loofa Sponge


2.4. Effect of Immobilization Time of Bacillus firmus Strain 37 Cells on Loofa Sponge for β-Cyclodextrin Production
 ) 10 days; (
) 10 days; (  ) 20 days; and (
) 20 days; and (  ) 30 days.
) 30 days.
 ) 10 days; (
) 10 days; (  ) 20 days; and (
) 20 days; and (  ) 30 days.
) 30 days.
3. Experimental
3.1. General
3.2. Culture Conditions and Microorganism Reactivation Procedure
3.3. Immobilization Procedure
3.4. Scanning Electron Microscopy
3.5. Evaluation of Bacillus firmus Strain 37 Preservation by Lyophilization
3.6. Cyclodextrin Production by Immobilized Cells
3.7. Optimization of Conditions for β-Cyclodextrin Production by Immobilized Cells
| Experiments | Temperature | pH | Dextrin | β-CD Production (mM) | |
|---|---|---|---|---|---|
| Experimental | Predicted | ||||
| 1 | −1 | −1 | −1 | 8.830 | 8.540 |
| 2 | 1 | −1 | −1 | 6.462 | 2.846 |
| 3 | −1 | 1 | −1 | 7.410 | 7.458 |
| 4 | 1 | 1 | −1 | 14.561 | 12.678 |
| 5 | −1 | −1 | 1 | 11.869 | 10.874 |
| 6 | 1 | −1 | 1 | 8.879 | 5.955 |
| 7 | −1 | 1 | 1 | 11.404 | 12.144 |
| 8 | 1 | 1 | 1 | 20.727 | 18.140 |
| 9 | −α | 0 | 0 | 9.129 | 8.288 |
| 10 | +α | 0 | 0 | 1.906 | 8.501 |
| 11 | 0 | −α | 0 | 1.640 | 5.983 |
| 12 | 0 | +α | 0 | 12.421 | 13.833 |
| 13 | 0 | 0 | −α | 6.380 | 9.249 |
| 14 | 0 | 0 | +α | 11.875 | 14.761 |
| 15 | 0 | 0 | 0 | 11.418 | 10.648 |
| 16 | 0 | 0 | 0 | 11.836 | 10.648 |
| 17 | 0 | 0 | 0 | 11.889 | 10.648 |
| 18 | 0 | 0 | 0 | 11.370 | 10.648 |
| 19 | 0 | 0 | 0 | 12.273 | 10.648 |
| 20 | 0 | 0 | 0 | 12.202 | 10.648 |
| 21 | 0 | 0 | 0 | 12.580 | 10.648 |
| 22 | 0 | 0 | 0 | 11.687 | 10.648 |
3.8. Influence of Immobilization Time on β-Cyclodextrin Production
3.9. Colorimetric Determination of β-Cyclodextrin
3.10. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Hays, H.C.W.; Millner, P.A.; Jones, J.K.; Rayner-Brandes, M.H. A novel and convenient self-drying system for bacterial preservation. J. Microbiol. Methods 2005, 63, 29–35. [Google Scholar] [CrossRef]
- Krumnow, A.A.; Sorokulova, I.B.; Olsen, E.; Globa, L.; Barbaree, J.M.; Vodyanoy, V.J. Preservation of bacteria in natural polymers. J. Microbiol. Methods 2009, 78, 189–194. [Google Scholar] [CrossRef]
- Morgan, C.A.; Herman, N.; White, P.A.; Vesey, G. Preservation of micro-organisms by drying: A review. J. Microbiol. Methods 2006, 66, 183–193. [Google Scholar] [CrossRef]
- Moriwaki, C.; Mazzer, C.; Pazzetto, R.; Matioli, G. Avaliação de métodos para manutenção e preservação de bactéria esporulada produtora da enzima CGTase. Acta Sci. Health Sci. 2009, 31, 113–119. [Google Scholar]
- Bjerketorp, J.; Hakansson, S.; Belkin, S.; Jansson, J.K. Advances in preservation methods: Keeping biosensor microorganisms alive and active. Curr. Opin. Biotechnol. 2006, 17, 43–49. [Google Scholar] [CrossRef]
- Ratnam, B.V.V.; Subba Rao, S.; Damodar Rao, M.; Narasimha Rao, M.; Ayyanna, C. Optimization of medium constituents and fermentation conditions for the production of ethanol from palmyra jaggery using response surface methodology. World J. Microbiol. Biotechnol. 2005, 21, 399–404. [Google Scholar] [CrossRef]
- Alam, M.Z.; Jamal, P.; Nadzir, M.M. Bioconversion of palm oil mill effluent for citric acid production: Statistical optimization of fermentation media and time by central composite design. World J. Microbiol. Biotechnol. 2008, 24, 1177–1185. [Google Scholar] [CrossRef]
- Kar, S.; Swain, M.R.; Ray, R.C. Statistical optimization of alpha-amylase production with immobilized cells of Streptomyces erumpens MTCC 7317 in Luffa cylindrica L. sponge discs. Appl. Biochem. Biotechnol. 2009, 152, 177–188. [Google Scholar] [CrossRef]
- Pazzetto, R.; Delani, T.C.O.; Fenelon, V.C.; Matioli, G. Cyclodextrin production by Bacillus firmus strain 37 cells immobilized on loofa sponge. Process Biochem. 2011, 46, 46–51. [Google Scholar] [CrossRef]
- Biwer, A.; Antranikian, G.; Heinzle, E. Enzimatic production of cyclodextrins. Appl. Microbiol. Biotechnol. 2002, 59, 609–617. [Google Scholar] [CrossRef]
- Del Valle, E.M.M. Cyclodextrins and their uses: A review. Process Biochem. 2004, 39, 1033–1046. [Google Scholar] [CrossRef]
- Yu, B.; Wang, J.; Zhang, H.; Jin, Z. Investigation of the interactions between the hydrophobic cavities of cyclodextrins and pullulanase. Molecules 2011, 16, 3010–3017. [Google Scholar] [CrossRef]
- Satoh, T.; Miyataka, H.; Yamamoto, K.; Hirano, T. Synthesis and physiological activity of novel tocopheryl glycosides. Chem. Pharm. Bull. 2001, 49, 948–953. [Google Scholar] [CrossRef]
- Shimoda, K.; Akagi, M.; Hamada, H. Production of β-maltooligosaccharides of α- and δ-tocopherols by Klebsiella pneumoniae and cyclodextrin glucanotransferase as anti-allergic agents. Molecules 2009, 14, 3106–3114. [Google Scholar] [CrossRef]
- Astray, G.; Gonzalez-Barreiro, C.; Mejuto, J.C.; Rial-Otero, R.; Simal-Gándara, J. A review on the use of cyclodextrins in food. Food Hidrocolloid 2009, 23, 1631–1640. [Google Scholar] [CrossRef]
- Abdel-Naby, M.A.; Reyad, R.M.; Abdel-Fattah, A.F. Biosynthesis of cyclodextrin glucosyltransferase by immobilized Bacillus amyloliquefaciens in batch and continuous cultures. Biochem. Eng. J. 2000, 5, 1–9. [Google Scholar] [CrossRef]
- Karel, S.F.; Libicki, S.B.; Robertson, C.R. The immobilization of whole cells: Engineering principles. Chem. Eng. Sci. 1985, 40, 1321–1354. [Google Scholar] [CrossRef]
- Hemachander, C.; Bose, N.; Puvanakrishnan, R. Whole cell immobilization of Ralstonia pickettii for lipase production. Process Biochem. 2001, 36, 629–633. [Google Scholar] [CrossRef]
- Iqbal, M.; Saeed, A.; Edyvean, R.G.J.; Sullivan, B.O.; Styring, P. Production of fungal biomass immobilized loofa sponge (FBILS)-discs for the removal of heavy metal ions and chlorinated compounds from aqueous solution. Biotechnol. Lett. 2005, 27, 1319–1323. [Google Scholar] [CrossRef]
- Meleigy, S.A.; Khalaf, M.A. Biosynthesis of gibberellic acid from milk permeate in repeated batch operation by a mutant Fusarium moniliforme cells immobilized on loofa sponge. Bioresour. Technol. 2009, 100, 374–379. [Google Scholar] [CrossRef]
- Ogbonna, J.C.; Tomiyama, S.; Tanaka, H. Development of a method for immobilization of non-flocculating cells in loofa (Luffa cylindrica) sponge. Process Biochem. 1996, 31, 737–744. [Google Scholar] [CrossRef]
- Domíngues, A.; Rivela, I.; Couto, S.R.; Sanromán, M.A. Design of a new rotating drum bioreactor for ligninolytic enzyme production by Phanerochaete chrysosporium grown on an inert support. Process Biochem. 2001, 37, 549–554. [Google Scholar] [CrossRef]
- Kourkoutasa, Y.; Bekatoroua, A.; Banatb, I.M.; Marchantb, R.; Koutinasa, A.A. Immobilization technologies and support materials suitable inalcohol beverages production: A review. Food Microbiol. 2004, 21, 377–397. [Google Scholar] [CrossRef]
- Mazzer, C.; Ferreira, L.R.; Rodella, J.R.T.; Moriwaki, C.; Matioli, G. Cyclodextrin production by Bacillus firmus strain 37 immobilized on inorganic matrices and alginate gel. Biochem. Eng. J. 2008, 41, 79–86. [Google Scholar] [CrossRef]
- Vignoli, J.A.; Celligoi, M.A.P.C.; Silva, R.S.F. Development of a statistical model for sorbitol production by free and immobilized Zymomonas mobilis in loofa sponge Luffa cylindrical. Process Biochem. 2006, 41, 240–243. [Google Scholar] [CrossRef]
- Kumar, P.; Satyanarayana, T. Optimization of culture variables for improving glucoamylase production by alginate-entrapped Thermomucor indicae-seudaticae using statistical methods. Bioresour. Technol. 2007, 98, 1252–1259. [Google Scholar] [CrossRef]
- Saudagar, P.S.; Shaligram, N.S.; Singhal, R.S. Immobilization of Streptomyces clavuligerus on loofa sponge for the production of clavulanic acid. Bioresour. Technol. 2008, 99, 2250–2253. [Google Scholar]
- Akhtar, N.; Iqbal, J.; Iqbal, M. Removal and recovery of nickel (II) from aqueous solution by loofa sponge-immobilized biomass of Chlorella sorokiniana: Characterization studies. J. Hazard. Mater. 2004, 108, 85–94. [Google Scholar] [CrossRef]
- Matioli, G.; Zanin, G.M.; Moraes, F.F. Characterization of cyclodextrin glycosyltransferase from Bacillus firmus strain No. 37. Appl. Biochem. Biotechnol. 2001, 91–93, 643–654. [Google Scholar] [CrossRef]
- Moriwaki, C.; Pelissari, F.M.; Gonçalves, R.A.C.; Gonçalves, J.E.; Matioli, G. Immobilization of Bacillus firmus strain 37 in inorganic matrix for cyclodextrin production. J. Mol. Catal. Enzyme 2007, 49, 1–7. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Pazzetto, R.; Ferreira, S.B.d.S.; Santos, E.J.S.; Moriwaki, C.; Guedes, T.A.; Matioli, G. Preservation of Bacillus firmus Strain 37 and Optimization of Cyclodextrin Biosynthesis by Cells Immobilized on Loofa Sponge. Molecules 2012, 17, 9476-9488. https://doi.org/10.3390/molecules17089476
Pazzetto R, Ferreira SBdS, Santos EJS, Moriwaki C, Guedes TA, Matioli G. Preservation of Bacillus firmus Strain 37 and Optimization of Cyclodextrin Biosynthesis by Cells Immobilized on Loofa Sponge. Molecules. 2012; 17(8):9476-9488. https://doi.org/10.3390/molecules17089476
Chicago/Turabian StylePazzetto, Rúbia, Sabrina Barbosa de Souza Ferreira, Elder James Silva Santos, Cristiane Moriwaki, Teresinha Aparecida Guedes, and Graciette Matioli. 2012. "Preservation of Bacillus firmus Strain 37 and Optimization of Cyclodextrin Biosynthesis by Cells Immobilized on Loofa Sponge" Molecules 17, no. 8: 9476-9488. https://doi.org/10.3390/molecules17089476
APA StylePazzetto, R., Ferreira, S. B. d. S., Santos, E. J. S., Moriwaki, C., Guedes, T. A., & Matioli, G. (2012). Preservation of Bacillus firmus Strain 37 and Optimization of Cyclodextrin Biosynthesis by Cells Immobilized on Loofa Sponge. Molecules, 17(8), 9476-9488. https://doi.org/10.3390/molecules17089476