Abstract
The amino acid and fatty acid composition of polypeptide k and oil isolated from the seeds of Momordica charantia was analysed. The analysis revealed polypeptide k contained 9 out of 11 essential amino acids, among a total of 18 types of amino acids. Glutamic acid, aspartic acid, arginine and glycine were the most abundant (17.08%, 9.71%, 9.50% and 8.90% of total amino acids, respectively). Fatty acid analysis showed unusually high amounts of C18-0 (stearic acid, 62.31% of total fatty acid). C18-1 (oleic acid) and C18-2 (linoleic acid) were the other major fatty acid detected (12.53% and 10.40%, respectively). The oil was devoid of the short fatty acids (C4-0 to C8-0). Polypeptide k and oil were also subjected to in vitro α-glucosidase and α-amylase inhibition assays. Both polypeptide k and seed oil showed potent inhibition of α-glucosidase enzyme (79.18% and 53.55% inhibition, respectively). α-Amylase was inhibited by 35.58% and 38.02%, respectively. Collectively, the in vitro assay strongly suggests that both polypeptide k and seed oil from Momordica charantia are potent potential hypoglycemic agents.
1. Introduction
Approximately 60–80% of the World’s population relies on plant-based medicines as their health care system. The traditional Indian system of medicine (Ayurveda) has a long history, however many of the plants used lack any adequate scientific documentation to support this use. Nevertheless, it is now clear that the medicinal value of these plants lies in the bioactive phytochemical constituents that produce definite biomedical effects on the human body [1]. Momordica charantia Linn (MC) or bitter gourd is one such plant that has been extensively used and reported for its hypoglycemic effects [2,3]. It has been commonly used as a traditional remedy in Asia, Africa, England, India and Sri Lanka [4]. In Ayurveda, the fruit is considered as to have many benifits such as tonic, stimulant, emetic, antibilous, laxative and alterative. However, some have claimed that MC will induce heartburn and worsen gastric ulcers [5].
MC contains several very promising bioactive compounds that activate adenosine monophosphate-activated protein kinase (AMPK), which is well known for regulating energy metabolism and enabling glucose uptake which is impaired in diabetics [5]. MC also contains a lectin that has insulin-like activity due to its affinity to the insulin receptors. This compound lowers blood glucose concentrations by acting on peripheral tissues and suppressing appetite [6]. Fruit and seeds of MC are traditionally used as a medicinal herb and/or vegetable for treatment of diabetes in Southeast Asian countries [7]. Its in vivo hypoglycemic activity has been reported for pulps, seeds and leaves [8]. Khanna et al. [9] isolated an active protein (polypeptide-p) from seeds by acid ethanol extraction, which decreased blood glucose in Streptozotocin-induced diabetic rats and increased glycolytic enzyme activity. Polypeptide-p has also shown to have hypoglycemic effects in juvenile and maturity-onset diabetic patients [9,10]. Polypeptide-k (PPK), which was later isolated from seeds of MC, also possesses blood glucose level-reducing activity which is more potent than that of polypeptide-p and helps in the prevention of diabetes [11]. Research and clinical trials have proven that this product can sucessfully regulate blood glucose [12]. The oil from the seeds of MC also has biomedical properties as reported by Noguchi et al. [13] and Braca et al. [14].
α-Glucosidase (EC 3.2.1.20, α-D-glucoside glucohydrolase) is an exo-carbohydrase that catalyzes the liberation of α-glucose from carbohydrates. This enzyme is widely distributed in microorganisms, plants, and animal tissues [15]. α-Amylase (EC 3.2.1.1) is an enzyme that hydrolyses the α-bonds of large α-linked polysaccharides. Found in many tissues, amylase is most prominent in pancreatic juice and saliva [16]. Thus, the objectives of this current investigation were to determine the chemical properties of PPK and oil isolated from MC seeds and determine their potential anti-diabetic activity in vitro through investigating their effects on inhibition of α-glucosidase and α-amylase.
2. Results and Discussion
The fatty acid analysis of MC seed oil is shown in Table 1. The most abundant fatty acid in MC seed oil is C18-0, followed by C18-1 and C18-2. The profile is different when compared to the common palm oil which contains predominantly C16-0 and C18-1. Both oils are devoid of short chain fatty acids (C4-0 to C8-0). The amino acid composition of PPKis shown in Table 2. The major amino acids are glutamic acid, aspartic acid, arginine and glycine (ranging from 8.9 to 17.08% of the total). Table 3 shows the anti-oxidant activity of polypeptide-k and MC seed oil. Standard α-tocopherol has the highest anti-oxidant activity of 96.58% when compared to PPK and MC seed oil (84.9 and 80.85% respectively). Palm oil displayed the lowest anti oxidant activity.

Table 1.
Fatty acid composition of oil from seeds of Momordica charantia (MCOP) and palm oil (PO, control).
| MCO | PO | |
|---|---|---|
| C4-0 (Butyric acid) | ND | ND |
| C6-0 (Caprioc acid) | ND | ND |
| C8-0 (Caprylic acid) | ND | ND |
| C10-0 (Capric acid) | 0.53 ± 0.03 | ND |
| C12-0 (Lauric acid) | 0.45 ± 0.04 | 0.3 ± 0.12 |
| C14-0 (Myristic acid) | ND | 1.1 ± 0.02 |
| C14-1 (Myristoliec acid) | ND | ND |
| C16-0 (Palmitic acid) | 4.72 ± 0.28 | 43.7 ± 1.95 |
| C16-1 (Palmitoleic acid) | ND | 0.5 ± 0.05 |
| C18-0 (Stearic acid) | 62.31 ± 3.74 | 4.9 ± 0.18 |
| C18-1 (Oleic acid) | 12.53 ± 0.70 | 37.8 ± 0.94 |
| C18-2 (Linoleic acid) | 10.40 ± 0.63 | 10.1 ± 1.0 |
| C20-0 (Arachidic acid) | 5.30 ± 0.39 | 0.43 ± 0.07 |
| Others | 2.76 ± 0.18 | 0.45 ± 0.16 |
Values are % of total fatty acid expressed as mean ± SD of three separate determinations. ND: not detected.
The chemical analysis of two constituents from the seeds of MC was investigated. The MC seed oil contains high amount of stearic acid (C18-0). This is very interesting as MC seed oil has been reported to posses wound healing and anti-diabetic properties [17]. Long chain saturated fatty acids are not recommended for human ingestion, but Bonanome and Grundy [18] reported reduction of plasma lipoprotein levels in 11 subjects during three dietary periods when given stearic acid. PPK isolated also from the seeds of MC has been reported to have potent anti-diabetic activity. It has been given to diabetic patients with good results [11]. In our previous study [12], we reported polypeptide k supplemented rolls accelerate the reduction of blood glucose in normal healthy individuals. As PPK contains a balanced amount or all amino acids, it can be a useful ingredient for many food, i.e., bread, noodles, flour, biscuits, etc. Indeed both MC seed oil and PPK had potent anti-oxidant properties which may be beneficial in reducing β-cell damage [11,12], thus improving the severity of diabetes. Interestingly, these results may be part of the key mechanism for the reduction of blood glucose observed in the clinical trials done previously, when 605 diabetic patients were administered PPK sublingually for 1 to 3 years for best effects (avoiding the first-pass metabolism effects) and blood glucose was reduced approximately 25 to 43.6% [11]. This study also revealed that patients taking PPK orally also had a reduction in elevated blood glucose [11]. Indeed, this product has been in Malaysian market for the past 10 years with very effective results.

Table 2.
Amino acid composition of polypeptide-k isolated from Momordica charantia.
| Amino acid | Polypeptide-k |
|---|---|
| Aspartic acid | 9.71 ± 0.43 |
| Glutamic acid | 17.08 ± 0.95 |
| Serine | 5.3 ± 0.15 |
| Glycine | 8.9 ± 0.57 |
| Histidine | 3.4 ± 0.23 |
| Arginine | 9.5 ± 1.57 |
| Threonine | 3.0 ± 0.01 |
| Alanine | 7.6 ± 1.21 |
| Proline | 4.1 ± 0.20 |
| Tyrosine | 2.7 ± 0.14 |
| Valine | 5.8 ± 0.35 |
| Methionine | 1.6 ± 0.25 |
| Cystine | 2.8 ± 0.23 |
| Isoleucine | 4.8 ± 0.12 |
| Leucine | 3.7 ± 0.28 |
| Phenylalanine | 4.2 ± 0.19 |
| Lysine | 4.4 ± 0.36 |
| Tryptophan | 1.0 ± 0.02 |
Values are % of total amino acid expressed as mean ± SD of three separate determinations.
PPK contains nine out of 11 essential amino acids, making it a good source of amino acids [19]. It also contains high amount of glutamic acid, glycine and arginine which are vital for many bodily functions. Glutamic acid and glycine are important for memory, learning and neurotransmitter [20]. Arginine plays an important role in blood circulation, healing of wounds, treatment of erectile dysfunction and immune functions [21]. Table 3 reveals that PPK and seed oil have potent anti-oxidant activity that may help in the reduction of blood glucose.

Table 3.
Total anti-oxidant activity of polypeptide-k and oil from Momordica charantia.
| Sample | Percentage anti oxidant activity (%) |
|---|---|
| α-Tocopherol (Standard) | 96.58 ± 1.76 |
| MCO | 80.54 ± 2.41 |
| PPK | 84.90 ± 1.71 |
| PO | 45.21 ± 6.90 |
Values are % of antioxidant expressed as mean ± SD of three separate determinations. MCO: Momordica charantia seed oil. PPK: Polypeptide-k. PO: palm oil.
The MC seed oil also contains many other phytocompounds that may have other biomedical properties. The oil contain high amount of trans-nerolidol, cis-dihydrocarveol and apiole [14]. Recently, Pripdeevech and Machan [22] reported potent anti-oxidant properties in different teas in Thailand and trans-nerolidol is one of the many volatile components identified. cis-Dihydrocarveol and apiole are related to anti-microbial activity [14]. Interestingly, seed oil also has the potential as it contains trans-Nerolidol and Apiole (2 major constituents) which are anti-microbial and help in wound healing [17].
The α-glucosidase inhibition is expressed in Table 4. PPK has the highest inhibition of 79.18% at 2 mg/mL as compared to MC seed oil of 53.55% at the same concentration. This inhibition was in a dose-dependent manner (Figure 1). The inhibition of α-amylase is illustrated in Table 5. Both PPK and MC seed oil inhibited the α-amylase activity from 18.20 to 35.58% and 24.54 to 38.02%, respectively. MC seed oil has slightly higher inhibition when compared to PPK. Inhibition of α-amylase also occurred in a dose-dependent manner (Figure 1).

Table 4.
Effect of polypeptide-k and oil from Momordica charantia on α-glucosidase activity.
| Concentration (mg/mL) | PPK α-Glucosidase activity (% Inhibition) | MCO α-Glucosidase activity (% Inhibition) |
|---|---|---|
| 0.267 | 70.6628 ± 5.0856 (45.55) | 97.0091 ± 1.0726 (20.71) |
| 0.356 | 51.4710 ± 9.9259 (53.89) | 90.7401 ± 1.1301 (25.84) |
| 0.475 | 55.4295 ± 2.9121 (54.33) | 85.6295 ± 3.0594 (30.09) |
| 0.633 | 48.5088 ± 3.6516 (58.73) | 80.4882 ± 3.4838 (34.30) |
| 0.844 | 42.7553 ± 4.6282 (65.79) | 74.0152 ± 2.1990 (39.55) |
| 1.125 | 37.2303 ± 4.8153 (70.21) | 68.8970 ± 2.5743 (43.75) |
| 1.5 | 32.4798 ± 2.0861 (74.01) | 64.5213 ± 2.0793 (47.31) |
| 2 | 26.0133 ± 2.5803 (79.18) | 56.8900 ± 2.9721 (53.55) |
All values are mean ± SD, n = 8.
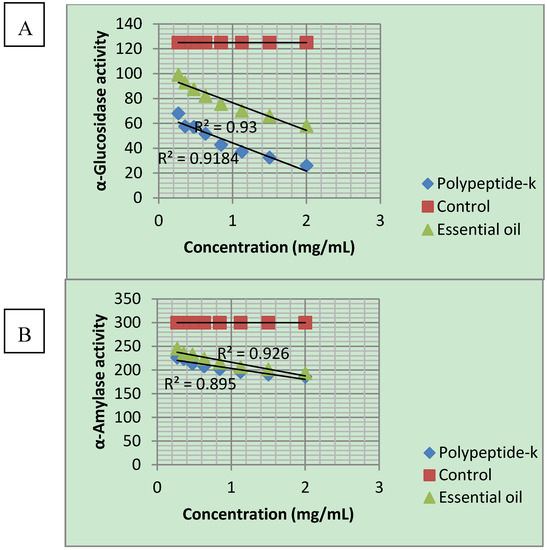
Figure 1.
Dose-dependent inhibition of α-glucosidase and α-amylase activities by polypeptide-k and Momordica charantia seed oil. A: α-glucosidase; B: α-amylase.
Figure 1.
Dose-dependent inhibition of α-glucosidase and α-amylase activities by polypeptide-k and Momordica charantia seed oil. A: α-glucosidase; B: α-amylase.

Table 5.
Effect of polypeptide-k and oil from Momordica charantia on α-amylase activity.
| Concentration (mg/mL) | PPK α-Amylase activity (% Inhibition) | MCO α-Amylase activity (% Inhibition) |
|---|---|---|
| 0.267 | 226.3502 ± 9.3639 (18.20) | 245.3911 ± 9.8317 (24.54) |
| 0.356 | 223.3028 ± 9.3302 (21.54) | 235.3548 ± 12.4998 (25.56) |
| 0.475 | 213.3901 ± 8.4586 (22.42) | 232.7123 ± 12.9344 (28.86) |
| 0.633 | 208.0655 ± 9.1613 (25.43) | 223.6822 ± 12.3970 (30.64) |
| 0.844 | 201.9705 ± 9.0938 (28.09) | 215.7165 ± 12.3385 (32.67) |
| 1.125 | 195.8756 ± 9.0263 (31.10) | 206.6906 ± 13.2289 (34.70) |
| 1.5 | 190.5341 ± 8.2053 (32.88) | 201.3527 ± 11.8301 (36.48) |
| 2 | 185.9291 ± 5.1072 (35.58) | 193.2560 ± 7.6110 (38.02) |
All values are mean ± SD, n = 8.
One therapeutic strategy for the treatment of diabetes is to decrease hyperglycemia post-ingestion. This can be done by inhibiting carbohydrate-hydrolyzing enzymes such as α-amylase and α-glucosidase [23]. In this current study, we demonstrated a large inhibition of 79% for α-glucosidase and 38% for α-amylase, suggesting that both polypeptide k and MC seed oil are potential potent anti-diabetic agents. By inhibiting these enzymes, they will delay carbohydrate digestion and prolong the overall time for carbohydrate digestion resulting in a reduction in the rate of glucose absorption and consequently blunting the post-parandial blood glucose rise [24], i.e., making the food lower in the glycemic index.
Previously, Ali et al. [23] reported several Malaysian plants (Phyllantus spp.) inhibited α-amylase activity by 24%, which they reported to be potent inhibition. In this study, we reported more potent inhibition of 35.58% and 38.02% for PPK and MC seed oil respectively. Interestingly, α-glucosidase revealed even bigger inhibition. Babu et al. [25] reported approximately 35% inhibition of Himalayan herbs constituents which are used in treating diabetes when compared to 79% in this current study. The inhibition of these enzymes may be the other key factors in the mechanism for the effects of PPK observed clinically [11,12]. The major setback of commercial anti-diabetic drugs is hypoglycemia if taken more than the recommended dose. Interestingly, in vivo in humans, no hypoglycemic effects were observed even when patients took more than the recommended dose [11]. This is the major advantage of PPK over all other commercial anti-diabetic drugs.
3. Experimental
3.1. General
Polypeptide-k and oil from seeds of MC were generous gifts from Magna Bio-Laboratories Sdn. Bhd. Malaysia (Batch 37/11, 121/11 and 375/11). Voucher specimens were deposited in the herbarium of the Institute of Bioscience, UPM, Malaysia. Polypeptide-k was isolated using the method of Kanna [11] and oil was isolated using methods of Noguchi et al. [13] and Kanna [17]. In this case, we used ripe bitter melon approximately 14–20 days after fruiting (3–3.5 months after planting). Other chemicals are of analytical grades and were obtained from Sigma Chemicals (St. Louis, MO, USA).
3.2. Antioxidant Activity
The antioxidant activity of extract was evaluated by the β-carotene-linoleate assay as described by Amin and Tan [26]. Briefly, β-carotene solution (1.0 mL, 0.2 mg/mL in chloroform) was pipetted into a round bottom flask (250 mL) containing linoleic acid (0.02 mL) and 100% Tween 20 (0.2 mL). The mixture was evaporated at 40 °C for 10 min and diluted with distilled water (100 mL) slowly and agitated vigorously to form an emulsion. Five mL aliquots of the emulsion were transferred into different test tubes containing 0.2 mL of samples in solvents at a final concentration of 1 mg/mL. The mixture was then gently shaken and placed in water bath at 45 °C for 2 h. The absorbance of the samples was measured at 470 nm using a spectrophotometer (UV-1610, Shimadzu, Kyoto, Japan) at initial time (t = 0) against a blank, consisting of an emulsion without β-carotene. Standard (α-tocopherol) of the same concentration of samples were used for comparison. A 0.2-mL amount of methanol in 5 mL of the above emulsion was used as the control. Measurements were carried out at 15 min intervals for 120 min. The antioxidant activity (AA) was measured in terms of successful bleaching of β-carotene.
3.3. Fatty Acid Composition of the Seed Oil
Total lipid extraction of the samples was carried out in triplicate as described previously [27,28], using a chloroform/methanol (2:1, v/v) solvent system. Transmethylation was carried out using 14% methanolic boron trifluoride. The derived fatty acid methyl esters (FAMEs) were separated on a Quadrex 007 series bonded phase fused silica capillary column (Quadrex Corporation, New Haven, CT, USA) (30 m × 0.25 mm ID, 0.20 mm film thickness, 007 Carbowax/BTR) in a 5890 Hewlett-Packard Gas-Liquid Chromatograph (Hewlett-Packard Co., Avondale, PA, USA). Individual fatty acids were identified and quantified by comparison with retention times and peak areas of FAMEs standards from Supleco Inc. (Bellefonte, PA, USA). Commercial palm oil from Malaysia was used as reference.
3.4. Amino Acid Determination of Polypeptide-k
The methods of Vidotti et al., [29] were employed, as previously modified [27,28]. Triplicate samples were hydrolysed with 6 N hydrochloric acid for 24 h at 110 °C. The hydrolysed samples were then analysed using an automatic amino acid analyzer L-8500 (Hitachi, Tokyo, Japan) with a ninhydrin reagent and lithium buffer system by injecting 20 μL [30]. The reproducibility of the results was within approximately 3%. The net height of each peak produced by the chart recorder of the analyzer (each representing an amino acid) was measured and calculated.
3.5. α-Glucosidase Inhibition
α-Glucosidase activity was measured using a protocol based on Ye et al. [16] with the following modifications: the inhibitory effect of the compounds on α-glucosidase were assessed by using 4-nitrophenyl-α-D-glucopyranoside (PNPG) as substrate (0.2 mL 20 mmol·L−1). Reaction system: 2.0 mL 0.1 mol·L−1 potassium phosphate buffer (pH 6.8), 0.1 mL sample solution, 50 μL reduced glutathione (1 mg·mL−1), 0.1 mL α-glucosidase solution (0.57 U·mL−1), which were well mixed, after being pre-incubated for 15 min at 37 °C, 0.2 mL of PNPG (20 mmol·L−1) was added, 37 °C for 15 min, after stopping the reaction by addition of 10 mL of 0.1 mol·L−1 Na2CO3. The amount of 4-nitrophenol was measured spectrophotometrically at 400 nm.
3.6. α-Amylase Inhibition
The α-amylase inhibition assay was performed using the chromogenic method adopted from Sigma–Aldrich, as adapted from Ali et al., [23]. Bovine pancreatic α-amylase (EC 3.2.1.1, Sigma) was dissolved in ice-cold distilled water to give a concentration of 4 unit/mL solution. Potato starch (0.5%, w/v) in 20 mM phosphate buffer (pH 6.9) containing 6.7 mM sodium chloride, was used as a substrate solution. α-Amylase activity was determined by measuring the absorbance of the mixture at 540 nm. Control incubations, representing 100% enzyme activity were conducted in an identical fashion replacing compound/oil with DMSO.
4. Conclusions
Collectively, the results from this present study strongly suggest that both polypeptide k and MC seed oil have potent anti-diabetic activity in vitro and also high anti-oxidant activity. Furthermore, oil from MC seed oil has the potential for wound healing [17] and polypeptide-k contains balanced amino acid which is suitable to be used as a source for amino acids in processed food.
Acknowledgments
The authors thank Magna Bio-Laboratories for supplying the research materials and Universiti Putra Malaysia for supporting this work.
References
- Shrivasta, S.; Leelavathi, S. Preliminary phytochemical evaluation of Leaf Extracts of Catunaregum spinosa Thunb. IJPSR 2010, 3, 114–118. [Google Scholar]
- Grover, J.K.; Yadav, S.P. Pharmacological actions and potential uses of Momordica charantia: A review. J. Ethnopharmacol. 2004, 93, 123–132. [Google Scholar] [CrossRef]
- Day, C.; Cartwright, T.; Provost, J.; Bailey, C.J. Hypoglycaemic effect of Momordica charantia extracts. Planta Med. 1990, 56, 426–429. [Google Scholar] [CrossRef]
- Ali, L.; Khan, A.K.A.; Mamun, M.I.R.; Mosihuzzaman, M.; Nahar, N.; Nur-e-Alam, M.; Rokeya, B. Studies on the hypoglycaemic effects of fruit pulp, Seed and whole plant of Momordica charantia on normal and diabetic model rats. Planta Med. 1993, 59, 408–412. [Google Scholar] [CrossRef]
- Kumar, D.S.; Sharathnath, K.V.; Yogeswaran, P.; Harani, A.; Sudhakar, K.; Sudha, P.; Banji, D. A medicinal potency of Momordica charantia. IJPSR 2010, 1, 95–100. [Google Scholar]
- Khan, M.A.; Singh, V.K. A folklore survey of some plants of Bhopal district forests, Madhya Pradesh, India, Described as antidiabetics. Fitoterapia 1996, 67, 416–421. [Google Scholar]
- Senanayake, G.V.K.; Maruyama, M.; Shibuya, K.; Sakono, M.; Fukuda, N.; Morishita, T.; Yukizaki, C.; Kawano, M.; Ohta, H. The effects of bitter melon (Momordica charantia) on serum and liver triglyceride levels in rats. J. Ethnopharmacol. 2004, 91, 257–262. [Google Scholar]
- Xiang, L.; Huang, X.; Chen, L.; Rao, P.; Ke, L. The reparative effects of Momordica charantia Linn. extract on HIT-T15 pancreatic -Cells. Asia Pac. J. Clin. Nutr. 2007, 16, 249–252. [Google Scholar]
- Kanna, P.; Jain, S.C.; Panagariya, A. Hypoglycemic activity of polypeptide-p from a plant source. J. Nat. Prod. 1981, 44, 648–655. [Google Scholar] [CrossRef]
- Virdi, J.; Sivakami, S.; Shahani, S.; Suthar, A.C.; Banavalikar, M.M.; Biyani, M.K. Antihyperglycemic effects of three extracts from Momordica charantia. J. Ethnopharmacol. 2003, 88, 107–111. [Google Scholar] [CrossRef]
- Kanna, P. Protein/polypeptide-k obtained from Momordica charantia. U.S. Patent 6,831,162, 2004. [Google Scholar]
- Lee, C.L.; Yong, Y.S.; Zuraini, A.; Yaacob, A.; Nazrul Hakim, M. Effects of polypeptide-k supplemented soft bun on blood glucose level in healthy adults. IJNAM 2011, 3, 7–10. [Google Scholar]
- Noguchi, R.; Yasui, Y.; Suzuki, R.; Hosokawa, M.; Fukunaga, K.; Miyashita, K. Dietary effects of bitter gourd oil on blood and liver lipids of rats. Arch. Biochem. Biophys. 2001, 396, 207–212. [Google Scholar]
- Braca, A.; Siciliano, T.; D’Arrigo, M.; Germanò, M.P. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia 2008, 79, 123–125. [Google Scholar]
- Kimura, K.; Lee, J.H.; Lee, I.S.; Lee, H.S.; Park, K.H.; Chiba, S.; Kim, D.M. Two potent competitive inhibitors discriminating alpha-glucosidase family I from family II. Carbohydr. Res. 2004, 339, 1035–1040. [Google Scholar] [CrossRef]
- Ye, X.P.; Song, C.Q.; Yuan, P.; Mao, R.G. α-Glucosidase and α-Amylase Inhibitory Activity of Common Constituents from Traditional Chinese Medicine Used for Diabetes Mellitus. Chin. J. Nat. Med. 2012, 8, 349–352. [Google Scholar]
- Kanna, P. Oil from Momordica charantia L.,Its method of preparation and uses. U.S. Patent 6,964,786, 2005. [Google Scholar]
- Bonanome, A.; Grundy, S.M. Effect of dietary stearic acid on plasma cholesterol and lipoprotein levels. N. Eng. J. Med. 1998, 318, 1244–1248. [Google Scholar] [CrossRef]
- Fürst, P.; Stehle, P. What are the essential elements needed for the determination of amino acid requirements in humans. J. Nutr. 2004, 134, S1558–S1565. [Google Scholar]
- lvarez, E.O.; Ruarte, M.B. Glutamic acid and histamine-sensitive neurons in the ventral hippocampus and the basolateral amygdala of the rat: Functional interaction on memory and learning processes. Behav. Brain Res. 2004, 152, 209–219. [Google Scholar]
- Toda, N.; Ayajiki, K.; Okamura, T. Nitric oxide and penile erectile function. Pharmacol. Ther. 2005, 106, 233–266. [Google Scholar]
- Pripdeevech, P.; Machan, T. Fingerprint of volatile flavour constituents and antioxidant activities of teas from Thailand. Food Chem. 2011, 125, 797–802. [Google Scholar] [CrossRef]
- Ali, H.; Houghton, P.J.; Soumyanath, A. α-mylase inhibitory activity of some Malaysian plants used to treat diabetes; With particular reference to Phyllanthus amarus. J. Ethnopharmacol. 2006, 107, 449–455. [Google Scholar] [CrossRef]
- Rhabasa-Lhoret, R.; Chiasson, J.L. α-Glucosidase inhibitors. In International Textbook of Diabetes Mellitus, 3rd; Defronzo, R.A., Ferrannini, E., Keen, H., Zimmet, P., Eds.; John Wiley & Sons Ltd.: London, UK, 2004; Volume 1, pp. 901–914. [Google Scholar]
- Babu, S.K.; Tiwari, A.K.; Srinivas, P.V.; Ali, A.Z.; Rajua, B.C.; Rao, J.M. Yeast and mammalian α-glucosidase inhibitory constituents from Himalayan rhubarb Rheum emodi Wall.ex Meisson. Bioorg. Med. Chem. Lett. 2004, 14, 3841–3845. [Google Scholar] [CrossRef]
- Amin, I.; Tan, S.H. Antioxidant activity of selected seaweeds. Mal. J. Nutr. 2002, 8, 167–177. [Google Scholar]
- Zuraini, A.; Somchit, M.N.; Solihah, M.H.; Goh, Y.M.; Arifah, A.K.; Zakaria, M.S.; Somchit, N.; Rajion, M.A.; Zakaria, Z.A.; Mat Jais, A.M. Fatty acid and amino acid composition of three local Malaysian Channa spp. Fish. Food Chem. 2006, 97, 674–678. [Google Scholar]
- Zakaria, Z.A.; Mat Jais, A.M.; Goh, Y.M.; Sulaiman, M.R.; Somchit, M.N. Amino acid and fatty acid composition of an aqueous extract of Channa striatus (Haruan) that exhibits antinociceptive activity. Clin. Exp. Pharmacol. Physiol. 2007, 34, 198–204. [Google Scholar]
- Vidotti, R.M.; Viegas, E.M.M.; Carneiro, D.J. Amino acid composition of processed fish silage using different raw materials. Anim. Feed Sci. Tech. 2003, 105, 199–204. [Google Scholar] [CrossRef]
- Yamamoto, M.; Unuma, T.; Akiyama, T. Postprandial changes in plasma free amino acid concentrations of rainbow trout fed different protein sources. Fishery Sci. 1998, 64, 474–481. [Google Scholar]
- Sample Availability: Samples of the compounds (Polypeptide k and Momordica charantia seed oil) are available from the author.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
