Cassia spectabilis (DC) Irwin et Barn: A Promising Traditional Herb in Health Improvement
Abstract
:1. Introduction
2. Botany
2.1. Scientific Name
2.2. Common Names
2.3. Synonyms
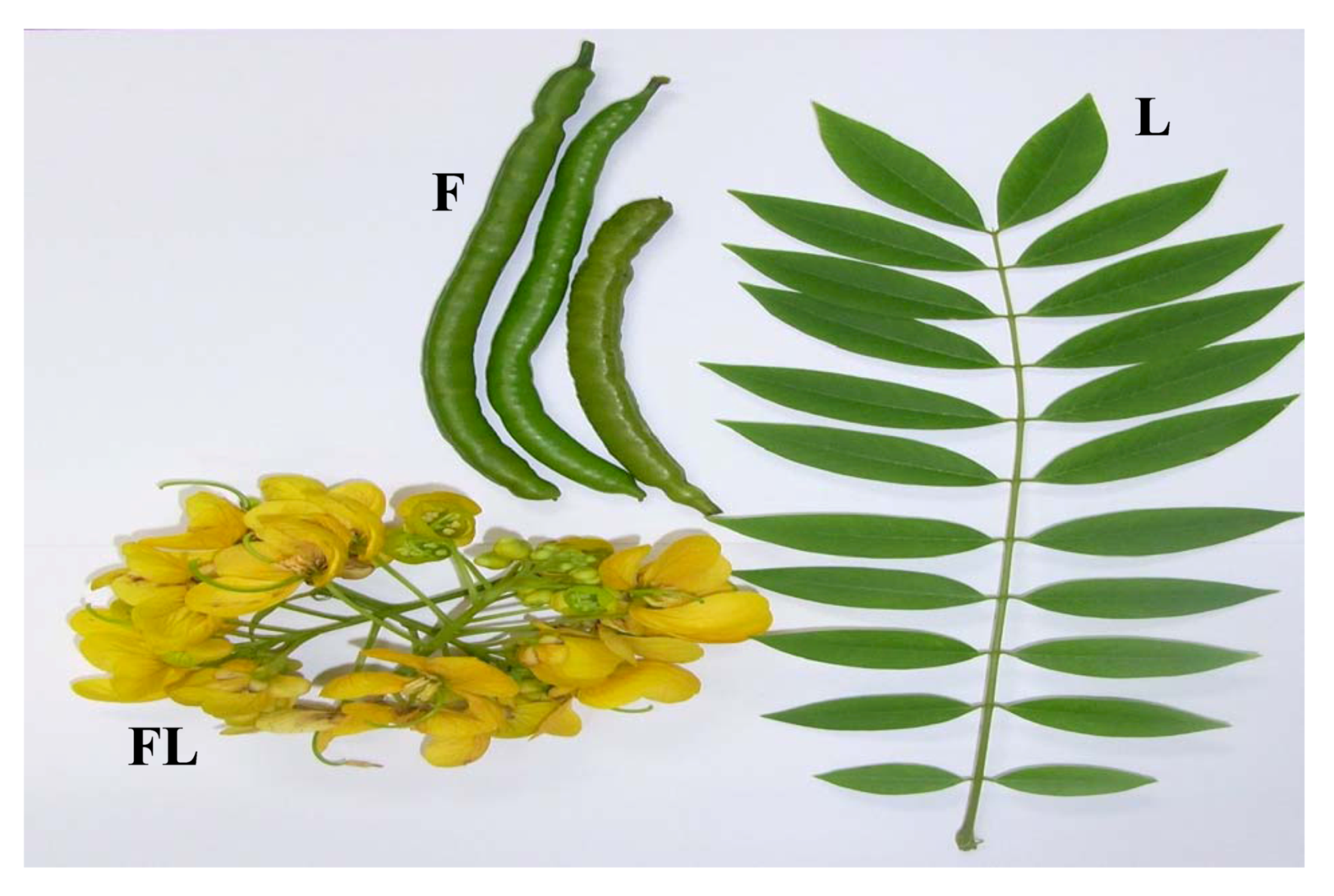
2.4. Classification

2.5. Distribution
2.6. Botanical Description
2.7. Propagation
2.8. Ethnomedicinal Uses
2.9. Phytochemistry

3. Pharmacological Activities of C. spectabilis
3.1. Standardizations of C. spectabilis Leaf
3.2. Antibiofilm Activity
3.3. Antifungal Activity
3.4. Antibacterial Activity
3.5. Antioxidant Activity
3.6. Anti-inflammatory and Anti-hyperalgesics Activity
3.7. Anticonvulsant Activities
4. Dosage/Mode of Usage
5. Toxicological Assessment
6. Precautions/Safety for Usage
7. Conclusions
Acknowledgements
References
- Mazumder, P.M.; Percha, V.; Farswan, M.; Upaganlawar, A. Cassia: A Wonder Gift to Medical Sciences. Int. J. Clin. Pharm. 2008, 1, 16–38. [Google Scholar]
- Duraipandiyan, V.; Ayyanar, M.; Ignacimuthu, S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement. Altern. Med. 2006, 6, 35–41. [Google Scholar] [CrossRef]
- Viegas, C., Jr.; Bolzani, V.S.; Furlan, M.; Furlan, M.S.; Barreiro, E.J.; Young, M.C.M.; Tomazela, D.; Eberlin, M.N. Further bioactive piperidine alkaloids from the flowers and green fruits of Cassia spectabilis. J. Nat. Prod. 2004, 67, 908–910. [Google Scholar]
- Lindley, J. Report upon the New or Rare Plants which Flowered in the Garden of the Horticultural Society at Chiswick, between March, 1825, and March, 1826, Part 1; London Horticultural Society: London, UK, 1830; pp. 46–75. [Google Scholar]
- AgroForestry Tree Datadase. A Tree Species Reference and Selection Guide. Available online: http://www.worldagroforestry.org (accessed on 1 May 2012).
- Sansores-Peraza, P.; Rosado-Valladi, M.; Brito-Loeza, W.; Mena-Rejon, G.J.; Quijano, L. Cassine, an antimicrobial alkaloid from Senna racemosa. Fitoterapia 2000, 71, 690–692. [Google Scholar]
- Freitas, R.M.; Souza, F.C.; Viana, G.S.; Fonteles, M.M. Acetylcholinesterase activities in hippocampus, frontal cortex and striatum of Wistar rats after pilocarpine-induced status epilepticus. Neurosci. Lett. 2006, 399, 76–78. [Google Scholar] [CrossRef]
- Lorenzi, H.; de Abreu Matos, F.J. Plantas Medicinais no Brasil: Nativas e Exóticas; Instituto Plantarum: Nova Odessa, Brazil, 2002; p. 291. [Google Scholar]
- Carlini, E.A. Plants and the central nervous system. Pharmacol. Biochem. Behav. 2003, 75, 501–512. [Google Scholar] [CrossRef]
- Christofidis, I.; Welter, A.; Jadot, J. Spectaline and iso-6-cassine, two new piperidin 3-ol alkaloids from the leaves of Cassia spectabilis. Tetrahedron 1977, 33, 977–979. [Google Scholar] [CrossRef]
- Christofidis, I.; Welter, A.; Jadot, J. Spectalinine and iso-6-carnavaline, two unprecedented piperidine alkaloids from the seeds of Cassia spectabilis. Tetrahedron 1977, 33, 3005–3006. [Google Scholar] [CrossRef]
- Mulchaldani, N.B.; Hassarajani, S.A. Cassinicine, a new alkaloid and anthraquinones from Cassia spectabilis and their biogenetic relationship. Planta Med. 1977, 32, 357–361. [Google Scholar] [CrossRef]
- Bolzani, V.S.; Gunatilaka, A.A.L.; Kingston, D.G.I. Bioactive and other piperidine alkaloids from Cassia leptophylla. Tetrahedron 1995, 51, 5929–5934. [Google Scholar]
- Alexandre-Moreira, M.S.; Viegas, C.Jr.; de Miranda, A.L.P.; Bolzani, V.S.; Barreiro, E.J. Antinociceptive profile of (−)-spectaline: A piperidine alkaloid from Cassia leptophylla. Planta Med. 2007, 69, 795–799. [Google Scholar]
- Viegas, C.Jr.; Alexandre-Moreira, M.S.; Fraga, C.A.M.; Barreiro, E.J.; Bolzani, V.S; de Miranda, A.L.P. Antinociceptive profile of 2,3,6-trisubstituted piperidine alkaloids: 3-O-acetylspectaline and semisynthetic derivatives of (−)-spectaline. Chem. Pharm. Bull. 2008, 54, 407–412. [Google Scholar]
- Viegas, C.Jr.; Silva, D.H.S.; Rivatto, M.; de Rezende, A.; Castro-Gamboa, I.; Bolzani, V.S.; Nair, M.G. Lipoperoxidation and cyclooxygenase enzyme inhibitory piperidine alkaloids from Cassia spectabilis green fruits. J. Nat. Prod. 2007, 70, 2026–2028. [Google Scholar]
- Pineyro-Lopez, A.; Waksman, N. Chemistry, Structure and Biological Activity of Anthracenones of the Karwinskia Genus. In Studies in Natural Product Chemistry; Atta-ur-Rahman, Ed.; Elsevier Science: Amsterdam, The Netherlands, 2000; Volume 22, pp. 555–606. [Google Scholar]
- Springob, K.; Kutchan, T.M. Introduction to different classes of natural products. In Plant-Derived Natural Products; Osbourn, A.E., Lanzotti, V., Eds.; Springer: New York, NY, USA, 2009; pp. 3–50. [Google Scholar]
- Kim, Y.M.; Lee, C.H.; Kim, H.G.; Lee, H.S. Anthraquinones isolated from Cassia tora(Leguminosae) seed show an antifungal property against phytopathogenic fungi. J. Agric. Food Chem. 2004, 52, 6096–6100. [Google Scholar]
- Yang, Y.C.; Lim, M.Y.; Lee, H.S. Emodin isolated from Cassia obtusifolia (Leguminosae) seed shows larvicidal against three mosquito species. J. Agric. Food Chem. 2003, 51, 7629–7631. [Google Scholar]
- Yen, G.C.; Chen, H.W.; Duh, P.D. Extraction and identification of an antioxidative component from jue ming zi (Cassia tora L.). J. Agric. Food Chem. 1998, 46, 820–824. [Google Scholar]
- Choi, J.S.; Chung, H.Y.; Jung, H.A.; Park, H.J.; Yokozawa, T. Comparative evaluation of antioxidant potential of alaternin (2-hydroxyemodin) and emodin. J. Agric. Food Chem. 2000, 48, 6347–6351. [Google Scholar] [CrossRef]
- Calixto, J.B.; Santos, A.R.S.; Filho, V.C.; Yunes, A.R. A review of the plants of the genus Phyllanthus: Their chemistry, pharmacology and therapeutic potential. Medical Res. Rev. 1998, 18, 225–228. [Google Scholar] [CrossRef]
- Pivatto, M.; Croth, A.E.M.; Lopes, N.P.; Castro-Gamboa, I.; de Rezende, A.; Viegas, C.Jr.; Young, M.C.M.; Furlan, M.; Bolzani, V.S. Electrospray ionization mass spectrometry screening of piperidine alkaloids from Senna spectabilis (Fabaceae) extracts: Fast identification of new constituents and co-metabolites. J. Braz. Chem. Soc. 2005, 16, 1431–1438. [Google Scholar] [CrossRef]
- de Oliveira Silva, F.; de Oliveira, I.R.; de Vasconcelos Silva, M.G.; Braz-Filho, R. Chemical compounds of leaves from Senna spectabilis (DC) Irwin & Barneby var. excelsa (Schrad.) Irwin & Barneby. Quimica Nova 2010, 33, 1874–1876. [Google Scholar]
- Torey, A.; Sasidharan, S.; Yeng, C.; Latha, L.Y. Standardization of Cassia Spectabilis with respect to authenticity, assay and chemical constituent analysis. Molecules 2010, 15, 3411–3420. [Google Scholar]
- Sangetha, S.; Zuraini, Z.; Suryani, S.; Sasidharan, S. In situ TEM and SEM studies on the antimicrobial activity and prevention of Candida albicans biofilm by Cassia spectabilis extract. Micron 2009, 40, 439–443. [Google Scholar] [CrossRef]
- Torey, A.; Sasidharan, S. Anti-candida albicans biofilm activity by Cassia Spectabilis standardized methanol extract: An ultrastructural study. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 875–882. [Google Scholar]
- Sangetha, S.; Zuraini, Z.; Sasidharan, S.; Suryani, S. Antibacterial, Antifungal and Cytotoxic Activities of Cassia spectabilis. Asian J. Pharm. Clin. Res. 2008, 1, 17–20. [Google Scholar]
- Sangetha, S.; Zuraini, Z.; Sasidharan, S.; Suryani, S. Fungicidal Effect and Oral Acute Toxicity of Cassia spectabilis Leaf Extract. Nippon Ishinkin Gakkai Zasshi 2008, 49, 299–304. [Google Scholar] [CrossRef]
- Silva, G.H.; Teles, H.L.; Zanardi, L.M.; Young, M.C.M.; Eberlin, M.N.; Hadad, R.; Pfenning, L.H.; Costa-Neto, C.M.; Castro-Gamboa, I.; da Silva Bolzani, V.; et al. Cadinane sesquiterpenoids of Phomopsis cassiae, an endophytic fungus associated with Cassia spectabilis (Leguminosae). Phytochemistry 2006, 67, 1964–1969. [Google Scholar]
- Krishnan, N.; Ramanathan, S.; Sasidharan, S.; Murugaiyah, V.; Mansor, S.M. Antimicrobial activity evaluation of Cassia Spectabilis leaf extracts. Int. J. Pharmacol. 2010, 6, 506–510. [Google Scholar]
- Sangetha, S.; Zuraini, Z.; Sasidharan, S.; Suryani, S. Free Radical Scavenging Activity of Cassia spectabilis and Cassia fistula. Int. J. Nat. Eng. Sci. 2008, 2, 111–114. [Google Scholar]
- da Silva, K.A.; Manjavachi, M.N.; Paszcuk, A.F.; Pivatto, M.; Viegas, C.Jr.; Bolzani, V.S.; Calixto, J.B. Plant derived alkaloid (−)-cassine induces anti-inflammatory and anti-hyperalgesics effects in both acute and chronic inflammatory and neuropathic pain models. Neuropharmacology 2012, 62, 967–977. [Google Scholar] [CrossRef]
- Bum, E.N.; Nkantchoua, G.N.; Njikam, N.; Taiwe, G.S.; Ngoupaye, G.T.; Pelanken, M.M.; Nanga; Maidawa, F.; Rakotonirina, A.; Rakotonirina, S.V. Anticonvulsant and sedative activity of leaves of Senna spectabilis in mice. Int. J. Pharmacol. 2010, 6, 123–128. [Google Scholar]
- de Oliveira Silva, F.; de Vasconcelos Silva, M.G.; Feng, D.; de Freitas, R.M. Evaluation of central nervous system effects of iso-6-cassine isolated from Senna spectabilis var. excelsa (Schrad) in mice. Fitoterapia 2011, 82, 255–259. [Google Scholar]
- Meyer, B.N.; Ferrigini, R.N.; Jacobsen, L.B.; Nicholas, D.E.; McLaughlin, J.L. Brine shrimp: A convenient general bioassay for active plant constituent. Planta Med. 1982, 45, 31–35. [Google Scholar] [CrossRef]
- Organization for Economic Cooperation and Development (OECD), Guidelines for the Testing of Chemicals, Revised draft guideline: Acute oral toxicity; OECD: Paris, France, 2000; p. 423.
- Sample Availability: Samples of the C. spectabilis are available from the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jothy, S.L.; Torey, A.; Darah, I.; Choong, Y.S.; Saravanan, D.; Chen, Y.; Latha, L.Y.; Deivanai, S.; Sasidharan, S. Cassia spectabilis (DC) Irwin et Barn: A Promising Traditional Herb in Health Improvement. Molecules 2012, 17, 10292-10305. https://doi.org/10.3390/molecules170910292
Jothy SL, Torey A, Darah I, Choong YS, Saravanan D, Chen Y, Latha LY, Deivanai S, Sasidharan S. Cassia spectabilis (DC) Irwin et Barn: A Promising Traditional Herb in Health Improvement. Molecules. 2012; 17(9):10292-10305. https://doi.org/10.3390/molecules170910292
Chicago/Turabian StyleJothy, Subramanion L., Angeline Torey, Ibrahim Darah, Yee Siew Choong, Dharmaraj Saravanan, Yeng Chen, Lachimanan Yoga Latha, Subramanian Deivanai, and Sreenivasan Sasidharan. 2012. "Cassia spectabilis (DC) Irwin et Barn: A Promising Traditional Herb in Health Improvement" Molecules 17, no. 9: 10292-10305. https://doi.org/10.3390/molecules170910292
APA StyleJothy, S. L., Torey, A., Darah, I., Choong, Y. S., Saravanan, D., Chen, Y., Latha, L. Y., Deivanai, S., & Sasidharan, S. (2012). Cassia spectabilis (DC) Irwin et Barn: A Promising Traditional Herb in Health Improvement. Molecules, 17(9), 10292-10305. https://doi.org/10.3390/molecules170910292




