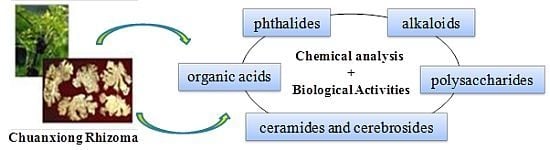
Advances in the Chemical Analysis and Biological Activities of Chuanxiong
Abstract
:1. Introduction
| Stage | Aug. | Sept. | Oct. | Nov. | Dec. | Jan. | Feb. | March | April | May | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Seeding | |||||||||||||
| Stem emergence and growth | |||||||||||||
| Senescene | |||||||||||||
| Emergence of the secondary stems | |||||||||||||
| Tillering | |||||||||||||
| Rhizome expansion | |||||||||||||
2. Chemical Compounds and Bioactivities
2.1. Phenols and Organic Acids
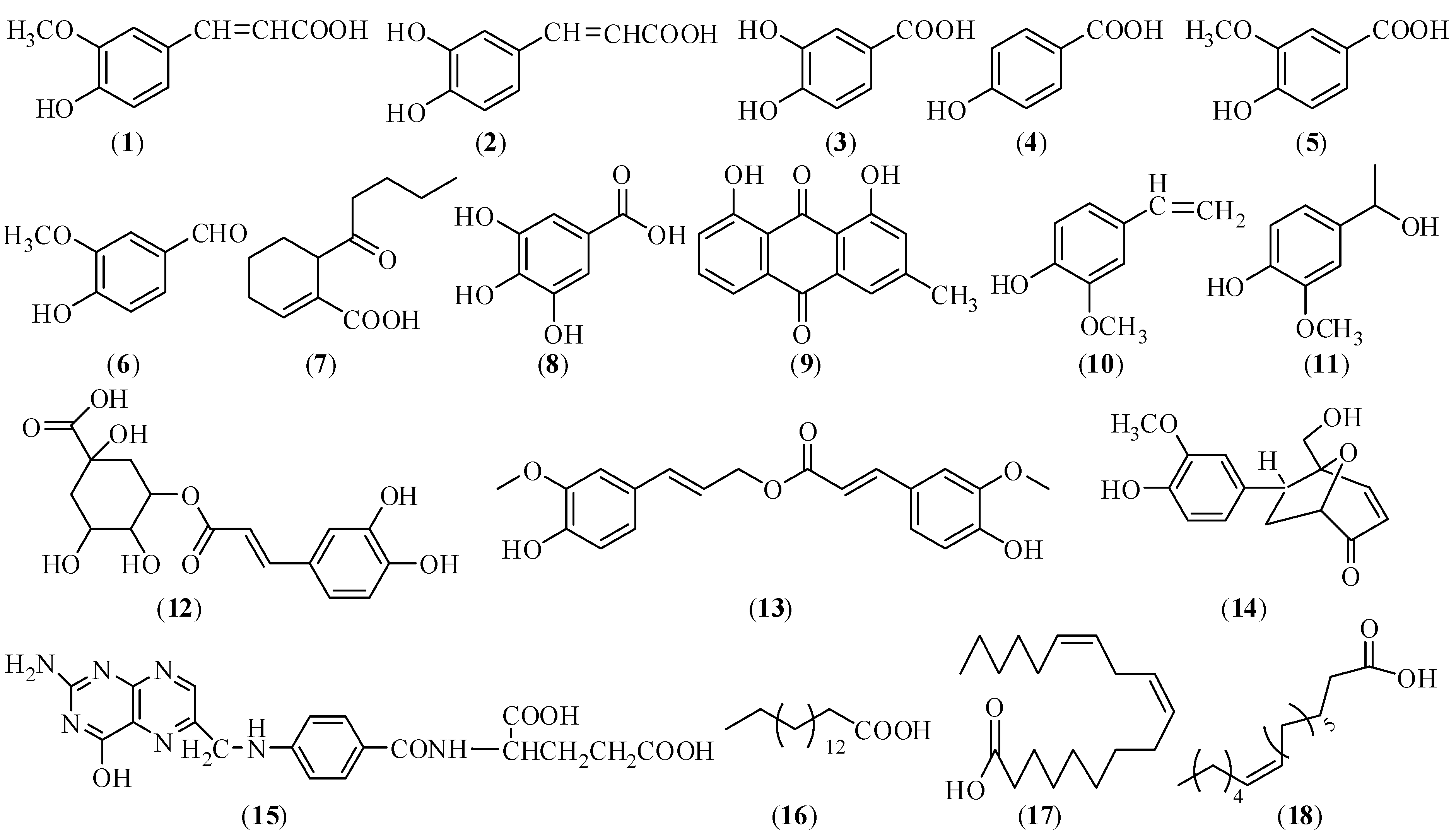
2.1.1. Chemical Structures
2.1.2. Sample Preparation for Chemical Analysis
2.1.3. Quantitative Analysis
| No. | Extraction solvent | Extraction method | Analytical method | Content (mg/g) | Ref. |
|---|---|---|---|---|---|
| 1 | 95% ethanol | Reflux | TLCS | 0.9395 | [67] |
| 2 | 70% ethanol | Sonication | HPCE | 0.82~1.19 | [66] |
| 3 | Methanol | Sonication | HPLC: PE-Pack C18 (4.6 mm × 150 mm, 5 µm), 1% glacial acetic acid:methanol (58:42), 0.5 mL/min, 313 nm | 0.146~0.778 | [68] |
| 4 | 70% ethanol | Ultrasonic agitation | CE | 0.82~1.19 | [42] |
| 5 | 95% ethanol | Soxhlet extraction | HPLC: Waters C18 (10 μm × 3.9 mm × 250 mm), 10% acetic acid:methanol (65:35), 1 mL/min, 320 nm | 1.234~1.368 | [69] |
| 6 | Methanol-water-36% acetic acid (30:67:3) | Sonication | HPLC: ODS C18 (250 mm × 4.6 mm), methanol:water:36% acetic acid (30:67:3), 1 mL/min, 322 nm | 0.653~1.327 | [51] |
| 7 | Methanol-36% acetic acid(95:5) | Sonication | HPLC: Kromasil C18 (250 mm ° 4.6 mm, 5 μm), acetonitrile:methanol:1% acetic acid (15:15:70), 0.6 mL/min | 0.327~0.723 | [70] |
| 8 | SFE | - | HPLC: Phenomenex (250 mm ° 4.6 mm, 5μm), methanol:water:glacial acetic acid (30:70:0.2), 1 mL/min, 320 nm | 0.8 | [71] |
| 9 | Water | Reflux | HPLC: DiamonsilTM C18 (250 mm ° 4.6 mm, 5 μm), methanol:water:glacial acetic acid (30:68:2), 1.0 mL/min, 320 nm | 1.87~2.17 | [72] |
| 10 | Methanol | Sonication | RP-HPLC: Inertsil C18 (250 mm × 4.6 mm, 5 μm), methanol:water:glacial acetic acid (35:65:0.5), 1.0 mL/min, 321 nm | 1.00~1.14 | [73] |
| 11 | Methanol-formic acid(95:5) | Sonication | HPLC: Kromasil C18 (250 mm × 4.6 mm, 5 μm), 1% acetic acid:acetonitrile, 1 mL/min, 320 nm | 0.107~2.374 | [74] |
| 12 | 40% ethanol | Water bath reflux | HPLC: Lichrosorb C18 (4.6 mm × 250 mm, 5 μm), 1% acetic acid:methanol (70:30), 1 mL/min, 320 nm | 1.141 | [75] |
| 13 | 70% methanol | Reflux | (1) HPLC: Agilent TC-C 18 (150 mm × 4.6 mm, 5 μm), acetonitrile:0.085% phosphoric acid (17:83), 1.0 mL/min, 316 nm(2) UPLC: Acquity UPLC HSS T3 (100 mm × 2.1 mm, 1.8 μm), acetonitrile:0.085% phosphoric acid (15:85), 0.3 mL/min, 316 nm | 1.211.24 | [76] |
2.1.4. Biological Activities
2.2. Phthalides
2.2.1. Chemical Structures

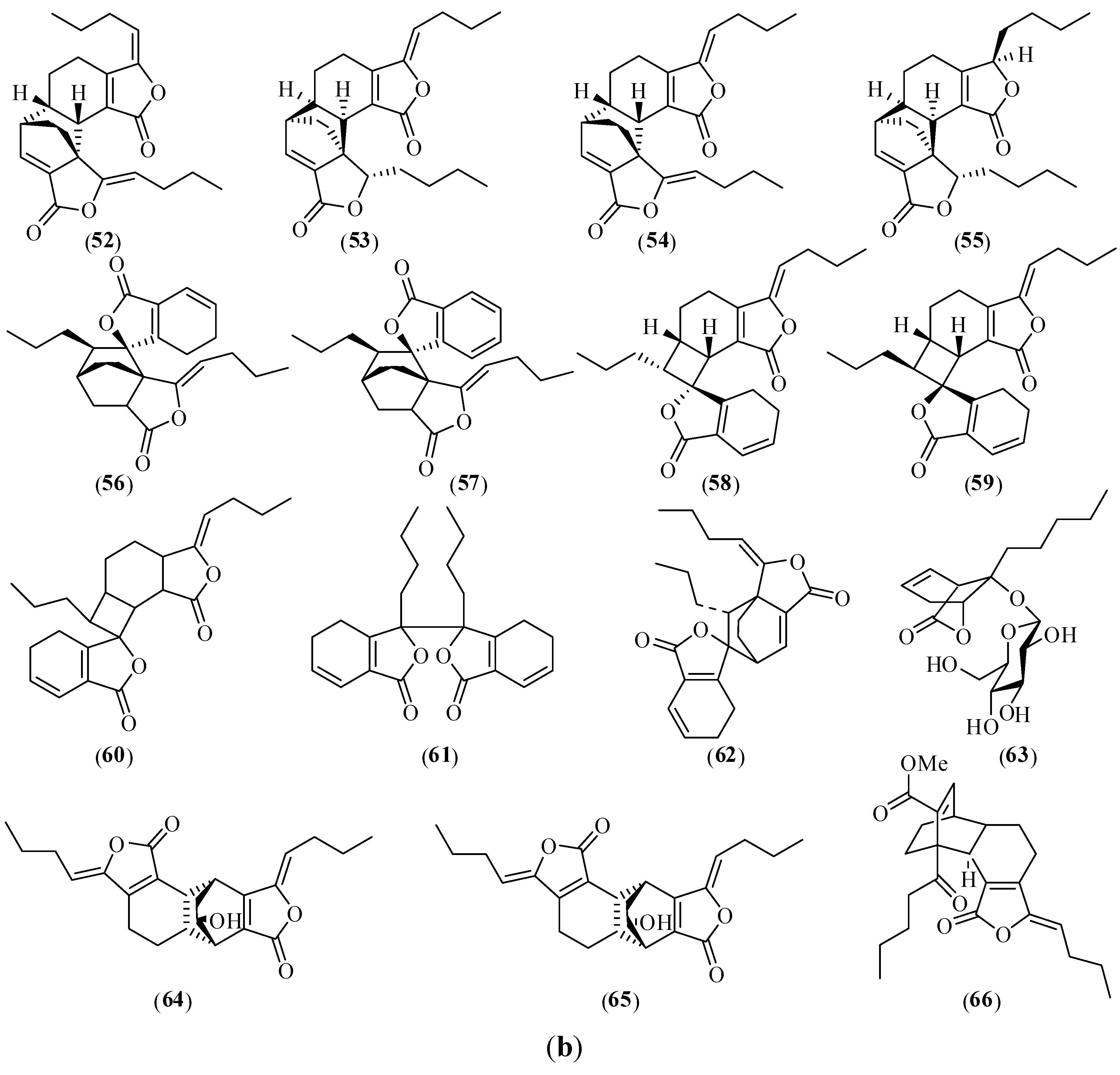
2.2.2. Sample Preparation for Chemical Analysis
2.2.3. Quantitative Analysis
2.2.4. Biological Activities
| No. | Analytes | Extraction solvent | Extraction method | Analytical method | Stationary phase | Mobile phase | Flow rate (mL/min) | λmax(nm) | Content(mg/g) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | ligustilide | - | - | HPLC | Nova-Pak C18(3.9 mm ° 150 mm) | Methanol and water with 10% isopropanol (53:47) | 0.8 | 280 | 15.7 | [116] |
| 2 | ligustilide | Methanol | Sonication | HPLC | Luna 5 μm silica(150 mm ° 4.6 mm) | n-hexane:ethyl acetate:chloroform (92:3:5) | 0.8 | 320 | 15.27 ± 1.86 | [117] |
| 3 | ligustilide | Acetonitrile | Shaking up | RP-HPLC | Hypersil ODS2(4.6 mm ° 200 mm, 5 μm) | Methanol:acetonitrile:water (33:21:46) | 0.8 | 275 | 347.9(in volatile oil) | [118] |
| 4 | ligustilide | Ethanol | Reflux | HPLC | C18(4.0 mm ° 200 mm, 5 μm) | Acetonitrile:water (both contain 0.1% acetic acid) | 0.76 | 280 | 8.2 | [119] |
| 5 | ligustilide | 70% ethanol | Reflux | HPLC | ODS C18(4.6 mm ° 200 mm, 5 μm) | Methanol:water:acetic acid (75.0:24.8:0.2) | 1.0 | 326 | 5.672~5.821 | [120] |
| 6 | ligustilide | Ethanol | Sonication | HPLC | Alltima C18(4.6 mm × 150 mm, 5 μm) | Acetonitrile:water(60:40) | 1.0 | 350 | 7.40 | [121] |
| 7 | butylphthalide | Acetonitrile | Shaking up | RP-HPLC | Kromasil C18(250 mm × 4.6 mm, 5 μm) | sodium acetate (0.05 mol/L):acetonitrile(45:55) | 1.0 | 228 | 131.2~138.3(in volatile oil) | [122] |
| 8 | butylphthalide | Ethyl ether | Sonication | RP-HPLC | Kromasil C18(250 mm × 4.6 mm, 5 μm) | Acetonitrile:acetic acid (pH 4.0, 45:55) | 1.0 | 228 | 7.86~8.01 | [123] |
2.3. Alkaloids
2.3.1. Chemical Structures

2.3.2. Sample Preparation for Chemical Analysis
2.3.3. Quantitative Analysis
| Extraction solvent | Extraction method | Analytical method | Content (mg/g) | Ref. |
|---|---|---|---|---|
| Benzene, ethyl ether, and ethyl acetate | Refluxing | HPLC-DAD | 1.2 ° 10−4 | [8] |
| Petroleum ether | Counter current | RP-HPLC | 0.12 ° 10−3~0.87 ° 10−3 | [154] |
| Ethanol | Sonication | HSCCC | 0.042 | [155] |
| 80% ethanol (containing 5% acetic acid) | Sonication | HPLC | 0.01256~0.07252 | [156] |
2.3.4. Biological Activities
2.4. Polysaccharides
2.4.1. Chemical Structures
2.4.2. Sample Preparation for Chemical Analysis
| Extraction method | Optimum technology | Extraction rate (%) | Ref. |
|---|---|---|---|
| Ultrasonic | Ultrasonic time: 40 min; ultrasonic power: 400 W; solid to liquid ratio: 1:10; extraction times: 2 | 2.74 | [176] |
| Pectinase | Compound pectinase: 1%; temperature: 60 °C; pH value: 3.5; the heating time: 150 min | 11.3 | [177] |
| Basic | Extraction temperature: 95 °C; Extraction time: 150 min; the concentration of NaOH: 0.8 mol/L; solid to liquid ratio: 1:200 g/mL | 2.69 | [178] |
| Enzymic | Cellulase: 0.15%; the compound pectinase: 10%; time: 210 min; pH: 3.4; temperature: 60 °C | 3.03 | [179] |
| Microwave assisted | Microwave power: 231 W; solid to liquid ratio: 1:40; extraction time: 10 min | 3.06 | [180] |
| Cellulose enzymic | Cellulase: 0.25%; time: 120 min; pH value: 4.0; temperature: 50 °C. | 7.26 | [181] |
| Basic | Extraction temperature: 90 °C; extraction time: 4 h. | 6.7 | [182] |
2.4.3. Quantitative Analysis
2.4.4. Biological Activities
2.5. Ceramides and Cerebrosides
2.5.1. Chemical Structures

2.5.2. Biological Activities
2.6. Other Compounds
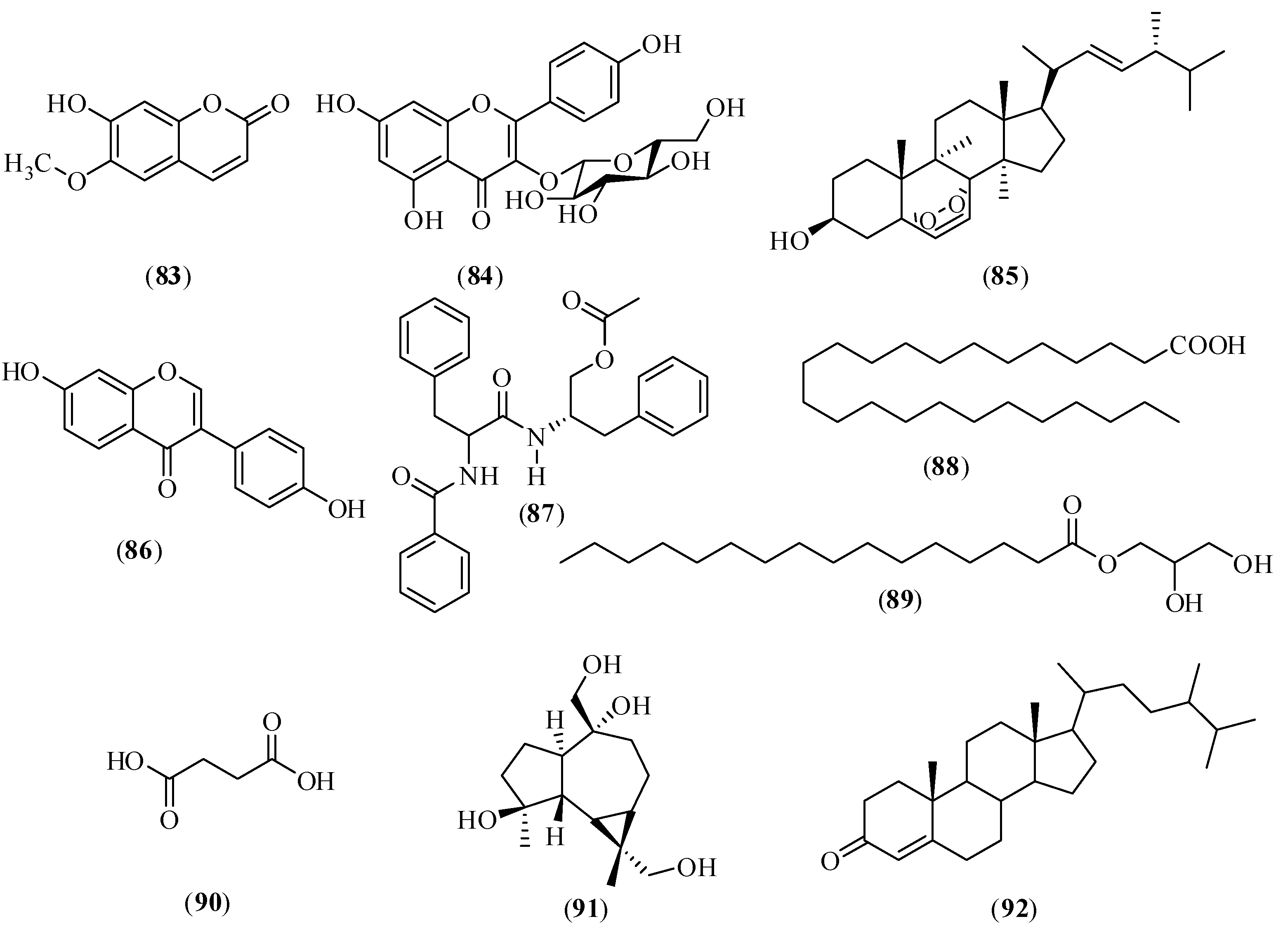
3. Analytical Methods for Simultaneous Determination of Different Types of Chemical Compounds
3.1. HPLC and HPLC-MS
| No. | Analytes | Detection mode | Stationary phase | Mobile phase | Ref. |
|---|---|---|---|---|---|
| 1 | 1, 6, 29, 30 and 67 | HPLC-MS | Zorbax SB-C18(250 mm × 4.5 mm, 5 µm) | Methanol:water:acetic acid (45:55:0.5, v/v/v) | [8] |
| 2 | 1, 6, 13, 19, 21, 27, 29, 30, 31, 39, 40, 47, 48, 53, 54, 56 and 58 | HPLC-DAD-MS | Waters symmetry C18(150 mm × 2.1 mm, 5 µm) | 0.25% aqueous acetic acid and methanol | [192] |
| 3 | 1 and 19 | HPLC-MS | C18(4.0 mm × 200 mm, 5 µm) | Acetonitrile with 0.1% acetic acid and 0.1% acetic acid | [119] |
| 4 | 1, 2, 3, 8 and 12 | HPLC-UV | Zorbax SB-C18(250 mm × 4.6 mm, 5 µm) | Water with 0.1% acetic acid and methanol | [59] |
| 5 | 19, 21, 29 and 30 | HPLC-DAD | Eclipse XDB-C8(4.6 mm i.d. × 150 mm) | Methanol and water with 1% formic acid | [110] |
| 6 | 1, 6, 13, 19, 21, 29, 30, 40, 48, 54, 58 and 67 | HPLC-UV | Waters symmetry C18(150 × 4.6 mm, 5 µm) | 0.25% aqueous acetic acid and methanol | [193] |
| 7 | 1, 13, 19, 20, 21, 27, 29, 30, 31, 39, E-40, Z-40, 47, 53, 54, 56, 58, 60, 61 and 62 | HPLC-DAD-MS | Alltima C18(4.6 mm × 250 mm, 5 µm) | 0.5% acetic acid in water and acetonitrile | [194] |
| 8 | 21, 19, 48, 39, 62 | HPLC-MSn | Eclipse XDB-C18(4.6 mm × 150 mm, 5 µm) | 0.25% acetic acid and methanol (containing 0.25% acetic acid) | [195,196] |
| 9 | 1, 13, 19, 20, 21, 27, 29, 30, 39, E-40 and Z-40 | HPLC-ESI-MS | Alltima C18(4.6 mm × 250 mm, 5 µm) | Water and acetonitrile | [197] |
| 10 | 1, 42 and 6,7-di-hydroxyligustilide | HPLC-DAD | Shinwa-ODS(250 mm × 4.6 mm, 5 µm) | Methanol and 0.1% acetic acid | [198] |
| 11 | 1, 19, 39, 40 and 67 | RP-HPLC-DAD | Grace Smart RP C18(250 mm × 4.6 mm, 5 µm) | Acetonitrile and 0.1% phosphoric acid | [119] |
| 12 | 19 and 21 | HPLC-DAD | Zorbax SB-C18(4.6 mm × 250 mm, 5 µm) | Acetonitrile and 1% acetic acid | [200] |
| 13 | 1, 2, 12, 19, 29, 30 and 40 | HPLC-DAD | Alltima-C18(250 mm × 4.6 mm, 5 μm) | 0.2% aqueous formic acid and acetonitrile | [201] |
3.2. GC-MS
| No. | Analytes | Detection mode | Stationary phase | Temperature | Ref. |
|---|---|---|---|---|---|
| 1 | 19, 39, 40, 47, 48 and senkyunolide | HP 5890 SERIES I GC | Gross-Linked Methyl Silicone Gum Phase (25 m × 0.2 mm) | Column: 80 °C; injector and detector: 250 °C; source: 200 °C; interface: 280 °C | [207] |
| 2 | 19, 20, 21, 22, 27, 29, 30, 39, 40 and 48 | HP6890 (GC) and a mass selective detector (HP5973) | HP-5 MS capillary column(30 m × 0.25 mm, 0.25 µm) | Column: 80 °C–280 °C; injector: 250 °C; source: 250 °C | [59] |
| 3 | 45 components were identified. | HP5988A GC-MS | SE-30 capillary column(30 m × 0.25 mm, 0. 25 µm) | Column: 90 °C–250 °C; injector: 260 °C | [208] |
| 4 | About 127 chemical components be separated and 81 of them identified. | ShimadzuGC-17A | OV-17 capillary column(30 m × 0.25 mm) | Column: 40 °C–230 °C; injector: 250 °C; source: 230 °C | [209] |
| 5 | 59 components were identified. | Agilent 6890N 5973N GC-MS | HP-1 capillary column(30 m × 0.25 mm) | Column: 40 °C–230 °C; injector: 280 °C; source: 230 °C; interface: 280 °C | [210] |
| 6 | 19 and 21 | Shimadzu GC-14B | SE-54 quartz capillary column(50 m × 0.2 mm) | Column: 240 °C; injector and detector: 280 °C | [211] |
| 7 | 52 volatile chemical components were determined. | Agilent 6890N 5973N GC-MS | HP-5MS capillary column(30 m × 0.25 mm) | Column: 60 °C–250 °C; injector: 250 °C; source: 230 °C; interface: 280 °C | [212] |
| 8 | 73 compounds were identified. | HP 5973 GC-MSD | HP-INNOWAX(30 m × 0.25 mm, 0.25 µm) | Column: 50 °C–210 °C; injector: 250 °C; source: 250 °C; interface: 280 °C | [213] |
| 9 | 62 components were identified. | Trace MS 2000 GC-MS | DB-5 capillary column(0.25 mm × 30 m, 0.25 µm) | Column: 50 °C–240 °C; injector: 270 °C; source: 200 °C; interface: 250 °C | [20] |
| 10 | 52 compounds were identified. | HP 6890 N GC | HP-5(30 m × 0.32 mm, 0.25 µm) | Column: 40 °C–100 °C; injector: 260 °C; source: 200 °C; interface: 220 °C | [108] |
3.3. CE
4. Fingerprinting
5. Conclusions
Acknowledgments
References
- The State Pharmacopoeia Commission of the People’s Republic of China. In Chinese Pharmacopoeia; Chemical Industry Press: Beijing, China, 2010; Volume 1, p. 38.
- The State Pharmacopoeia Commission of the People’s Republic of China. In Chinese Pharmacopoeia; Chemical Industry Press: Beijing, China, 2005; Volume 1, p. 28.
- Wagner, H.; Bauer, R.; Melchart, D.; Xiao, P.G.; Staudinger, A. Chromatographic Fingerprint Analysis of Herbal Medicines: Thin-layer and High Performance Liquid Chromatography of Chinese Drugs, 2nd ed; Springer Wien New York: Berlin, Germany, 2011; Volume 1, pp. 181–190. [Google Scholar]
- The Compile Commission of Zhonghua Bencao of the State Administration of TCM of China. In Zhonghua Bencao; Shanghai Science and Technology Press: Shanghai, China, 1999; Volume 5, p. 976.
- Song, P.S.; Ma, X.; Zhang, B.C.; Wang, Q.Z. Textual research and historical evolution of Xiongqiong (Chuanxiong). China J. Chin. Mat. Med. 2000, 25, 434–446. [Google Scholar]
- Zhang, Y.S. Yixue Qiyuan; People’s Medical Publishing House: Beijing, China, 1978; p. 63. [Google Scholar]
- Ramalingam, M.; Yong-Ki, P. Free radical scavenging activities of Cnidium officinale Makino and Ligusticum chuanxiong Hort. methanolic extracts. Pharmacogn. Mag. 2010, 6, 323–330. [Google Scholar] [CrossRef]
- Li, H.X.; Ding, M.Y.; Lü, K.; Yu, J.Y. Determination of the active ingredients in Chuanxiong by HPLC, HPLC-MS, and EI-MS. J. Liq. Chromatogr. R. T. 2001, 24, 2017–2031. [Google Scholar] [CrossRef]
- Masaru, K.; Hiroshi, M. Structures of three new ligustilide derivatives from Ligusticum wallichii. Chem. Pharm. Bull. (Tokyo) 1987, 35, 4789–4792. [Google Scholar] [CrossRef]
- Masaru, K.; Miyuki, F.; Hiroshi, M. Studies on the constituents of umbelliferae plants. XV.1) constituents of Cnidium officinale: Occurrence of pregnenolone, coniferylferulate and hydroxyphthalides. Chem. Pharm. Bull. (Tokyo) 1987, 35, 1427–1433. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, H.W.; Sung, J.S.; Bang, J.W. Inter-genomic relationships among three medicinal herbs: Cnidium officinale, Ligusticum chuanxiong and Angelica polymorpha. Genes Genom. 2010, 32, 95–101. [Google Scholar] [CrossRef]
- Park, Y.K. The study on antioxidative effects and quality comparison of Ligusticum chuanxiong and Cnidium officinale (1). Korean J. Herbol. 1998, 13, 103–108. [Google Scholar]
- Lee, H.W.; Cho, H.G.; Park, Y.K. The study on antioxidative effects and quality comparison of Ligusticum chuanxiong and Cnidium officinale (2). Vascular relaxant effect of Cnidii rhizome and Cnidii rhizome-Angelicae radix compound. Korean J. Herbol. 1999, 14, 55–60. [Google Scholar]
- Jeong, J.B.; Ju, S.Y.; Park, J.H.; Lee, J.R.; Yun, K.W.; Kwon, S.T.; Lim, J.H.; Chung, G.Y.; Jeong, H.J. Antioxidant activity in essential oils of Cnidium officinale makino and Ligusticum chuanxiong Hort and their inhibitory effects on DNA damage and apoptosis induced by ultraviolet B in mammalian cell. Cancer Epidemiol. 2009, 33, 41–46. [Google Scholar] [CrossRef]
- Chen, X.F.; Ding, D.R.; Huang, W.X.; Liu, S.R.; Liu, S.X. The growth and development characteristics of Chuanxiong. China J. Chin. Mat. Med. 1997, 22, 527–574. [Google Scholar]
- Li, S.L.; Lin, G.; Tam, Y.K. Time-course accumulation of main bioactive components in the rhizome of Ligusticum chuanxiong. Planta Med. 2006, 72, 278–280. [Google Scholar] [CrossRef]
- Zhang, Y.; Fan, Q.J.; Zheng, S.L.; Zhou, H.; Yuan, J.C.; Ma, Y.Y.; Jiang, G.H. The effects of additional fertilizer in spring on the content of chuanxiong production, ferulic acid and total alkaloid. China J. Chin. Mat. Med. 2008, 33, 1944–1947. [Google Scholar]
- Hou, J. Quality evaluating of germplasm resources of Chuanxiong in different producing areas. Master Thesis, Chengdu University of TCM, Chengdu, China. 2007. [Google Scholar]
- Shi, X.J.; Chen, L.; Peng, C. Comparative study of chemical composition of chuanxiong from different commodities specifications. J. Sichuan Tradit. Chin. Med. 2011, 29, 58–61. [Google Scholar]
- Wu, Q.; Yang, X.W. GC-MS analysis of essential oil from rhizomes of Ligusticum chuanxiong cultivated in GAP base for Chinese medicinal materials of China. China J. Chin. Mat. Med. 2008, 33, 276–280. [Google Scholar]
- Gao, X.M. Traditional Chinese Pharmacy; China Press Traditional Chinese Medicine: Beijing, China, 2006; p. 364. [Google Scholar]
- Zhang, L.; Jiang, Y.R.; Guo, C.Y.; Wu, C.F.; Chen, K.J.; Yin, H.J. Effects of active components of Red Paeonia and Rhizoma chuanxiong on angiogenesis in atherosclerosis plaque in rabbits. Chin. J. Integr. Med. 2009, 15, 359–364. [Google Scholar] [CrossRef]
- Liang, M.J.; He, L.C.; Yang, G.D. Screening, analysis and in vitro vasodilatation of effective components from Ligusticum chuanxiong. Life Sci. 2005, 78, 128–133. [Google Scholar] [CrossRef]
- Li, M.; Handa, S.; Ikeda, Y.; Goto, S. Specific inhibiting characteristics of tetramethylpyrazine, one of the active ingredients of the Chinese herbal medicine “Chuanxiong”, on platelet thrombus formation under high shear rates. Thromb. Res. 2001, 104, 15–28. [Google Scholar] [CrossRef]
- Chen, K.J.; Chen, K. Ischemic stroke treated with Ligusticum chuanxiong. Chin. Med. J. (Engl.) 1992, 105, 870–873. [Google Scholar]
- Yang, X.; Zeng, X.; Wu, T. Chuanxiong preparations for preventing stroke. Cochrane Database Syst. Rev. 2010. [Google Scholar] [CrossRef]
- Chan, S.S.; Jones, R.L.; Lin, G. Synergistic interaction between the Ligusticum chuanxiong constituent butylidenephthalide and the nitric oxide donor sodium nitroprusside in relaxing rat isolated aorta. J. Ethnopharmacol. 2009, 122, 308–312. [Google Scholar] [CrossRef]
- Hou, Y.Z.; Zhao, G.R.; Yang, J.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Protective effect of Ligusticum chuanxiong and Angelica sinensis on endothelial cell damage induced by hydrogen peroxide. Life Sci. 2004, 75, 1775–1786. [Google Scholar] [CrossRef]
- Hou, Y.Z.; Zhao, G.R.; Yuan, Y.J.; Zhu, G.G.; Hiltunen, R. Inhibition of rat vascular smooth muscle cell proliferation by extract of Ligusticum chuanxiong and Angelica sinensis. J. Ethnopharmacol. 2005, 100, 140–144. [Google Scholar] [CrossRef]
- Jin, Y.; Liang, T.; Fu, Q.; Xiao, Y.S.; Feng, J.T.; Ke, Y.X.; Liang, X.M. Fingerprint analysis of Ligusticum chuanxiong using hydrophilic interaction chromatography and reversed-phase liquid chromatography. J. Chromatogr. A 2009, 1216, 2136–2141. [Google Scholar] [CrossRef]
- Lu, Y.; Hu, Y.L.; Kong, X.F.; Wang, D.Y. Selection of component drug in activating blood flow and removing blood stasis of Chinese herbal medicinal formula for dairy cow mastitis by hemorheological method. J. Ethnopharmacol. 2008, 116, 313–317. [Google Scholar] [CrossRef]
- Shu, D.; He, J.; Chen, J. Neuroprotective effects and mechanisms of Chuanxiong Chatiao pulvis against MPTP-induced dopaminergic neurotoxicity in mice model of Parkinson’s disease. China J. Chin. Mat. Med. 2009, 34, 2494–2497. [Google Scholar]
- Lin, Y.L.; Wang, G.J.; Huang, C.L.; Lee, Y.C.; Liao, W.C.; Lai, W.L.; Lin, Y.J.; Huang, N.K. Ligusticum chuanxiong as a potential neuroprotectant for preventing serum deprivation-induced apoptosis in rat pheochromocytoma cells: functional roles of mitogen-activated protein kinases. J. Ethnopharmacol. 2009, 122, 417–423. [Google Scholar] [CrossRef]
- Or, T.C.; Yang, C.L.; Law, A.H.; Li, J.C.; Lau, A.S. Isolation and identification of anti-inflammatory constituents from Ligusticum chuanxiong and their underlying mechanisms of action on microglia. Neuropharmacology 2011, 60, 823–831. [Google Scholar] [CrossRef]
- Li, Y.; Xu, C.; Zhang, Q.; Liu, J.Y.; Tan, R.X. In vitro anti-Helicobacter pylori action of 30 Chinese herbal medicines used to treat ulcer diseases. J. Ethnopharmacol. 2005, 98, 329–333. [Google Scholar] [CrossRef]
- Lee, T.F.; Lin, Y.L.; Huang, Y.T. Studies on antiproliferative effects of phthalides from Ligusticum chuanxiong in hepatic stellate cells. Planta Med. 2007, 73, 527–534. [Google Scholar] [CrossRef]
- Chor, S.Y.; Hui, A.Y.; To, K.F.; Chan, K.K.; Go, Y.Y.; Chan, H.L.; Leung, W.K.; Sung, J.J. Anti-proliferative and pro-apoptotic effects of herbal medicine on hepatic stellate cell. J. Ethnopharmacol. 2005, 100, 180–186. [Google Scholar] [CrossRef]
- Yuan, J.F.; Zhang, Z.Q.; Fan, Z.C.; Yang, J.X. Antioxidant effects and cytotoxicity of three purified polysaccharides from Ligusticum chuanxiong Hort. Carbohydr. Polym. 2008, 74, 822–827. [Google Scholar] [CrossRef]
- Wu, J.G.; Wei, Y.J.; Ran, X.; Zhang, H.; Nian, H.; Qin, L.P. Inhibitory effects of essential oil from rhizomes of Ligusticum chuanxiong on hypertrophic scarring in the rabbit ear model. Pharm. Biol. 2011, 49, 764–769. [Google Scholar] [CrossRef]
- Zeng, Z.D.; Liang, Y.Z.; Chau, F.T.; Chen, S.; Daniel, M.K.W.; Chan, C.O. Mass spectral profiling: An effective tool for quality control of herbal medicines. Anal. Chim. Acta 2007, 604, 89–98. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Xie, P.S.; Chau, F. Chromatographic fingerprinting and related chemometric techniques for quality control of traditional Chinese medicines. J. Sep. Sci. 2010, 33, 410–421. [Google Scholar] [CrossRef]
- Ji, S.G.; Chai, Y.F.; Wu, Y.T.; Yin, X.P.; Liang, D.S.; Xu, Z.X.; Li, X. Determination of ferulic acid in Angelica sinensis and Chuanxiong by capillary zone electrophoresis. Biomed. Chromatogr. 1999, 13, 333–334. [Google Scholar] [CrossRef]
- Wang, W.X.; Gu, M.; Jiang, X.G.; Gu, Z.L.; Fan, P.S. Studies on chemical constituents of Ligusticum chuanxiong. Chin. Tradit. Herb. Drugs 2002, 33, 4–5. [Google Scholar]
- Wang, P.S.; Gao, X.L.; Fushan, A.B.; Guanhou, D.Z. The chemical composition of traditional Chinese medicineChuanxiong—Six phenolic compounds. Chin. Tradit. Herb. Drugs 1985, 16, 45–47. [Google Scholar]
- Beijing Institute on the Pharmaceutical Industry. Chemical research of the constituents of Chuanxiong. Pharm. Bull. 1980, 15, 471.
- Beijing Institute on the Pharmaceutical Industry. The effective constituents study of Chuanxiong. Pharm. Bull. 1979, 14, 670–675.
- Wang, P.S.; Gao, X.L.; Fushan, A.B.; Guanhou, D.Z. The chemical composition of traditional Chinese medicineChuanxiong—A terpenoid. Chin. Tradit. Herb. Drugs 1985, 16, 30–32. [Google Scholar]
- Ke, R.T.; Zeng, G.F. The chemical composition study ofChuanxiong. Acta Chim. Sin. 1957, 23, 246. [Google Scholar]
- Cao, F.Y.; Liu, W.X.; Wen, Y.S.; He, Z.R.; Qin, W.J. The chemical composition study of Chuanxiong. Chin. Tradit. Herb. Drugs 1983, 14, 1–3. [Google Scholar]
- Qian, F.; Yan, E.C. Qualitative and quantitative analysis of ferulic acid in Chuanxiong by TLC scanning method. Chin. Tradit. Pat. Med. 1990, 12, 9–10. [Google Scholar]
- Wang, F.; Kuang, W.H. RP-HPLC determination of ferulic acid of Ligusticum chuanxiong Hort from different areas. Chin. J. Mod. Appl. Pharm. 2002, 19, 310–311. [Google Scholar]
- Sheng, Y.X.; Li, L.; Wang, Q.; Guo, H.Z.; Guo, D.A. Simultaneous determination of gallic acid, albiflorin, paeoniflorin, ferulic acid and benzoic acid in Si-Wu decoction by high-performance liquid chromatography DAD method. J. Pharm. Biomed. Anal. 2005, 37, 805–810. [Google Scholar] [CrossRef]
- Zhang, T.H.; Yang, X.L.; Zhang, P.; Zhu, M.; He, Z.G.; Bi, K.S. Determination of ferulic acid in rat plasma by liquid chromatography-tandem mass spectrometry method: application to a pharmacokinetic study. Anal. Lett. 2009, 42, 2157–2169. [Google Scholar] [CrossRef]
- Li, L.J.; Feng, J.; Cheng, H.; Chen, Q.F.; Zhong, Z.H.; Kong, H.X.; Wu, J.L. Separation and determination of salicylic acid, cinnamic acid, ferulic acid and vanillic acid with sample stacking-non-aqueous electrophoresis. Chin. J. Anal. Chem. 2007, 35, 401–404. [Google Scholar]
- Xian, D.L.; Huang, K.L.; Hu, W.G.; Xiao, J.Y.; Jiao, F.P. Evaluation of ferulic acid-biomembrane interaction by liposome electrokinetic chromatography. Chin. J. Anal. Chem. 2007, 35, 1521–1524. [Google Scholar] [CrossRef]
- Kong, L.; Yu, Z.Y.; Bao, Y.M.; Su, X.Y.; Zou, H.F.; Li, X. Screening and analysis of an antineoplastic compound in Rhizoma Chuanxiong by means of in vitro metabolism and HPLC-MS. Anal. Bioanal. Chem. 2006, 386, 264–274. [Google Scholar] [CrossRef]
- Li, H.X.; Ding, M.Y.; Yu, J.Y. Separation and identification of the phthalic anhydride derivatives of Ligusticum Chuanxiong Hort by GC-MS, TLC, HPLC-DAD, and HPLC-MS. J. Chromatogr. Sci. 2002, 40, 156–161. [Google Scholar]
- Li, F.; Cao, Q.E.; Ding, Z. Separation and determination of three phenylpropanoids in the traditional Chinese medicine and its preparations by capillary electrophoresis. J. Chromatogr. Sci. 2007, 45, 354–359. [Google Scholar]
- Zhao, Y.X.; Ding, M.Y.; Liu, D.L. Phenolic acids analysis in Ligusticum chuanxiong using HPLC. J. Chromatogr. Sci. 2005, 43, 389–393. [Google Scholar]
- Antolovich, M.; Prenzler, P.D.; Robards, K.; Ryan, D. Sample preparation in the determination of phenolic compounds in fruits. Analyst 2000, 125, 989–1009. [Google Scholar] [CrossRef]
- Huang, W.Y.; Sheu, S.J. Separation and identification of the organic acids in Angelicae Radix and Ligustici Rhizoma by HPLC and CE. J. Sep. Sci. 2006, 29, 2616–2624. [Google Scholar] [CrossRef]
- Tian, S.; Nakamura, K.; Kayahara, H. Analysis of phenolic compounds in white rice, brown rice, and germinated brown rice. J. Agric. Food Chem. 2004, 52, 4808–4813. [Google Scholar] [CrossRef]
- Goncalves, J.; Mendes, B.; Silva, C.L.; Camara, J.S. Development of a novel microextraction by packed sorbent-based approach followed by ultrahigh pressure liquid chromatography as a powerful technique for quantification phenolic constituents of biological interest in wines. J. Chromatogr. A 2012, 1229, 13–23. [Google Scholar] [CrossRef]
- Lü, G.; Cheng, S.; Chan, K.; Leung, K.S.; Zhao, Z. Determination of free ferulic acid and total ferulic acid in Chuanxiong by high-performance liquid chromatography for quality assessment. China J. Chin. Mat. Med. 2010, 35, 194–198. [Google Scholar]
- Li, Y.; Lu, D.G.; Lei, Y.Q.; Lei, P.; Liu, S.; Li, X.Z. Comparison of ferulic acid and paeoniflorin between traditional slice decoction and dispensing granule decoction of Siwu Tang. J. Chin. Med. Mat. 2008, 31, 125–128. [Google Scholar]
- Xu, Z.M.; Ji, S.G. Separation and determination of ferulic acid in Chuanxiong by high performance capillary electrophoresis. J. Pharm. Pract. 1998, 16, 165–166. [Google Scholar]
- Liu, Y.P.; Li, Z.L.; Zhang, T.M.; Ling, Y.K.; Wan, L. The determination of ferulic acid in Chuanxiong different parts by TLC scanning method. China J. Chin. Mat. Med. 1995, 20, 9–10. [Google Scholar]
- Luo, L.; Guo, R.; Wu, H.; Zhang, A.H.; Qiu, L.Y.; Qian, Q.X. The effect of Paozhi on the content of ferulic acid in chuanxiong. J. Chin. Med. Mat. 1998, 21, 184–185. [Google Scholar]
- Wang, W.X.; Gu, M.; Xu, X.Y.; Tang, L.H.; Gu, Z.L. The determination of ferulic acid in Chuanxiong by HPLC. Chin. Wild Plant Res. 2000, 19, 44–45. [Google Scholar]
- Liu, Y.; Jia, M.R. Determination of Rhizoma Chuanxiong, Naixiong and Shanchuanxiong by RP-HPLC. West China J. Pharm. Sci. 2004, 19, 363–365. [Google Scholar]
- Li, L.; Dou, C.J.; Yao, L. Determination of ferulic acid in Ligusticum chuanxiong by supercritical fluid CO2 extraction method and HPLC. Chem. Anal. Meterage 2006, 15, 42–45. [Google Scholar]
- Zhu, L.B.; Zou, X.F. Determination of the contents of ferulic acid in Rhizoma Chuanxiong from different regions by HPLC. Heilongjiang Med. J. 2008, 21, 13–14. [Google Scholar]
- Cai, D.F.; Zhang, Q.; Zou, Y.; Wang, J.C. Determination of the content of ferulic acid in Rhizoma Chuanxiong by RP-HPLC. J. Qiqihar Med. Coll. 2008, 29, 835–836. [Google Scholar]
- Wang, M.W.; Zhang, Y.; Zhang, J.; Xiao, Y.Y.; Ma, Y.Y.; Jiang, G.H. Determination of the content of total ferulic acid in Rhizoma Chuanxiong by HPLC. West China J. Pharm. Sci. 2008, 23, 100–102. [Google Scholar]
- Zhang, X.L. Determination of the content of ferulic acid in Chuanxiong by HPLC. Chin. Pharm. Aff. 2009, 23, 469–471. [Google Scholar]
- Fu, X.Y.; Huang, Q.L. Comparative determination of ferulic acid in two kinds of Chuanxiong by HPLC and UPLC. J. Chin. Med. Mat. 2011, 34, 1070–1072. [Google Scholar]
- Wang, X.M.; Jiao, L.; Liu, X.L.; Li, H. Quantitative ananlysis of ferulic acid in Ligusticum chuanxiong Hort. by near infrared diffuse reflectance spectroscopy. Chin. J. Pharm. Anal. 2011, 31, 1016–1019. [Google Scholar]
- Tang, Y.P.; Duan, J.A.; Fan, X.S.; Shang, E.X.; Su, S.L.; Ding, A.W. Effects of aromatic acids on stimulating blood circulation and removing blood stasis. World Sci. Technol. 2008, 10, 38–42. [Google Scholar] [CrossRef]
- Hou, Y.Z.; Yang, J.; Zhao, G.R.; Yuan, Y.J. Ferulic acid inhibits vascular smooth muscle cell proliferation induced by angiotensin II. Eur. J. Pharmacol. 2004, 499, 85–90. [Google Scholar] [CrossRef]
- Kobayashi, S.; Mimura, Y.; Naitoh, T.; Kimura, I.; Kimura, M. Chemical structure-activity of cnidium rhizome-derived phthalides for the competence inhibition of proliferation in primary cultures of mouse aorta smooth muscle cells. Jpn. J. Pharmacol. 1993, 63, 353–359. [Google Scholar] [CrossRef]
- Mathew, S.; Abraham, T.E. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit. Rev. Biotechnol. 2004, 24, 59–83. [Google Scholar] [CrossRef]
- Lin, Z.H.; Zhu, D.N.; Yan, Y.Q.; Yu, B.Y. Neuroprotection by herbal formula FBD and its active compounds. Pharm. Biol. 2009, 47, 608–614. [Google Scholar] [CrossRef]
- Barone, E.; Calabrese, V.; Mancuso, C. Ferulic acid and its therapeutic potential as a hormetin for age-related diseases. Biogerontology 2009, 10, 97–108. [Google Scholar] [CrossRef]
- Madhujith, T.; Shahidi, F. Antioxidant and antiproliferative potential of pearled barley (Hordeum vulgarae). Pharm. Biol. 2008, 46, 88–95. [Google Scholar] [CrossRef]
- Chawla, A.S.; Singh, M.; Murthy, M.S.; Gupta, M.; Singh, H. Anti-inflammatory action of ferulic acid and its esters in carrageenan induced rat paw oedema model. Indian J. Exp. Biol. 1987, 25, 187–189. [Google Scholar]
- Chotimarkorn, C.; Ushio, H. The effect of trans-ferulic acid and gamma-oryzanol on ethanol-induced liver injury in C57BL mouse. Phytomedicine 2008, 15, 951–958. [Google Scholar] [CrossRef]
- Nakashima, H.; Murakami, T.; Yamamoto, N.; Naoe, T.; Kawazoe, Y.; Konno, K.; Sakagami, H. Lignified materials as medicinal resources. V. Anti-HIV (human immunodeficiency virus) activity of some synthetic lignins. Chem. Pharm. Bull. (Tokyo) 1992, 40, 2102–2105. [Google Scholar] [CrossRef]
- Kikuzaki, H.; Hisamoto, M.; Hirose, K.; Akiyama, K.; Taniguchi, H. Antioxidant properties of ferulic acid and its related compounds. J. Agric. Food Chem. 2002, 50, 2161–2168. [Google Scholar] [CrossRef]
- Castelluccio, C.; Paganga, G.; Melikian, N.; Bolwell, G.P.; Pridham, J.; Sampson, J.; Rice-Evans, C. Antioxidant potential of intermediates in phenylpropanoid metabolism in higher plants. FEBS Lett. 1995, 368, 188–192. [Google Scholar] [CrossRef]
- Kono, Y.; Kobayashi, K.; Tagawa, S.; Adachi, K.; Ueda, A.; Sawa, Y.; Shibata, H. Antioxidant activity of polyphenolics in diets. Rate constants of reactions of chlorogenic acid and caffeic acid with reactive species of oxygen and nitrogen. Biochim. Biophys. Acta 1997, 1335, 335–342. [Google Scholar] [CrossRef]
- Gulcin, I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid). Toxicology 2006, 217, 213–220. [Google Scholar] [CrossRef]
- Koshihara, Y.; Neichi, T.; Murota, S.; Lao, A.; Fujimoto, Y.; Tatsuno, T. Caffeic acid is a selective inhibitor for leukotriene biosynthesis. Biochim. Biophys. Acta 1984, 792, 92–97. [Google Scholar] [CrossRef]
- Nardini, M.; Leonardi, F.; Scaccini, C.; Virgili, F. Modulation of ceramide-induced NF-kappaB binding activity and apoptotic response by caffeic acid in U937 cells: comparison with other antioxidants. Free Radic. Biol. Med. 2001, 30, 722–733. [Google Scholar] [CrossRef]
- Mori, H.; Tanaka, T.; Shima, H.; Kuniyasu, T.; Takahashi, M. Inhibitory effect of chlorogenic acid on methylazoxymethanol acetate-induced carcinogenesis in large intestine and liver of hamsters. Cancer Lett. 1986, 30, 49–54. [Google Scholar] [CrossRef]
- Tanaka, T.; Kojima, T.; Kawamori, T.; Wang, A.; Suzui, M.; Okamoto, K.; Mori, H. Inhibition of 4-nitroquinoline-1-oxide-induced rat tongue carcinogenesis by the naturally occurring plant phenolics caffeic, ellagic, chlorogenic and ferulic acids. Carcinogenesis 1993, 14, 1321–1325. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Suzuki, O.; Igarashi, K. Protective effects of chlorogenic acid on paraquat-induced oxidative stress in rats. Biosci. Biotechnol. Biochem. 1996, 60, 765–768. [Google Scholar] [CrossRef]
- Hemmerle, H.; Burger, H.J.; Below, P.; Schubert, G.; Rippel, R.; Schindler, P.W.; Paulus, E.; Herling, A.W. Chlorogenic acid and synthetic chlorogenic acid derivatives: Novel inhibitors of hepatic glucose-6-phosphate translocase. J. Med. Chem. 1997, 40, 137–145. [Google Scholar] [CrossRef]
- Nardini, M.; D’Aquino, M.; Tomassi, G.; Gentili, V.; di Felice, M.; Scaccini, C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radic. Biol. Med. 1995, 19, 541–552. [Google Scholar] [CrossRef]
- Hsu, C.L.; Huang, S.L.; Yen, G.C. Inhibitory effect of phenolic acids on the proliferation of 3T3-L1 preadipocytes in relation to their antioxidant activity. J. Agric. Food Chem. 2006, 54, 4191–4197. [Google Scholar] [CrossRef]
- Naito, T.; Katsuhara, T.; Niitsu, K.; Ikeya, Y.; Okada, M.; Mitsuhashi, H. Phthalide dimers from Ligusticum chuanxiong Hort. Heterocycles 1991, 32, 2433–2442. [Google Scholar] [CrossRef]
- Naito, T.; Katsuhara, T.; Niitsu, K.; Ikeya, Y.; Okada, M.; Mitsuhashi, H. Two phthalides from Ligusticum chuanxiong. Phytochemistry 1992, 31, 639–642. [Google Scholar] [CrossRef]
- Naito, T.; Niitsu, K.; Ikeya, Y.; Okada, M.; Mitsuhashi, H. A phthalide and 2-farnesyl-6-methyl benzoquinone from Ligusticum chuanxiong. Phytochemistry 1992, 31, 1787–1789. [Google Scholar] [CrossRef]
- Naito, T.; Ikeya, Y.; Okada, M.; Mistuhashi, H.; Maruno, M. Two phthalides from Ligusticum chuanxiong. Phytochemistry 1996, 41, 233–236. [Google Scholar]
- Li, Y.H.; Peng, S.L.; Zhou, Y.; Yu, K.B.; Ding, L.S. Two new phthalides from Ligusticum chuanxiong. Planta Med. 2006, 72, 652–656. [Google Scholar] [CrossRef]
- Chang, X.L.; Jiang, Z.Y.; Ma, Y.B.; Zhang, X.M.; Tsim, K.W.; Chen, J.J. Two new compounds from the roots of Ligusticum chuanxiong. J. Asian Nat. Prod. Res. 2009, 11, 805–810. [Google Scholar] [CrossRef]
- Guo, Z.F.; Wang, L.; Guo, T.T.; Song, C.Y.; Zhang, L.X. Analysis of the Chuanxiong ultrasonic extracts by GC-MS. J. Hebei Univ. (Nat. Sci. Ed.) 2009, 29, 177–183. [Google Scholar]
- Mitsuhashi, H.; Muramatsu, T.; Nagai, U.; Nakano, T.; Ueno, K. Studies on the constituents of Umbelliferae plants. VIII. Distribution of alkylphthalides in umbelliferae plants. Chem. Pharm. Bull. (Tokyo) 1963, 11, 1317–1319. [Google Scholar] [CrossRef]
- Lim, L.S.; Shen, P.; Gong, Y.H.; Yong, E.L. Dimeric progestins from rhizomes of Ligusticum chuanxiong. Phytochemistry 2006, 67, 728–734. [Google Scholar] [CrossRef]
- Mitsuhashi, H.; Nagai, U.; Muramatsu, T. Studies on the constituents of Umbelliferae plants (III). Chem. Pharm. Bull. (Tokyo) 1961, 9, 115–119. [Google Scholar] [CrossRef]
- Cao, J.M.; Wang, Z.H.; Sun, G.P.; Ding, M.Y.; Yang, X.D.; Kou, H.J. Study on extraction and separation method for lactone components in Chuanxiong. Chin. J. Anal. Lab 2005, 24, 59–62. [Google Scholar]
- Wang, X.Y.; Zheng, X.H.; Zhao, X.F.; Wang, R.; Wang, S.X. Optimization of the solid phase extraction of esters in the Chuanxiong water extracts. Chin. Tradit. Pat. Med. 2010, 32, 589–591. [Google Scholar]
- Zhang, Z.D.; Chen, J.P.; Li, C.P.; Yan, P.F.; Wang, A.J.; Urs, W.B. Microwave-assisted extraction of lactone from Ligusticum Chuanxiong Hort using ionic liquid. Chin. J. Process Eng. 2010, 10, 498–502. [Google Scholar]
- Zhang, X.Z.; Xu, Q.; Xiao, H.B.; Liang, X.M. Preparation of senkyunolide I by reversed-phase high performance liquid chromatography. Chin. J. Chromatogr. 2004, 22, 41–43. [Google Scholar]
- Ito, Y. Golden rules and pitfalls in selecting optimum conditions for high-speed counter-current chromatography. J. Chromatogr. A 2005, 1065, 145–168. [Google Scholar] [CrossRef]
- Liu, W.; Wu, P.; Zhuo, C.; Zhang, J.Q.; Shen, P.N. One step separation and preparation of senkyunolide A and Z-ligustilide in Ligusticum chuanxiong Hort by high speed counter current chromatography. Chin. Tradit. Pat. Med. 2010, 32, 764–767. [Google Scholar]
- Wu, G.T.; Shi, L.F.; Hu, J.H.; Li, L. Determination of ligustilide content in Chuanxiong by supercritical fluid extraction method. Acta Pharm. Sin. 1998, 33, 457–460. [Google Scholar]
- Chao, Z.Z.; Chao, R.B. Determination of ligustilide content in Chuanxiong by HPLC. West China J. Pharm. Sci. 2004, 19, 197–198. [Google Scholar]
- Lin, Y.Z.; Tang, X.; Bi, K.S. Determination of ligustilide in the volatile oil of Chuanxiong content by RP-HPLC. China J. Chin. Mat. Med. 2004, 29, 63–66. [Google Scholar]
- Kong, L.; Yu, Z.Y.; Zou, H.F.; Sun, N.C.; Wu, L.H.; Ni, J.Y. Determination of the active ingredient of ferulic acid and ligustilide in Chuanxiong by HPLC-MS. Chin. J. Anal. Chem. 2004, 32, 1421–1425. [Google Scholar]
- Hu, J.; Feng, L.L.; Liu, Y.; Cui, F.D. Determination and the study of alcohol extraction process of ligustilide in Danggui and Chuanxiong. J. Shenyang Pharm. Univ. 2005, 22, 145–148. [Google Scholar]
- Cheng, S.Q.; Lv, G.H.; Liang, S.X.; Wang, Y.; Xu, Y.C.; Zhao, Z.Z. Determination of ligustilide for quality assessment of Ligusticum chuanxiong. China J. Chin. Mat. Med. 2006, 31, 1143–1146. [Google Scholar]
- Shan, J.J.; Di, L.Q. Determination the butylphthalide content in the volatile oil of chuanxiong by RP-HPLC method. J. Nanjing TCM Univ. 2006, 22, 94–95. [Google Scholar]
- Shan, J.J.; Di, L.Q.; Luo, X.H.; Chen, H. Determination the butylphthalide content of chuanxiong by RP-HPLC method. Chin. Tradit. Herb. Drugs 2006, 37, 281–282. [Google Scholar]
- Zhang, J.L.; He, X.F.; Zhou, Z.H. HPLC determination of five constituents in plants of genus Ligusticum. Acta Pharm. Sin. 1996, 31, 622–625. [Google Scholar]
- Tang, Y.P.; Zhu, M.; Yu, S.; Hua, Y.Q.; Duan, J.A.; Su, S.L.; Zhang, X.; Lu, Y.; Ding, A.W. Identification and comparative quantification of bio-active phthalides in essential oils from si-wu-tang, fo-shou-san, radix angelica and rhizoma chuanxiong. Molecules 2010, 15, 341–351. [Google Scholar] [CrossRef]
- Shi, L.F.; Deng, Y.Z.; Wu, B.S. Studies on chemical constituents and their stability of the essential oil from dry rhizome of Ligusticum chuanxiong Hort. Chin. J. Pharm. Anal. 1995, 15, 26–30. [Google Scholar]
- Li, G.S.; Ma, C.J.; Li, X.Y.; Liu, K. Studies on the stability of ligustilide and the analysis of its isomerized products by GC-MS. Chin. Tradit. Herb. Drugs 2000, 31, 405–407. [Google Scholar]
- Zschocke, S.; Liu, J.H.; Stuppner, H.; Bauer, R. Comparative study of roots of Angelica sinensis and related umbelliferous drugs by thin layer chromatography, high-performance liquid chromatography, and liquid chromatography-mass spectrometry. Phytochem. Anal. 1998, 9, 283–290. [Google Scholar] [CrossRef]
- Yi, T.; Leung, K.S.; Lu, G.H.; Zhang, H.; Chan, K. Identification and comparative determination of senkyunolide A in traditional Chinese medicinal plants Ligusticum chuanxiong and Angelica sinensis by HPLC coupled with DAD and ESI-MS. Chem. Pharm. Bull. (Tokyo) 2005, 53, 1480–1483. [Google Scholar] [CrossRef]
- Yi, T.; Leung, K.S.; Lu, G.H.; Chan, K.; Zhang, H. Simultaneous qualitative and quantitative analyses of the major constituents in the rhizome of Ligusticum Chuanxiong using HPLC-DAD-MS. Chem. Pharm. Bull. (Tokyo) 2006, 54, 255–259. [Google Scholar] [CrossRef]
- Wang, Z.J.; Wo, S.K.; Wang, L.; Lau, C.B.; Lee, V.H.; Chow, M.S.; Zuo, Z. Simultaneous quantification of active components in the herbs and products of Si-Wu-Tang by high performance liquid chromatography-mass spectrometry. J. Pharm. Biomed. Anal. 2009, 50, 232–244. [Google Scholar] [CrossRef]
- Gijbels, M.J.M.; Scheffer, J.J.C.; Svendsen, A.B. Analysis of phthalides from umbelliferae by combined liquid-solid and gas-liquid chromatography. Chromatographia 1981, 14, 452–454. [Google Scholar] [CrossRef]
- Almeida, C.; Kehraus, S.; Prudencio, M.; Konig, G.M. Marilones A-C, phthalides from the sponge-derived fungus Stachylidium sp. Beilstein J. Org. Chem. 2011, 7, 1636–1642. [Google Scholar] [CrossRef]
- Beck, J.J.; Chou, S.C. The structural diversity of phthalides from the Apiaceae. J. Nat. Prod. 2007, 70, 891–900. [Google Scholar] [CrossRef]
- Cao, Y.X.; Zhang, W.; He, J.Y.; He, L.C.; Xu, C.B. Ligustilide induces vasodilatation via inhibiting voltage dependent calcium channel and receptor-mediated Ca2+ influx and release. Vasc. Pharmacol. 2006, 45, 171–176. [Google Scholar] [CrossRef]
- Hu, Y.; Liang, Y.Z.; Li, B.Y.; Li, X.N.; Du, Y.P. Multicomponent spectral correlative chromatography applied to complex herbal medicines. J. Agric. Food Chem. 2004, 52, 7771–7776. [Google Scholar] [CrossRef]
- Tao, J.Y.; Ruan, Y.P.; Mei, Q.B.; Liu, S.; Tian, Q.L.; Chen, Y.Z.; Zhang, H.D.; Duan, Z.X. Studies on the antiasthmatic action of ligustilide of dang-gui, Angelica sinensis (Oliv.) Diels. Acta Pharm. Sin. 1984, 19, 561–565. [Google Scholar]
- Zhang, L.; Du, J.R.; Wang, J.; Yu, D.K.; Chen, Y.S.; He, Y.; Wang, C.Y. Z-ligustilide extracted from Radix Angelica Sinensis decreased platelet aggregation induced by ADP ex vivo and arterio-venous shunt thrombosis in vivo in rats. Yakugaku Zasshi 2009, 129, 855–859. [Google Scholar] [CrossRef]
- Du, J.R.; Yu, Y.; Ke, Y.; Wang, C.Y.; Zhu, L.; Qian, Z.M. Ligustilide attenuates pain behavior induced by acetic acid or formalin. J. Ethnopharmacol. 2007, 112, 211–214. [Google Scholar] [CrossRef]
- Lu, Q.; Qiu, T.Q.; Yang, H. Ligustilide inhibits vascular smooth muscle cells proliferation. Eur. J. Pharmacol. 2006, 542, 136–140. [Google Scholar] [CrossRef]
- Ozaki, Y.; Sekita, S.; Harada, M. Centrally acting muscle relaxant effect of phthalides (ligustilide, cnidilide and senkyunolide) obtained from Cnidium officinale Makino. Yakugaku Zasshi 1989, 109, 402–406. [Google Scholar]
- Matsumoto, K.; Kohno, S.; Ojima, K.; Tezuka, Y.; Kadota, S.; Watanabe, H. Effects of methylenechloride-soluble fraction of Japanese angelica root extract, ligustilide and butylidenephthalide, on pentobarbital sleep in group-housed and socially isolated mice. Life Sci. 1998, 62, 2073–2082. [Google Scholar] [CrossRef]
- Wu, X.M.; Qian, Z.M.; Zhu, L.; Du, F.; Yung, W.H.; Gong, Q.; Ke, Y. Neuroprotective effect of ligustilide against ischaemia-reperfusion injury via up-regulation of erythropoietin and down-regulation of RTP801. Br. J. Pharmacol. 2011, 164, 332–343. [Google Scholar] [CrossRef]
- Peng, H.Y.; Du, J.R.; Zhang, G.Y.; Kuang, X.; Liu, Y.X.; Qian, Z.M.; Wang, C.Y. Neuroprotective effect of Z-ligustilide against permanent focal ischemic damage in rats. Biol. Pharm. Bull. 2007, 30, 309–312. [Google Scholar] [CrossRef]
- Kuang, X.; Yao, Y.; Du, J.R.; Liu, Y.X.; Wang, C.Y.; Qian, Z.M. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res. 2006, 1102, 145–153. [Google Scholar] [CrossRef]
- Liu, L.; Ning, Z.Q.; Shan, S.; Zhang, K.; Deng, T.; Lu, X.P.; Cheng, Y.Y. Phthalide lactones from Ligusticum chuanxiong inhibit lipopolysaccharide-induced TNF-alpha production and TNF-alpha-mediated NF-kappaB activation. Planta Med. 2005, 71, 808–813. [Google Scholar] [CrossRef]
- Chan, S.S.; Cheng, T.Y.; Lin, G. Relaxation effects of ligustilide and senkyunolide A, two main constituents of Ligusticum chuanxiong, in rat isolated aorta. J. Ethnopharmacol. 2007, 111, 677–680. [Google Scholar] [CrossRef]
- Liang, M.J.; He, L.C. Inhibitory effects of ligustilide and butylidenephthalide on bFGF-stimulated proliferation of rat smooth muscle cells. Acta Pharm. Sin. 2006, 41, 161–165. [Google Scholar]
- Wang, Y.X.; Gao, X.L.; Wang, P.S.; Fushan, A.B.; Shanxia, X.S. Study of the another active constituents of chuanxiong. Chin. Tradit. Herb. Drugs 1985, 16, 17–18. [Google Scholar]
- Liu, B.; John, R.D.; Rachel, P. Extraction of tetramethylpyrazine from Ligusticum chuanxiong Hort using phytosol solvent and supercritical fluid. Chin. J. Pharm. 1999, 30, 196–199. [Google Scholar]
- Yang, H.; Liu, L.; Zhang, H.J. Study for the extraction process and quality standards of tetramethylpyrazine. Chin. J. Ethnomed. Ethnopharm. 2009, 4, 24–40. [Google Scholar]
- Li, X.Y.; Guan, Y.T.; Huang, A.B.; Liang, X.F.; Lei, F.H. Study on optimum ultrasonic extraction process of ligustrazine hydrochloride from Ligusticum chuanxiong Hort. by orthogonal design. Lishizhen Med. Mat. Med. Res. 2009, 20, 1990–1992. [Google Scholar]
- Dai, L. Study on ultrafine vibration extraction technology of Rhizoma Chuanxiong. China J. Chin. Mat. Med. 2009, 34, 977–979. [Google Scholar]
- Sun, X.G.; Wang, T.; Zhu, J.S. Determination of TMP content in Chuanxiong by counter current extraction-High performance liquid chromatography. Acta Univ. Med. Tongji 2001, 30, 209–210. [Google Scholar]
- Li, H.B.; Chen, F. Preparative isolation and purification of chuanxiongzine from the medicinal plant Ligusticum chuanxiong by high-speed counter-current chromatography. J. Chromatogr. A 2004, 1047, 249–253. [Google Scholar]
- Ma, L.; Wang, X.J.; Zhang, J.; Zhao, Q.D.; Chen, Z.J. Explore the best extractive craft of tetramethylpyrazine from Rhizoma Chuanxiong. Lishizhen Med. Mat. Med. Res. 2007, 18, 3057–3058. [Google Scholar]
- He, Z.Y.; Liu, Y.; Jia, M.R.; Qiang, Y.; Ma, Y.Y.; Chen, K.; Zhang, Y.; Jiang, G. Determination of the total alkaloids contents from Ligusticum chuanxiong (Naichuanxiong, Shanchuanxiong and Rhizoma chuanxiong). West China J. Pharm. Sci. 2004, 19, 292–293. [Google Scholar]
- Wang, S.B.; Song, H.G. Colorimetric determination of total alkaloids in Ligusticum chuanxiong with reinecke salt. Cent. South Pharm. 2009, 7, 824–826. [Google Scholar]
- Li, S.L. Determination of alkaloid content in different processed products of Chuanxiong. J. Henan Coll. Tradit. Chin. Med. 2001, 16, 18–19. [Google Scholar]
- Sheu, J.R.; Kan, Y.C.; Hung, W.C.; Lin, C.H.; Yen, M.H. The antiplatelet activity of tetramethylpyrazine is mediated through activation of NO synthase. Life Sci. 2000, 67, 937–947. [Google Scholar] [CrossRef]
- Pang, P.K.; Shan, J.J.; Chiu, K.W. Tetramethylpyrazine, a calcium antagonist. Planta Med. 1996, 62, 431–435. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wei, T.T.; Hou, J.W.; Li, G.S.; Yu, S.Z.; Xin, W.J. Tetramethylpyrazine scavenges superoxide anion and decreases nitric oxide production in human polymorphonuclear leukocytes. Life Sci. 2003, 72, 2465–2472. [Google Scholar] [CrossRef]
- Huang, Y.T.; Chang, F.C.; Chen, K.J.; Hong, C.Y. Acute hemodynamic effects of tetramethylpyrazine and tetrandrine on cirrhotic rats. Planta Med. 1999, 65, 130–134. [Google Scholar] [CrossRef]
- Liu, C.F.; Lin, C.C.; Ng, L.T.; Lin, S.C. Hepatoprotective and therapeutic effects of tetramethylpyrazine on acute econazole-induced liver injury. Planta Med. 2002, 68, 510–514. [Google Scholar] [CrossRef]
- Wu, H.J.; Hao, J.; Wang, S.Q.; Jin, B.L.; Chen, X.B. Protective effects of ligustrazine on TNF-alpha-induced endothelial dysfunction. Eur. J. Pharmacol. 2012, 674, 365–369. [Google Scholar] [CrossRef]
- Feng, L.; Xiong, Y.; Cheng, F.; Zhang, L.; Li, S.; Li, Y. Effect of ligustrazine on ischemia-reperfusion injury in murine kidney. Transplant. Proc. 2004, 36, 1949–1951. [Google Scholar] [CrossRef]
- Gao, C.; Feng, L.; Li, Y.P.; Cheng, Y. Effect of ligustrazine on chronic allograft nephropathy in rats. Transplant. Proc. 2007, 39, 3415–3419. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wei, T.T.; Hou, J.W.; Li, G.S.; Yu, S.Z.; Xin, W.J. Iron-induced oxidative damage and apoptosis in cerebellar granule cells: Attenuation by tetramethylpyrazine and ferulic acid. Eur. J. Pharmacol. 2003, 467, 41–47. [Google Scholar] [CrossRef]
- Liao, S.L.; Kao, T.K.; Chen, W.Y.; Lin, Y.S.; Chen, S.Y.; Raung, S.L.; Wu, C.W.; Lu, H.C.; Chen, C.J. Tetramethylpyrazine reduces ischemic brain injury in rats. Neurosci. Lett. 2004, 372, 40–45. [Google Scholar] [CrossRef]
- Fan, L.H.; Wang, K.Z.; Cheng, B.; Wang, C.S.; Dang, X.Q. Anti-apoptotic and neuroprotective effects of Tetramethylpyrazine following spinal cord ischemia in rabbits. BMC Neurosci. 2006, 7, 48. [Google Scholar] [CrossRef]
- Cheng, X.R.; Zhang, L.; Hu, J.J.; Sun, L.; Du, G.H. Neuroprotective effects of tetramethylpyrazine on hydrogen peroxide-induced apoptosis in PC12 cells. Cell Biol. Int. 2007, 31, 438–443. [Google Scholar] [CrossRef]
- Fan, Z.C.; Zhang, Z.Q. Polysaccharides from Ligusticum chuanxiong. Chin. Tradit. Herb. Drugs 2006, 37, 973–976. [Google Scholar]
- Fan, Z.C.; Zhang, Z.Q. Extraction, purification and anti-oxidative activities of polysaccharides from Ligusticum chuanxiong Hort. Nat. Prod. Res. Dev. 2005, 17, 561–567. [Google Scholar]
- Zhao, L. The study on the separation, extraction and the relevant nature of Chuanxiong (Ligusticum Chuanxiong Hort.) saccharide substance. Master Thesis, Northeast Normal University, Changchun, China. 2006. [Google Scholar]
- Sun, X.C.; Yan, J.; He, G.; Zhang, L.L.; Yi, Y.; Gou, X.J. Purification and analysis of monosaccharide composition of Ligusticum chuanxiong polysaccharide. J. Sichuan Agric. Univ. 2011, 29, 56–60. [Google Scholar]
- Xiang, M.; Wang, X.J.; Wang, W.X. Optimization of the ultrasonic extraction process of Chuanxiong polysaccharide. Chin. Tradit. Pat. Med. 2008, 30, 1621–1623. [Google Scholar]
- Li, L.; Wang, W.X.; Wang, X.J. Study of the pectinase extraction process of Chuanxiong polysaccharide. J. Chin. Med. Mat. 2008, 31, 600–602. [Google Scholar]
- Tang, Q.; Wang, W.X.; Wang, X.J. Extraction of polysaccharides from Ligusticum chuanxiong Hort by basic method. Lishizhen Med. Mat. Med. Res. 2008, 19, 2096–2097. [Google Scholar]
- Ding, W.W.; Wang, W.X.; Wang, X.J. Extraction of polysaccharides from Ligusticum chuanxiong Hort by enzymatic method. J. Xihua Univ. (Nat. Sci.) 2008, 27, 42–43. [Google Scholar]
- Huang, H.F.; Wang, W.X. Extraction of polysaccharides from Ligusticum chuanxiong Hort bymicrowave-assisted method. Lishizhen Med. Mat. Med. Res. 2009, 20, 2734–2735. [Google Scholar]
- Sun, X.; Wang, W.X. Extraction of polysaccharides from Ligusticum chuanxiong Hort by cellulose enzyme method. J. Xihua Univ. (Nat. Sci.) 2009, 28, 103–106. [Google Scholar]
- Aihemaiti, P.R.H.T.; Aihemaiti, S.D.; Xu, S.S.; Ji’er, G.L.; Wu, Q. Explore for the extracted process of the Chuanxiong polysaccharide separation. Friend Sci. Amateurs 2011, 3–4. [Google Scholar]
- Wang, W.X.; Wang, X.J.; Huang, X.; Yang, W.Y. Comparison study of decolorization ways for polysaccharide from Ligusticum Chuanxiong Hort. Ion Exch. Adsorp. 2010, 24, 74–82. [Google Scholar]
- Zhang, Z.Q.; Fan, Z.C.; Yan, H.T. Flow injection on-line hydrolytic spectrophotometry for the rapid determination of polysaccharide in Ligusticum chuanxiong Hort. Chin. J. Pharm. Anal. 2004, 24, 320–323. [Google Scholar]
- Zhang, W.J.; Wang, P.; Yang, M.J.; Wang, Y.G.; Ju, Y.; Du, R.H. Analysis and comparison of polysaccharide activity of chuanxiong and chishao. J. Chin. Med. Mat. 2011, 34, 1569–1574. [Google Scholar]
- Yang, N.Y.; Ren, D.C.; Duan, J.A.; Xu, X.H.; Xie, N.; Tian, L.J. Ceramides and cerebrosides from Ligusticum chuanxiong Hort. Helv. Chim. Acta 2009, 92, 291–297. [Google Scholar] [CrossRef]
- Han, X.; Cheng, H. Characterization and direct quantitation of cerebroside molecular species from lipid extracts by shotgun lipidomics. J. Lipid Res. 2005, 46, 163–175. [Google Scholar]
- Chen, Y.; Lü, J.L.; Duan, J.A.; Jiang, L.H.; Guo, J.M. Distribution of cerebroside from a viewpoint of organic evolution and advances of its biological activity. J. Int. Pharm. Res. 2009, 36, 121–126. [Google Scholar]
- Nie, H.D.; Hao, R. Study of the chemical constituents of the Chuanxiong ground part. Med. Inform. 2011, 326, 326–328. [Google Scholar]
- Hao, S.J.; Zhang, Z.X.; Tian, Y.; Ma, Y.P.; Peng, B.; Shen, S.; Zhang, L.; Wang, J.H. Study on chemical constituents of Ligusticum chuanxiong Hort. Mod. Chin. Med. 2010, 12, 22–38. [Google Scholar]
- Miao, C.; Wu, S.; Luo, B.; Wang, J.; Chen, Y. A new sesquiterpenoid from Ligusticum chuanxiong Hort. Fitoterapia 2010, 81, 1088–1090. [Google Scholar] [CrossRef]
- Li, S.L.; Chan, S.S.; Lin, G.; Ling, L.; Yan, R.; Chung, H.S.; Tam, Y.K. Simultaneous analysis of seventeen chemical ingredients of Ligusticum chuanxiong by on-line high performance liquid chromatography-diode array detector-mass spectrometry. Planta Med. 2003, 69, 445–451. [Google Scholar] [CrossRef]
- Yan, R.; Li, S.L.; Chung, H.S.; Tam, Y.K.; Lin, G. Simultaneous quantification of 12 bioactive components of Ligusticum chuanxiong Hort. by high-performance liquid chromatography. J. Pharm. Biomed. Anal. 2005, 37, 87–95. [Google Scholar] [CrossRef]
- Sun, G.P.; Yang, X.D.; Wang, Q.E.; Ding, M.Y. Quantitative determination of total senkyunolides in Rhizoma Chuanxiong by UV spectrophotometry. Chin. J. Anal. Lab 2007, 26, 9–11. [Google Scholar]
- Teng, J.W.; Li, D.L.; Luo, A.D. Identification and characterization of supercritical fluid extracts of Rhizoma Chuanxiong by high performance liquid chromatography-Ion trap mass spectrometry. J. Chin. Mass Spectrom. Soc. 2007, 26, 356–359. [Google Scholar]
- Teng, J.W.; Chen, H.W.; Li, D.L.; Luo, A.D. Identification and characterization of supercritical fluid extracts of Rhizoma Chuanxiong by high performance liquid chromatography ion trap mass spectrometry. Front. Chem. China 2006, 1, 454–458. [Google Scholar] [CrossRef]
- Yi, T.; Leung, K.S.; Lu, G.H.; Zhang, H. Comparative analysis of Ligusticum chuanxiong and related umbelliferous medicinal plants by high performance liquid chromatography-Electrospray ionization mass spectrometry. Planta Med. 2007, 73, 392–398. [Google Scholar] [CrossRef]
- Yao, Z.L.; Du, S.Y.; Lu, Y.; Xu, B.; Chen, X.L. Simultaneous determination of active ingredient content of five kinds of different sources of Chuanxiong by RP-HPLC. China J. Chin. Mat. Med. 2010, 35, 2696–2699. [Google Scholar]
- Wang, Y.M.; Zhang, H.F.; Yan, B.Q.; Chen, X.H.; Bi, K.S. Determination of ferulic acid, 6,7-di-hydroxyligustilide and 4-hydroxy-3-butylphthalide in Rhizoma Chuanxiong by HPLC. Chin. J. Pharm. Anal. 2009, 29, 2109–2112. [Google Scholar]
- Wu, P.L.; Liu, W.; Zhang, J.Q.; Shen, P.R.; Zhuo, C. Determination of senkyunolide A and Z-ligustilide in Ligusticum Chuanxiong Hort. by HPLC. Chin. J. Pharm. 2010, 41, 290–292. [Google Scholar]
- Li, W.X.; Wang, H.; Tang, Y.P.; Guo, J.M.; Qian, D.W.; Ding, A.W.; Duan, J.A. The quantitative comparative analysis for main bio-active components in Angelica sinensis, Ligusticum chuanxiong and the herb pair Gui-Xiong. J. Liq. Chromatogr. R. T. 2012, in press. [Google Scholar]
- Zschocke, S.; Klaiber, I.; Bauer, R.; Vogler, B. HPLC-coupled spectroscopic techniques (UV, MS, NMR) for the structure elucidation of phthalides in Ligusticum chuanxion. Mol. Divers. 2005, 9, 33–39. [Google Scholar] [CrossRef]
- Zhang, X.Z.; Xiao, H.B.; Xu, Q.; Li, X.L.; Wang, J.N.; Liang, X.M. Characterization of phthalides in Ligusticum chuanxiong by liquid chromatographic-atmospheric pressure chemical ionization-mass spectrometry. J. Chromatogr. Sci. 2003, 41, 428–433. [Google Scholar]
- Hu, L.H.; Chen, X.G.; Kong, L.; Su, X.Y.; Ye, M.L.; Zou, H.F. Improved performance of comprehensive two-dimensional HPLC separation of traditional Chinese medicines by using a silica monolithic column and normalization of peak heights. J. Chromatogr. A 2005, 1092, 191–198. [Google Scholar] [CrossRef]
- Chen, X.G.; Kong, L.; Su, X.Y.; Fu, H.J.; Ni, J.Y.; Zhao, R.H.; Zou, H.F. Separation and identification of compounds in Rhizoma chuanxiong by comprehensive two-dimensional liquid chromatography coupled to mass spectrometry. J. Chromatogr. A 2004, 1040, 169–178. [Google Scholar] [CrossRef]
- Chen, Q.H.; Li, P.; Li, B.; Yang, H.D.; Li, X.L.; Chen, F.C. A GC-MS-SIM simultaneous determination of ligustilide and butylidenephthalide from Ligusticum chuanxiong using SFE. Chromatographia 2010, 72, 963–967. [Google Scholar] [CrossRef]
- Li, Z.W.; Zhang, Q. Separation and identification of lactone components in Ligusticum chuanxiong and Cnidium officinale by GC-MS. Chin. J. Pharm. Anal. 1993, 13, 187–189. [Google Scholar]
- Zhou, B.J.; Zhang, Z.Y.; Shi, Y.; Gu, W.X.; Zhang, S.Y. Extraction, separation and GC/MS analysis of Chuanxiong volatile components extracted by supercritical CO2 fluid extraction and molecular distillation. J. First Mil. Med. Univ. 2002, 22, 652–653. [Google Scholar]
- Gong, F.; Liang, Y.Z.; Chau, F.T. Combination of GC-MS with local resolution for determining volatile components in si-wu decoction. J. Sep. Sci. 2003, 26, 112–122. [Google Scholar] [CrossRef]
- Xu, J.Y.; Zhu, Z.S.; Yu, W.B. Component analysis by GC-MS of the Chuanxiong pieces extracted with alcohol. Guangdong Pharm. J. 2005, 15, 3–4. [Google Scholar]
- Zhang, D.L.; Li, G.S.; Ren, Z.Y.; Qu, G.W. GC quantitative determination of Z-ligustilide and senkyunolide A in essential oil of Ligusticum chuanxiong Hort. Chin. J. Pharm. Anal. 2006, 26, 895–897. [Google Scholar]
- Li, X.R.; Liang, Y.Z.; Guo, F.Q. Analysis of volatile oil in Rhizoma ligustici chuanxiong-Radix paeoniae rubra by gas chromatography-mass spectrometry and chemometric resolution. Acta Pharmacol. Sin. 2006, 27, 491–498. [Google Scholar] [CrossRef]
- Zhang, C.; Qi, M.L.; Shao, Q.L.; Zhou, S.; Fu, R.N. Analysis of the volatile compounds in Ligusticum chuanxiong Hort. using HS-SPME-GC-MS. J. Pharm. Biomed. Anal. 2007, 44, 464–470. [Google Scholar] [CrossRef]
- Wang, N.; Zhang, Y.; Li, X.; Tong, Y.P.; Kong, H.W.; Xu, G.W. Analysis of volatile oils of Ligusticum chuanxiong Hort. from different geographical origins by comprehensive two-dimensional gas chromatography/time-of-flight mass spectrometry. Se Pu 2010, 28, 329–335. [Google Scholar]
- Chu, Q.C.; Zhang, D.L.; Zhang, H.T.; Ye, J.N. Study on Rhizoma Chuanxiong based on capillary electrophoresis with amperometric detection. Chin. Chem. Lett. 2010, 21, 217–220. [Google Scholar] [CrossRef]
- Jorgenson, J.W.; Lukacs, K.D. Zone electrophoresis in open-tubular glass capillaries. Anal. Chem. 1981, 53, 1298–1302. [Google Scholar] [CrossRef]
- Hau, F.C.R.; Morrison, P.D.; Small, D.M.; Marriott, P.J. Investigation of folic acid stability in fortified instant noodles by use of capillary electrophoresis and reversed-phase high performance liquid chromatography. J. Chromatogr. A 2008, 1213, 93–99. [Google Scholar] [CrossRef]
- Wei, F.H.; Xiao, Y.C.; Luo, Q.Q.; Sun, X.G. HPCE studies of the chemical profile of Chuanxiong water extraction. J. Chin. Med. Mat. 2008, 31, 1149–1151. [Google Scholar]
- Zhang, K.; Gao, R.Y.; Jiang, Z.J.; Yao, C.Y.; Zhang, Z.C.; Wang, Q.S.; Yan, C. Pressurized capillary electrochromatography separation of peptides with strong cation exchange and hydrophilic interaction. J. Sep. Sci. 2003, 26, 1389–1394. [Google Scholar] [CrossRef]
- Lu, H.X.; Wang, J.B.; Wang, X.C.; Lin, X.C.; Wu, X.P.; Xie, Z.H. Rapid separation and determination of structurally related anthraquinones in Rhubarb by pressurized capillary electrochromatography. J. Pharm. Biomed. Anal. 2007, 43, 352–357. [Google Scholar] [CrossRef]
- Xie, G.X.; Zhao, A.H.; Li, P.; Li, L.; Jia, W. Fingerprint analysis of Rhizoma chuanxiong by pressurized capillary electrochromatography and high-performance liquid chromatography. Biomed. Chromatogr. 2007, 21, 867–875. [Google Scholar] [CrossRef]
- Gan, F.; Ye, R.Y. New approach on similarity analysis of chromatographic fingerprint of herbal medicine. J. Chromatogr. A 2006, 1104, 100–105. [Google Scholar] [CrossRef]
- Wei, G.; Cai, Q.Q.; Huang, Y.C.; Liu, C.L. Analysis on correlation of HPLC fingerprint on decoction pieces and decoction of Rhizoma Ligusticum. Chin. J. Exp. Tradit. Med. Formulae 2011, 17, 46–49. [Google Scholar]
- Sun, Y.; Guo, T.; Sui, Y.; Li, F.M. HPCE-FP methodology study of Chuanxiong. China J. Chin. Mat. Med. 2003, 28, 167–171. [Google Scholar]
- Qin, H.L.; Deng, A.J.; Du, G.H.; Wang, P.; Zhang, J.L.; Li, Z.H. Fingerprinting analysis of Rhizoma Chuanxiong of commercial types using H-1 nuclear magnetic resonance spectroscopy and high performance liquid chromatography method. J. Integr. Plant Biol. 2009, 51, 537–544. [Google Scholar] [CrossRef]
- Li, S.L.; Lin, G.; Chung, H.S.; Tamy, Y.K. Study on fingerprint of Rhizoma Chuanxiong by HPLC-DAD-MS. Acta Pharm. Sin. 2004, 39, 621–626. [Google Scholar]
- Gong, F.; Liang, Y.Z.; Xie, P.S.; Chau, F.T. Information theory applied to chromatographic fingerprint of herbal medicine for quality control. J. Chromatogr. A 2003, 1002, 25–40. [Google Scholar] [CrossRef]
- Yang, B.J.; Chen, J.H.; Lee, F.S.C.; Wang, X.R. GC-MS fingerprints for discrimination of Ligusticum chuanxiong from Angelica. J. Sep. Sci. 2008, 31, 3231–3237. [Google Scholar] [CrossRef]
- Qin, H.L.; Li, Z.H.; Wang, P.; Yang, J.R. Specific control substance analysis and its creation of the fingerprint of Huanglian. Acta Acad. Med. Sin. 2004, 26, 622–627. [Google Scholar]
- Su, D.M.; Han, J.L.; Yu, S.S.; Qin, H.L. Assessment of 1H-NMR spectroscopy for specific metabolite fingerprinting of Angelica sinensis. Nat. Prod. Commun. 2008, 3, 727–730. [Google Scholar]
- Wren, S.A. Peak capacity in gradient ultra performance liquid chromatography (UPLC). J. Pharm. Biomed. Anal. 2005, 38, 337–343. [Google Scholar] [CrossRef]
- Wang, C.; Feng, R.N.; Sun, D.J.; Li, Y.; Bi, X.X.; Sun, C.H. Metabolic profiling of urine in young obese men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC/Q-TOF MS). J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 2871–2876. [Google Scholar] [CrossRef]
- Wang, X.J.; Sun, W.J.; Sun, H.; Lv, H.T.; Wu, Z.M.; Wang, P.; Liu, L.; Cao, H.X. Analysis of the constituents in the rat plasma after oral administration of Yin Chen Hao Tang by UPLC/Q-TOF-MS/MS. J. Pharm. Biomed. Anal. 2008, 46, 477–490. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, N.; Choi, F.F.K.; Qiao, C.F.; Song, J.Z.; Li, S.L.; Liu, X.; Cai, Z.W.; Fu, P.P.; Lin, G.; et al. A new approach for simultaneous screening and quantification of toxic pyrrolizidine alkaloids in some potential pyrrolizidine alkaloid-containing plants by using ultra performance liquid chromatography-tandem quadrupole mass spectrometry. Anal. Chim. Acta 2010, 681, 33–40. [Google Scholar] [CrossRef]
- Krzyzanowska, J.; Janda, B.; Pecio, L.; Stochmal, A.; Oleszek, W.; Czubacka, A.; Przybys, M.; Doroszewska, T. Determination of polyphenols in Mentha longifolia and M. piperita field-grown and in vitro plant samples using UPLC-TQ-MS. J. AOAC Int. 2011, 94, 43–50. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Li, W.; Tang, Y.; Chen, Y.; Duan, J.-A. Advances in the Chemical Analysis and Biological Activities of Chuanxiong. Molecules 2012, 17, 10614-10651. https://doi.org/10.3390/molecules170910614
Li W, Tang Y, Chen Y, Duan J-A. Advances in the Chemical Analysis and Biological Activities of Chuanxiong. Molecules. 2012; 17(9):10614-10651. https://doi.org/10.3390/molecules170910614
Chicago/Turabian StyleLi, Weixia, Yuping Tang, Yanyan Chen, and Jin-Ao Duan. 2012. "Advances in the Chemical Analysis and Biological Activities of Chuanxiong" Molecules 17, no. 9: 10614-10651. https://doi.org/10.3390/molecules170910614
APA StyleLi, W., Tang, Y., Chen, Y., & Duan, J.-A. (2012). Advances in the Chemical Analysis and Biological Activities of Chuanxiong. Molecules, 17(9), 10614-10651. https://doi.org/10.3390/molecules170910614