Applied Biological and Physicochemical Activity of Isoquinoline Alkaloids: Oxoisoaporphine and Boldine
Abstract
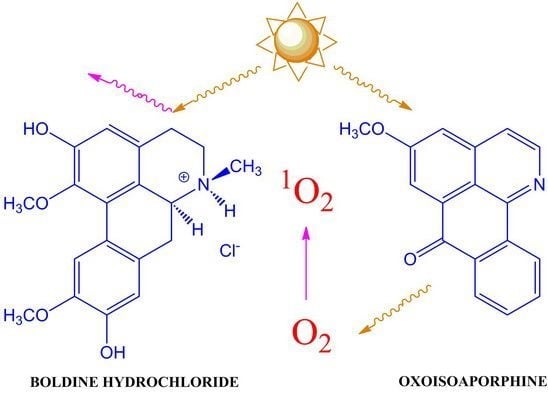
:1. Introduction

2. Results and Discussion
2.1. SPF in Vitro Test
| Irradiation time (min) | Boldine | B1 | B2 | B3 | B4 |
|---|---|---|---|---|---|
| 0 | 6.30 | 6.33 | 6.51 | 6.39 | 6.43 |
| 5 | 4.12 | 4.08 | 4.20 | 4.00 | 4.02 |
| 10 | 3.71 | 3.93 | 4.07 | 3.95 | 3.97 |
| 15 | 3.70 | 3.90 | 4.00 | 3.80 | 3.86 |
| 20 | 3.23 | 3.25 | 2.68 | 2.71 | 2.77 |
| 25 | 2.96 | 2.80 | 2.58 | 2.68 | 2.70 |
| 30 | 2.33 | 2.35 | 2.41 | 2.31 | 2.36 |
2.2. Photohemolysis
| Samples | % Hemolysis in darkness | % Hemolysis | % Photohemolysis |
|---|---|---|---|
| preirradiated | |||
| Boldine | 0.00 ± 0.00 | 0.00 ± 0.00 | 2.31 ± 2.35 × 10−3 |
| B1 | 0.00 ± 0.00 |  | 0.00 ± 0.00 |
| B2 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| B3 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
| B4 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 |
2.3. Toxicity Test: Eggs of Artemia salina
| Samples | Lethal dose 50 (LD50) | Lethal Dose 50 (LD50) Preirradiated | Lethal dose 50 (LD50) Irradiated |
|---|---|---|---|
| Boldine | 900 | 400 | 685 |
| B1 | 860 | 335 | 602 |
| B2 | 900 | 305 | 580 |
| B3 | 900 | 360 | 662 |
| B4 | 850 | 366 | 560 |
2.4. Method of Fibroblast Cell Survival

2.5. Antioxidant Capacity and Singlet Oxygen Formation
| Compounds | Concentration (μM) | AA |
|---|---|---|
| 1 | 100 | 0.35 |
| 2 | 100 | 0.40 |
| 3 | 100 | 0.35 |
| Trolox | 100 | 89.72 |
| Solvent | Polarity | Φ4 | Φ5 | Φ6 | ΦPhenal |
|---|---|---|---|---|---|
| Cyclohexane | 0.006 | 0.95 | 0.92 | ||
| Toluene | 0.099 | 1.00 | 0.95 | 1.00 | 0.95 |
| Tetrahydrofuran | 0.207 | 1.00 | 0.87 | ||
| N,N'-dimethylacetamide | 0.377 | 1.00 | 0.93 | 0.87 | |
| Acetonitrile | 0.460 | 0.98 | 1.00 | 0.98 | |
| Propylene carbonate | 0.475 | 1.00 | 1.00 | ||
| Methanol | 0.762 | 1.00 | 0.97 | 0.93 | 0.98 |
| 2,2,3.3-Tetrafluoropropanol | 0.886 | 1.00 | 1.00 |
3. Experimental
3.1. Synthesis of Boldine Derivatives
3.2. SPF in Vitro Test

3.3. Method of Fibroblast Cell Survival [23]
3.4. Photohemolysis
3.5. Toxicity Test: Eggs of Artemia saline
3.6. Synthesis of Oxozhines

3.7. Singlet Oxygen Production
3.8. Singlet Oxygen Quantum Yield
3.9. DPPH
3.10. Autooxidation of β-Carotene [28]

3.11. Statistical Analysis
4. Conclusions
Acknowledgments
References
- Masao, T.; Okamoto, Y.; Kikuchi, T.; Osaki, K.; Nishikawa, M.; Kamiya, K.; Sasaky, Y.; Matoba, K.; Goto, K. Studies on the Alkaloids of Menispermaceous Plants. CCLIX. Alkaloid of Menispermum dauricum DC. Structures of Acutumine and Acutumidine, Chlorine-Containing Alkaloids with a Novel Skeleton. Chem. Pharm. Bull. 1971, 19, 770–791. [Google Scholar]
- Kunitomo, J.; Satoh, M. A New Type of Isoquinoline Alkaloid. Chem. Pharm. Bull. 1982, 30, 2659–2660. [Google Scholar] [CrossRef]
- Zhang, X.; Ye, W.; Zhao, S.; Che, C.-T. Isoquinoline and isoindole alkaloids from Menispermum Dauricum. Phytochemistry 2004, 65, 929–932. [Google Scholar] [CrossRef]
- Yu, B.-W.; Meng, L.-H.; Chen, J.-Y.; Zhou, T.-X.; Cheng, K.-F.; Ding, J.; Qin, G.-W. Cytotoxic Oxoisoaporphine Alkaloids from Menispermum Dualism. J. Nat. Prod. 2001, 64, 968–970. [Google Scholar]
- Qian, J.-Q. Cardiovascular pharmacological effects of bisbenzylisoquinoline alkaloid derivatives. Acta Pharmacol. Sin. 2002, 23, 1086–1092. [Google Scholar]
- Wang, F.; Qu, L.; Lv, Q.; Guo, L.-J. Effect of phenolic alkaloids from Menispermum dauricum on myocardial-cerebral ischemia-reperfusion injury in rabbits. Acta Pharmacol. Sin. 2001, 22, 1130–1134. [Google Scholar]
- Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C.; Astudillo, S.L.; Feresin, G.E.; Tapia, A. Free-radical scavengers and antioxidants from Peumus boldus Mol. (“Boldo”). Free Radic. Res. 2003, 37, 447–452. [Google Scholar]
- Schmidt, R.; Tanielian, C.; Dunsbach, R.; Wolff, C. Phenalenone, a universal reference compound for the determination of quantum yields of singlet oxygen O2(1Δg) sensitization. J. Photochem. Photobiol. A 1994, 79, 11–17. [Google Scholar]
- Luis, J.G.; Grillo, T.A. New diterpenes from Salvia munzii: Chemical and biogenetic aspects. Tetrahedron 1993, 49, 6277–6284. [Google Scholar] [CrossRef]
- Cooke, R.G.; Edwards, J.M. Naturally occurring phenalenones and related compounds. Prog. Chem. Org. Nat. Prod. 1981, 40, 153–190. [Google Scholar]
- Lázaro, A.; Corominas, M.; Martí, C.; Flors, L.; Izquierdo, L.; Grillo, T.; Luis, G.; Nonell, S. Light- and singlet oxygen-mediated antifungal activity of phenylphenalenone phytoalexins. Photochem. Photobiol. Sci. 2004, 3, 706–710. [Google Scholar] [CrossRef]
- Zanocco, A.L.; Lemp, E.; Günter, G. A kinetic study of the reaction between boldine and singlet oxygen [O2(1ωg)]. J. Chem. Soc. Perkin Trans. 2 1997, 2, 1299–1302. [Google Scholar]
- Suau, R.; López-Romero, J.M.; Rico, R.; Alonso, F.J.; Lobo, C. A New Approach to the Synthesis of 4,5-Dioxoaporphine Alkaloids from Preformed Biaryl Bond Precursors. Tetrahedron 1996, 52, 11307–11320. [Google Scholar] [CrossRef]
- Speisky, H.; Cassels, B.K.; Lissi, E.A.; Videla, L.A. Antioxidant properties of the alkaloid boldine in systems undergoing lipid peroxidation and enzyme inactivation. Biochem. Pharmacol. 1991, 41, 1575–1581. [Google Scholar] [CrossRef]
- Martínez, S.; Madrero, Y.; Elorriaga, M.; Noguera, M.A.; Cassels, B.; Sobarzo, E.; D’Ocon, P.; Ivorra, M.D. Halogenated derivatives of boldine with high selectivity for α1A-adrenoceptors in rat cerebral cortex. Life Sci. 1999, 64, 1205–1214. [Google Scholar] [CrossRef]
- Hidalgo, M.E.; Farah, M.; Carrasco, L.; Fernández, E. Photostability and photoprotection factor of boldine and glaucine. J. Photochem. Photobiol. B 2005, 80, 65–69. [Google Scholar] [CrossRef]
- Hidalgo, M.; Alarcón, M.; Ojeda, J.; Fernández, E.; Sobarzo-Sánchez, E.; de la Fuente, J. Spectroscopic and Photochemical Properties of some Annulated Boldine Derivatives. J. Braz. Chem. Soc. 2010, 21, 2205–2210. [Google Scholar]
- Winterbourn, C. Oxidative reactions of haemoglobin. Methods Enzymol. 1990, 186, 265–272. [Google Scholar] [CrossRef]
- McLaughlin, J.L.; Chang, C.-J.; Smith, D.L. Bench Top Bioassays for the Discovery of Bioactive Natural Products: An Update. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 1991; Volume 9, pp. 388–409. [Google Scholar]
- Hidalgo, M.E.; González, I.; Toro, F.; Fernández, E.; Speisky, H.; Jiménez, I. Boldine as a Sunscreen. Cosmet. Toiletries 1998, 113, 59–66. [Google Scholar]
- Sobarzo-Sánchez, E.; Jullian, C.; Cassels, B.K.; Saitz-Barría, C. New Heterocyclic skeletons derived from the aporphine alkaloid boldine. Synth. Commun. 2002, 32, 3687–3693. [Google Scholar] [CrossRef]
- Sobarzo-Sánchez, E.; Julian, C.; Cassels, K.B.; Saitz, C. Oxazine- and oxazole-fused derivatives of the alkaloid boldine and their complete structural and spectral assignments by HMQC and HMBC experiments. Magn. Reson. Chem. 2001, 39, 361–366. [Google Scholar]
- Meybeck, A. Objetive methods for the evaluation of sunscreens. Cosmet. Toiletries 1983, 98, 51–60. [Google Scholar]
- Tobella, L.; Cabrera, S.; Moreno, R. Efectos de la radiación UVA y UVB sobre linfocitos humanos. Rev. Med. Chile 1994, 122, 861–872. [Google Scholar]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolics phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- De la Fuente, J.; Jullian, C.; Saitz, C.; Sobarzo-Sánchez, E.; Neira, V.; González, C.; López, R.; Pessoa-Mahana, H. Photoreduction of oxoisoaporphines. Another example of a formal hydride-transfer mechanism. Photochem. Photobiol. Sci. 2004, 3, 194–199. [Google Scholar]
- Lee, M.; Wang, Z.Y.; Li, H.; Chen, L.; Sun, Y.; Gobbo, S.; Balentine, D.A.; Yang, C.S. Analysis of plasma and urinary tea polyphenols in human subjects. Cancer Epidemiol. Biomarkers Prev. 1995, 4, 393–399. [Google Scholar]
- Blake, J.; Pratt, D.; Lin, S. Thermolyses of O-phenyl oxime ethers. A new source of iminyl radicals and a new source of aryloxyl radicals. J. Org. Chem. 2004, 69, 3112–3120. [Google Scholar]
- Baiano, A.; Gambacorta, G.; Terracone, C.; Previtali, M.A.; Lamacchia, C.; la Notte, E. Changes in Phenolic Content and Antioxidant Activity of Italian Extra-Virgin Olive Oils during Storage. J. Food Sci. 2009, 74, 177–183. [Google Scholar]
- Sample Availability: Contact the authors.
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sobarzo-Sánchez, E.; Soto, P.G.; Valdés Rivera, C.; Sánchez, G.; Hidalgo, M.E. Applied Biological and Physicochemical Activity of Isoquinoline Alkaloids: Oxoisoaporphine and Boldine. Molecules 2012, 17, 10958-10970. https://doi.org/10.3390/molecules170910958
Sobarzo-Sánchez E, Soto PG, Valdés Rivera C, Sánchez G, Hidalgo ME. Applied Biological and Physicochemical Activity of Isoquinoline Alkaloids: Oxoisoaporphine and Boldine. Molecules. 2012; 17(9):10958-10970. https://doi.org/10.3390/molecules170910958
Chicago/Turabian StyleSobarzo-Sánchez, Eduardo, Patricio González Soto, Cristóbal Valdés Rivera, Georgina Sánchez, and María Eliana Hidalgo. 2012. "Applied Biological and Physicochemical Activity of Isoquinoline Alkaloids: Oxoisoaporphine and Boldine" Molecules 17, no. 9: 10958-10970. https://doi.org/10.3390/molecules170910958
APA StyleSobarzo-Sánchez, E., Soto, P. G., Valdés Rivera, C., Sánchez, G., & Hidalgo, M. E. (2012). Applied Biological and Physicochemical Activity of Isoquinoline Alkaloids: Oxoisoaporphine and Boldine. Molecules, 17(9), 10958-10970. https://doi.org/10.3390/molecules170910958