Curcumin Induces Cell Death and Restores Tamoxifen Sensitivity in the Antiestrogen-Resistant Breast Cancer Cell Lines MCF-7/LCC2 and MCF-7/LCC9
Abstract
:1. Introduction
2. Results and Discussion
2.1. Results
2.1.1. Curcumin Suppresses Proliferation of MCF-7/LCC2 and MCF-7/LCC9

2.1.2. Curcumin Inhibits the Colony-Forming Ability of MCF-7/LCC2 and MCF-7/LCC9
2.1.3. Curcumin Induces Apoptosis in MCF-7/LCC2 and MCF-7/LCC9

2.1.4. Curcumin Induces G2/M Phase Arrest in MCF-7/LCC2 and MCF-7/LCC9
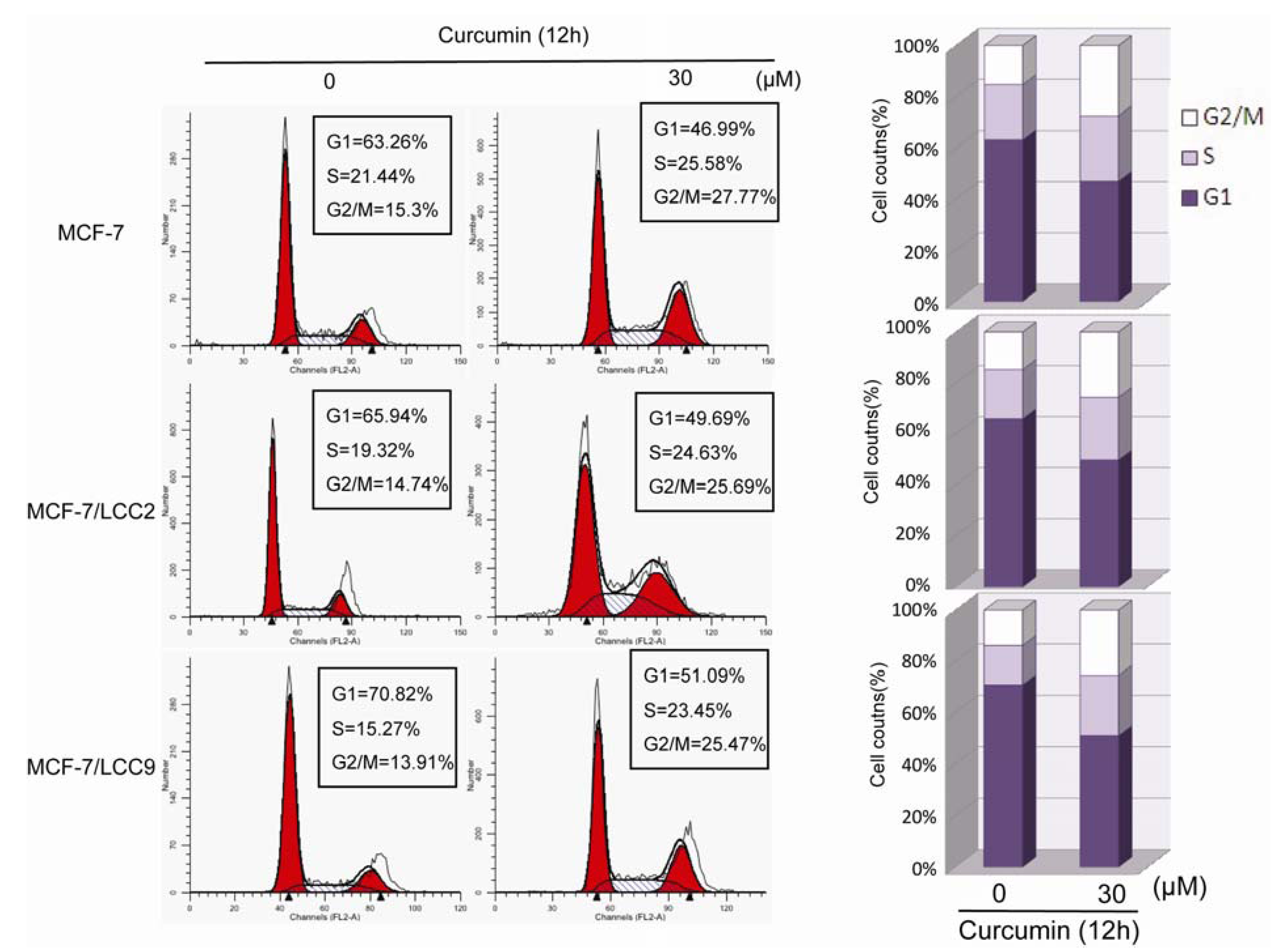
2.1.5. Curcumin Sensitizes Tamoxifen Action in MCF-7 and Reverses Tamoxifen Resistance in MCF-7/LCC2 and MCF-7/LCC9

2.1.6. Curcumin Suppresses the Protein and mRNA Expression of Cell Signals Associated with Proliferation in MCF-7/LCC2 and MCF-7/LCC9
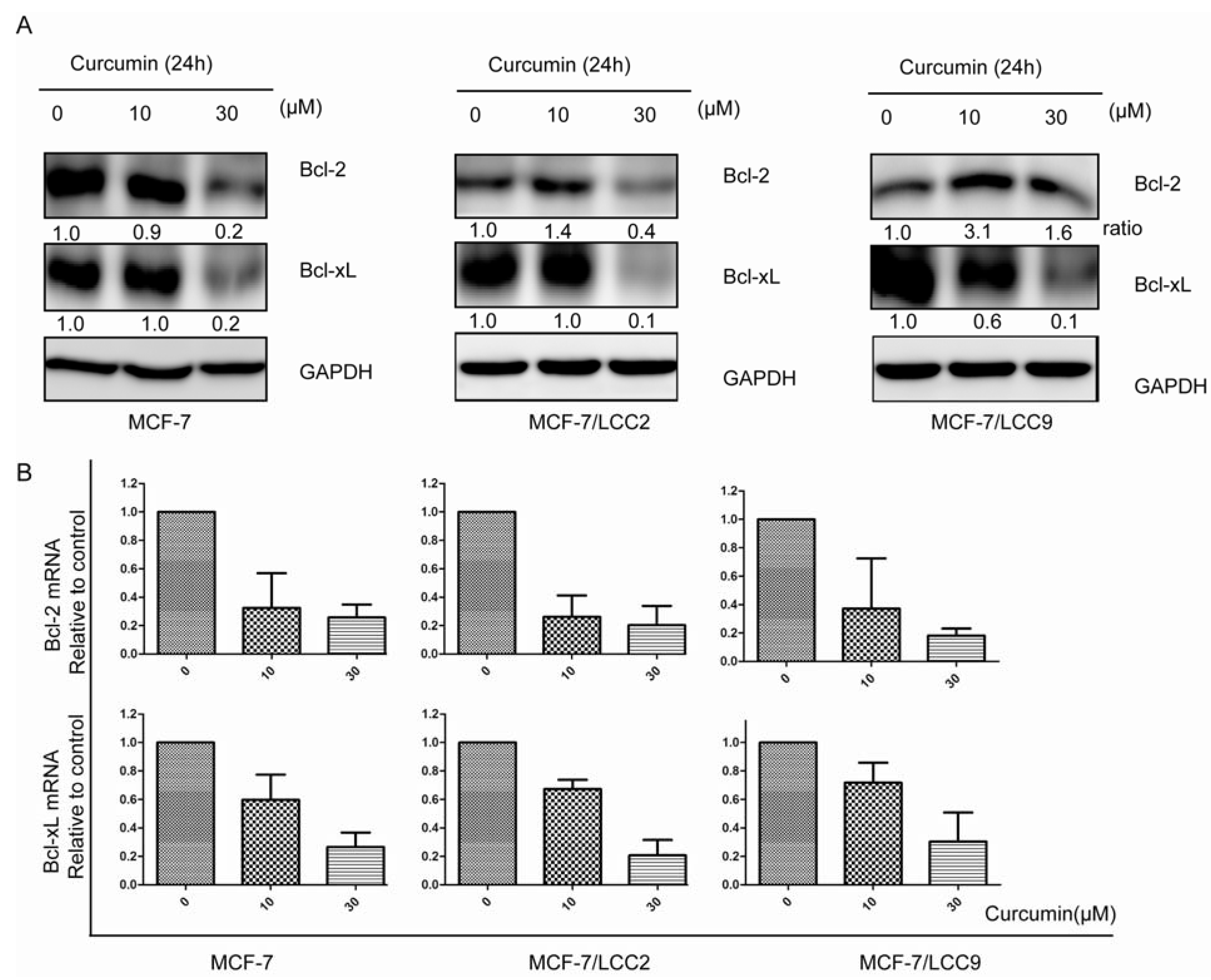
2.1.7. Curcumin Downregulates the Protein and mRNA Expression of Cell Survival Signals in MCF-7/LCC2 and MCF-7/LCC9

2.1.8. Curcumin Inhibits IKK-NF-κB Signaling Pathway in MCF-7/LCC2 and MCF-7/LCC9

2.1.9. Curcumin Inhibits Src Activation and Downregulates FAK Expression in MCF-7/LCC2 and MCF-7/LCC9
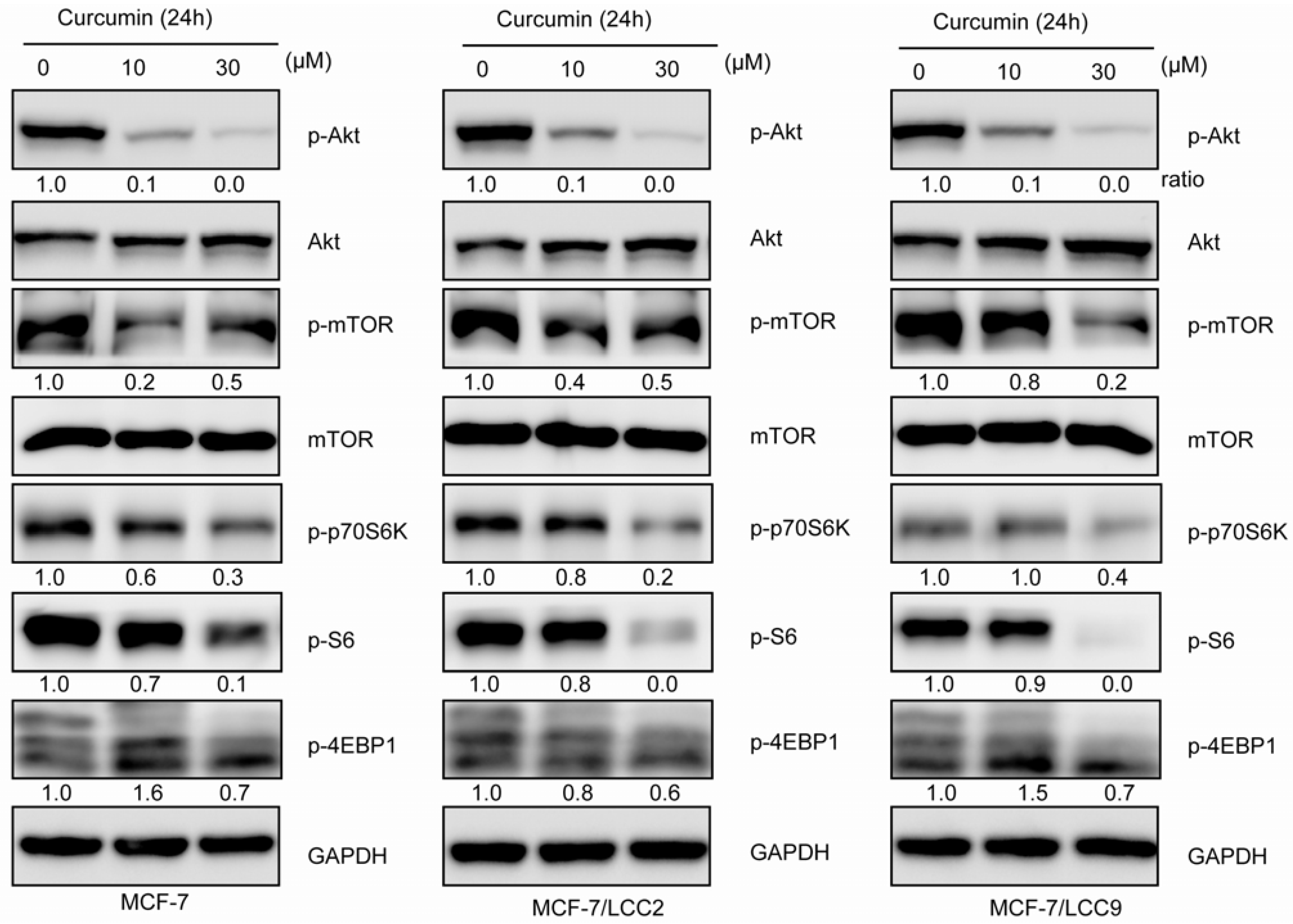
2.1.10. Curcumin Inhibits Akt/mTOR Signaling in MCF-7/LCC2 and MCF-7/LCC9

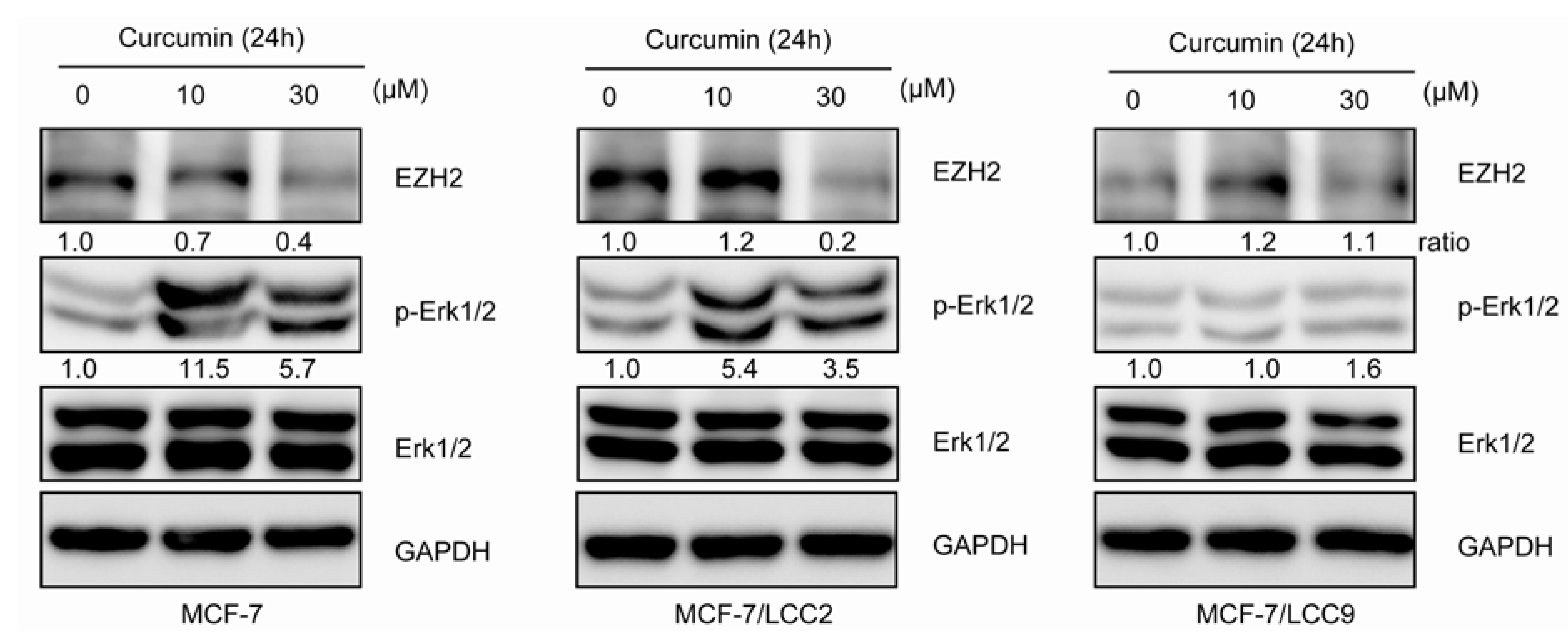
2.1.11. Curcumin Suppresses the Expression of EZH2 and Induces ERK Activation in MCF-7/LCC2
2.2. Discussion
3. Experimental
3.1. Cell Culture and Chemicals
3.2. Sulforhodamine B (SRB) Assay
3.3. Drug Treatment Assay
3.4. Colony Formation Assay
3.5. Cell Cycle Analysis
3.6. Apoptosis Analysis
3.7. Reverse Transcription-Polymerase Chain Reaction Assay
3.8. Western Blot Analysis and Antibodies
3.9. Statistics
4. Conclusions
Supplementary Files
Acknowledgments
Conflicts of Interest
- Sample Availability: Not available.
References
- Siegel, R.; Naishadham, D.; Jemal, A. Cancer statistics, 2012. CA Cancer J. Clin. 2012, 62, 10–29. [Google Scholar] [CrossRef]
- Sorlie, T.; Perou, C.M.; Tibshirani, R.; Aas, T.; Geisler, S.; Johnsen, H.; Hastie, T.; Eisen, M.B.; van de Rijn, M.; Jeffrey, S.S.; et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl. Acad. Sci. USA 2001, 98, 10869–10874. [Google Scholar]
- Anderson, W.F.; Chatterjee, N.; Ershler, W.B.; Brawley, O.W. Estrogen receptor breast cancer phenotypes in the Surveillance, Epidemiology, and End Results database. Breast Cancer Res. Treat. 2002, 76, 27–36. [Google Scholar] [CrossRef]
- Masuda, N.; Sagara, Y.; Kinoshita, T.; Iwata, H.; Nakamura, S.; Yanagita, Y.; Nishimura, R.; Iwase, H.; Kamigaki, S.; Takei, H.; et al. Neoadjuvant anastrozole versus tamoxifen in patients receiving goserelin for premenopausal breast cancer (STAGE): A double-blind, randomised phase 3 trial. Lancet Oncol. 2012, 13, 345–352. [Google Scholar] [CrossRef]
- Ali, S.; Coombes, R.C. Endocrine-responsive breast cancer and strategies for combating resistance. Nat. Rev. Cancer 2002, 2, 101–112. [Google Scholar] [CrossRef]
- Ring, A.; Dowsett, M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer 2004, 11, 643–658. [Google Scholar] [CrossRef]
- Musgrove, E.A.; Sutherland, R.L. Biological determinants of endocrine resistance in breast cancer. Nat. Rev. Cancer 2009, 9, 631–643. [Google Scholar] [CrossRef]
- Yde, C.W.; Emdal, K.B.; Guerra, B.; Lykkesfeldt, A.E. NFkappaB signaling is important for growth of antiestrogen resistant breast cancer cells. Breast Cancer Res. Treat. 2012, 135, 67–78. [Google Scholar] [CrossRef]
- Schiff, R.; Reddy, P.; Ahotupa, M.; Coronado-Heinsohn, E.; Grim, M.; Hilsenbeck, S.G.; Lawrence, R.; Deneke, S.; Herrera, R.; Chamness, G.C.; et al. Oxidative stress and AP-1 activity in tamoxifen-resistant breast tumors in vivo. J. Natl. Cancer Inst. 2000, 92, 1926–1934. [Google Scholar] [CrossRef]
- Vallabhaneni, S.; Nair, B.C.; Cortez, V.; Challa, R.; Chakravarty, D.; Tekmal, R.R.; Vadlamudi, R.K. Significance of ER-Src axis in hormonal therapy resistance. Breast Cancer Res. Treat. 2011, 130, 377–385. [Google Scholar] [CrossRef]
- Steelman, L.S.; Navolanic, P.; Chappell, W.H.; Abrams, S.L.; Wong, E.W.; Martelli, A.M.; Cocco, L.; Stivala, F.; Libra, M.; Nicoletti, F.; et al. Involvement of Akt and mTOR in chemotherapeutic- and hormonal-based drug resistance and response to radiation in breast cancer cells. Cell Cycle 2011, 10, 3003–3015. [Google Scholar] [CrossRef]
- Butt, A.J.; McNeil, C.M.; Musgrove, E.A.; Sutherland, R.L. Downstream targets of growth factor and oestrogen signalling and endocrine resistance: the potential roles of c-Myc, cyclin D1 and cyclin E. Endocr. Relat. Cancer 2005, 12, S47–S59. [Google Scholar] [CrossRef]
- Abukhdeir, A.M.; Vitolo, M.I.; Argani, P.; de Marzo, A.M.; Karakas, B.; Konishi, H.; Gustin, J.P.; Lauring, J.; Garay, J.P.; Pendleton, C.; et al. Tamoxifen-stimulated growth of breast cancer due to p21 loss. Proc. Natl. Acad. Sci. USA 2008, 105, 288–293. [Google Scholar]
- Larsen, M.S.; Bjerre, K.; Giobbie-Hurder, A.; Laenkholm, A.V.; Henriksen, K.L.; Ejlertsen, B.; Lykkesfeldt, A.E.; Rasmussen, B.B. Prognostic value of Bcl-2 in two independent populations of estrogen receptor positive breast cancer patients treated with adjuvant endocrine therapy. Acta Oncol. 2012, 51, 781–789. [Google Scholar] [CrossRef]
- Arpino, G.; Wiechmann, L.; Osborne, C.K.; Schiff, R. Crosstalk between the estrogen receptor and the HER tyrosine kinase receptor family: Molecular mechanism and clinical implications for endocrine therapy resistance. Endocr. Rev. 2008, 29, 217–233. [Google Scholar]
- Creighton, C.J.; Casa, A.; Lazard, Z.; Huang, S.; Tsimelzon, A.; Hilsenbeck, S.G.; Osborne, C.K.; Lee, A.V. Insulin-like growth factor-I activates gene transcription programs strongly associated with poor breast cancer prognosis. J. Clin. Oncol. 2008, 26, 4078–4085. [Google Scholar] [CrossRef]
- Leary, A.F.; Sirohi, B.; Johnston, S.R. Clinical trials update: Endocrine and biological therapy combinations in the treatment of breast cancer. Breast Cancer Res. 2007, 9. [Google Scholar] [CrossRef]
- Lai, H.W.; Chien, S.Y.; Kuo, S.J.; Tseng, L.M.; Lin, H.Y.; Chi, C.W.; Chen, D.R. The potential utility of curcumin in the treatment of HER-2-overexpressed breast cancer: An in vitro and in vivo comparison study with herceptin. Evid. Based Complement. Alternat. Med. 2012, 2012. [Google Scholar] [CrossRef]
- Zhou, Q.M.; Wang, X.F.; Liu, X.J.; Zhang, H.; Lu, Y.Y.; Huang, S.; Su, S.B. Curcumin improves MMC-based chemotherapy by simultaneously sensitising cancer cells to MMC and reducing MMC-associated side-effects. Eur. J. Cancer 2011, 47, 2240–2247. [Google Scholar]
- Sen, G.S.; Mohanty, S.; Hossain, D.M.; Bhattacharyya, S.; Banerjee, S.; Chakraborty, J.; Saha, S.; Ray, P.; Bhattacharjee, P.; Mandal, D.; et al. Curcumin enhances the efficacy of chemotherapy by tailoring p65NFkappaB-p300 cross-talk in favor of p53-p300 in breast cancer. J. Biol. Chem. 2011, 286, 42232–42247. [Google Scholar]
- Nagaraju, G.P.; Aliya, S.; Zafar, S.F.; Basha, R.; Diaz, R.; El-Rayes, B.F. The impact of curcumin on breast cancer. Integr. Biol. (Camb) 2012, 4, 996–1007. [Google Scholar]
- Zhou, Y.; Eppenberger-Castori, S.; Eppenberger, U.; Benz, C.C. The NFkappaB pathway and endocrine-resistant breast cancer. Endocr. Relat. Cancer 2005, 12, S37–S46. [Google Scholar] [CrossRef]
- Riggins, R.B.; Zwart, A.; Nehra, R.; Clarke, R. The nuclear factor kappa B inhibitor parthenolide restores ICI 182,780 (Faslodex; fulvestrant)-induced apoptosis in antiestrogen-resistant breast cancer cells. Mol. Cancer Ther. 2005, 4, 33–41. [Google Scholar]
- Perkins, N.D. The diverse and complex roles of NF-kappaB subunits in cancer. Nat. Rev. Cancer 2012, 12, 121–132. [Google Scholar]
- Bachmeier, B.E.; Mohrenz, I.V.; Mirisola, V.; Schleicher, E.; Romeo, F.; Hohneke, C.; Jochum, M.; Nerlich, A.G.; Pfeffer, U. Curcumin downregulates the inflammatory cytokines CXCL1 and -2 in breast cancer cells via NFkappaB. Carcinogenesis 2008, 29, 779–789. [Google Scholar]
- Kim, S.R.; Park, H.J.; Bae, Y.H.; Ahn, S.C.; Wee, H.J.; Yun, I.; Jang, H.O.; Bae, M.K.; Bae, S.K. Curcumin down-regulates visfatin expression and inhibits breast cancer cell invasion. Endocrinology 2012, 153, 554–563. [Google Scholar] [CrossRef]
- Morgan, L.; Gee, J.; Pumford, S.; Farrow, L.; Finlay, P.; Robertson, J.; Ellis, I.; Kawakatsu, H.; Nicholson, R.; Hiscox, S. Elevated Src kinase activity attenuates Tamoxifen response in vitro and is associated with poor prognosis clinically. Cancer Biol. Ther. 2009, 8, 1550–1558. [Google Scholar] [CrossRef]
- Chen, Y.; Alvarez, E.A.; Azzam, D.; Wander, S.A.; Guggisberg, N.; Jorda, M.; Ju, Z.; Hennessy, B.T.; Slingerland, J.M. Combined Src and ER blockade impairs human breast cancer proliferation in vitro and in vivo. Breast Cancer Res. Treat. 2011, 128, 69–78. [Google Scholar] [CrossRef]
- Lin, H.J.; Su, C.C.; Lu, H.F.; Yang, J.S.; Hsu, S.C.; Ip, S.W.; Wu, J.J.; Li, Y.C.; Ho, C.C.; Wu, C.C.; et al. Curcumin blocks migration and invasion of mouse-rat hybrid retina ganglion cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and Rock-1 gene expression. Oncol. Rep. 2010, 23, 665–670. [Google Scholar]
- Hiscox, S.; Jordan, N.J.; Smith, C.; James, M.; Morgan, L.; Taylor, K.M.; Green, T.P.; Nicholson, R.I. Dual targeting of Src and ER prevents acquired antihormone resistance in breast cancer cells. Breast Cancer Res. Treat. 2009, 115, 57–67. [Google Scholar] [CrossRef]
- Saini, S.; Arora, S.; Majid, S.; Shahryari, V.; Chen, Y.; Deng, G.; Yamamura, S.; Ueno, K.; Dahiya, R. Curcumin modulates microRNA-203-mediated regulation of the Src-Akt axis in bladder cancer. Cancer Prev Res. (Phila) 2011, 4, 1698–1709. [Google Scholar]
- Tokunaga, E.; Kimura, Y.; Mashino, K.; Oki, E.; Kataoka, A.; Ohno, S.; Morita, M.; Kakeji, Y.; Baba, H.; Maehara, Y. Activation of PI3K/Akt signaling and hormone resistance in breast cancer. Breast Cancer 2006, 13, 137–144. [Google Scholar] [CrossRef]
- Leung, E.; Kim, J.E.; Rewcastle, G.W.; Finlay, G.J.; Baguley, B.C. Comparison of the effects of the PI3K/mTOR inhibitors NVP-BEZ235 and GSK2126458 on tamoxifen-resistant breast cancer cells. Cancer Biol. Ther. 2011, 11, 938–946. [Google Scholar]
- Block, M.; Grundker, C.; Fister, S.; Kubin, J.; Wilkens, L.; Mueller, M.D.; Hemmerlein, B.; Emons, G.; Gunthert, A.R. Inhibition of the AKT/mTOR and erbB pathways by gefitinib, perifosine and analogs of gonadotropin-releasing hormone I and II to overcome tamoxifen resistance in breast cancer cells. Int. J. Oncol. 2012, 41, 1845–1854. [Google Scholar]
- Clark, C.A.; McEachern, M.D.; Shah, S.H.; Rong, Y.; Rong, X.; Smelley, C.L.; Caldito, G.C.; Abreo, F.W.; Nathan, C.O. Curcumin inhibits carcinogen and nicotine-induced Mammalian target of rapamycin pathway activation in head and neck squamous cell carcinoma. Cancer Prev. Res. (Phila) 2010, 3, 1586–1595. [Google Scholar]
- Johnson, S.M.; Gulhati, P.; Arrieta, I.; Wang, X.; Uchida, T.; Gao, T.; Evers, B.M. Curcumin inhibits proliferation of colorectal carcinoma by modulating Akt/mTOR signaling. Anticancer Res. 2009, 29, 3185–3190. [Google Scholar]
- Yu, S.; Shen, G.; Khor, T.O.; Kim, J.H.; Kong, A.N. Curcumin inhibits Akt/mammalian target of rapamycin signaling through protein phosphatase-dependent mechanism. Mol. Cancer Ther. 2008, 7, 2609–2620. [Google Scholar] [CrossRef]
- Cao, R.; Wang, L.; Wang, H.; Xia, L.; Erdjument-Bromage, H.; Tempst, P.; Jones, R.S.; Zhang, Y. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science 2002, 298, 1039–1043. [Google Scholar] [CrossRef]
- Granit, R.Z.; Gabai, Y.; Hadar, T.; Karamansha, Y.; Liberman, L.; Waldhorn, I.; Gat-Viks, I.; Regev, A.; Maly, B.; Darash-Yahana, M.; et al. EZH2 promotes a bi-lineage identity in basal-like breast cancer cells. Oncogene 2012. [Google Scholar] [CrossRef]
- Holm, K.; Grabau, D.; Lovgren, K.; Aradottir, S.; Gruvberger-Saal, S.; Howlin, J.; Saal, L.H.; Ethier, S.P.; Bendahl, P.O.; Stal, O.; et al. Global H3K27 trimethylation and EZH2 abundance in breast tumor subtypes. Mol. Oncol. 2012, 6, 494–506. [Google Scholar] [CrossRef]
- Jansen, M.P.; Reijm, E.A.; Sieuwerts, A.M.; Ruigrok-Ritstier, K.; Look, M.P.; Rodriguez-Gonzalez, F.G.; Heine, A.A.; Martens, J.W.; Sleijfer, S.; Foekens, J.A.; et al. High miR-26a and low CDC2 levels associate with decreased EZH2 expression and with favorable outcome on tamoxifen in metastatic breast cancer. Breast Cancer Res. Treat. 2012, 133, 937–947. [Google Scholar] [CrossRef] [Green Version]
- Reijm, E.A.; Jansen, M.P.; Ruigrok-Ritstier, K.; van Staveren, I.L.; Look, M.P.; van Gelder, M.E.; Sieuwerts, A.M.; Sleijfer, S.; Foekens, J.A.; Berns, E.M. Decreased expression of EZH2 is associated with upregulation of ER and favorable outcome to tamoxifen in advanced breast cancer. Breast Cancer Res. Treat. 2011, 125, 387–394. [Google Scholar] [CrossRef]
- Hua, W.F.; Fu, Y.S.; Liao, Y.J.; Xia, W.J.; Chen, Y.C.; Zeng, Y.X.; Kung, H.F.; Xie, D. Curcumin induces down-regulation of EZH2 expression through the MAPK pathway in MDA-MB-435 human breast cancer cells. Eur. J. Pharmacol. 2010, 637, 16–21. [Google Scholar] [CrossRef]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [Google Scholar] [CrossRef]
- Chen, Y.R.; Tan, T.H. Inhibition of the c-Jun N-terminal kinase (JNK) signaling pathway by curcumin. Oncogene 1998, 17, 173–178. [Google Scholar]
- Brunner, N.; Frandsen, T.L.; Holst-Hansen, C.; Bei, M.; Thompson, E.W.; Wakeling, A.E.; Lippman, M.E.; Clarke, R. MCF7/LCC2: A 4-hydroxytamoxifen resistant human breast cancer variant that retains sensitivity to the steroidal antiestrogen ICI 182,780. Cancer Res. 1993, 53, 3229–3232. [Google Scholar]
- Brunner, N.; Boysen, B.; Jirus, S.; Skaar, T.C.; Holst-Hansen, C.; Lippman, J.; Frandsen, T.; Spang-Thomsen, M.; Fuqua, S.A.; Clarke, R. MCF7/LCC9: An antiestrogen-resistant MCF-7 variant in which acquired resistance to the steroidal antiestrogen ICI 182,780 confers an early cross-resistance to the nonsteroidal antiestrogen tamoxifen. Cancer Res. 1997, 57, 3486–3493. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Jiang, M.; Huang, O.; Zhang, X.; Xie, Z.; Shen, A.; Liu, H.; Geng, M.; Shen, K. Curcumin Induces Cell Death and Restores Tamoxifen Sensitivity in the Antiestrogen-Resistant Breast Cancer Cell Lines MCF-7/LCC2 and MCF-7/LCC9. Molecules 2013, 18, 701-720. https://doi.org/10.3390/molecules18010701
Jiang M, Huang O, Zhang X, Xie Z, Shen A, Liu H, Geng M, Shen K. Curcumin Induces Cell Death and Restores Tamoxifen Sensitivity in the Antiestrogen-Resistant Breast Cancer Cell Lines MCF-7/LCC2 and MCF-7/LCC9. Molecules. 2013; 18(1):701-720. https://doi.org/10.3390/molecules18010701
Chicago/Turabian StyleJiang, Min, Ou Huang, Xi Zhang, Zuoquan Xie, Aijun Shen, Hongchun Liu, Meiyu Geng, and Kunwei Shen. 2013. "Curcumin Induces Cell Death and Restores Tamoxifen Sensitivity in the Antiestrogen-Resistant Breast Cancer Cell Lines MCF-7/LCC2 and MCF-7/LCC9" Molecules 18, no. 1: 701-720. https://doi.org/10.3390/molecules18010701
APA StyleJiang, M., Huang, O., Zhang, X., Xie, Z., Shen, A., Liu, H., Geng, M., & Shen, K. (2013). Curcumin Induces Cell Death and Restores Tamoxifen Sensitivity in the Antiestrogen-Resistant Breast Cancer Cell Lines MCF-7/LCC2 and MCF-7/LCC9. Molecules, 18(1), 701-720. https://doi.org/10.3390/molecules18010701



