Additional Minor Diterpene Glycosides from Stevia rebaudiana Bertoni
Abstract
:1. Introduction
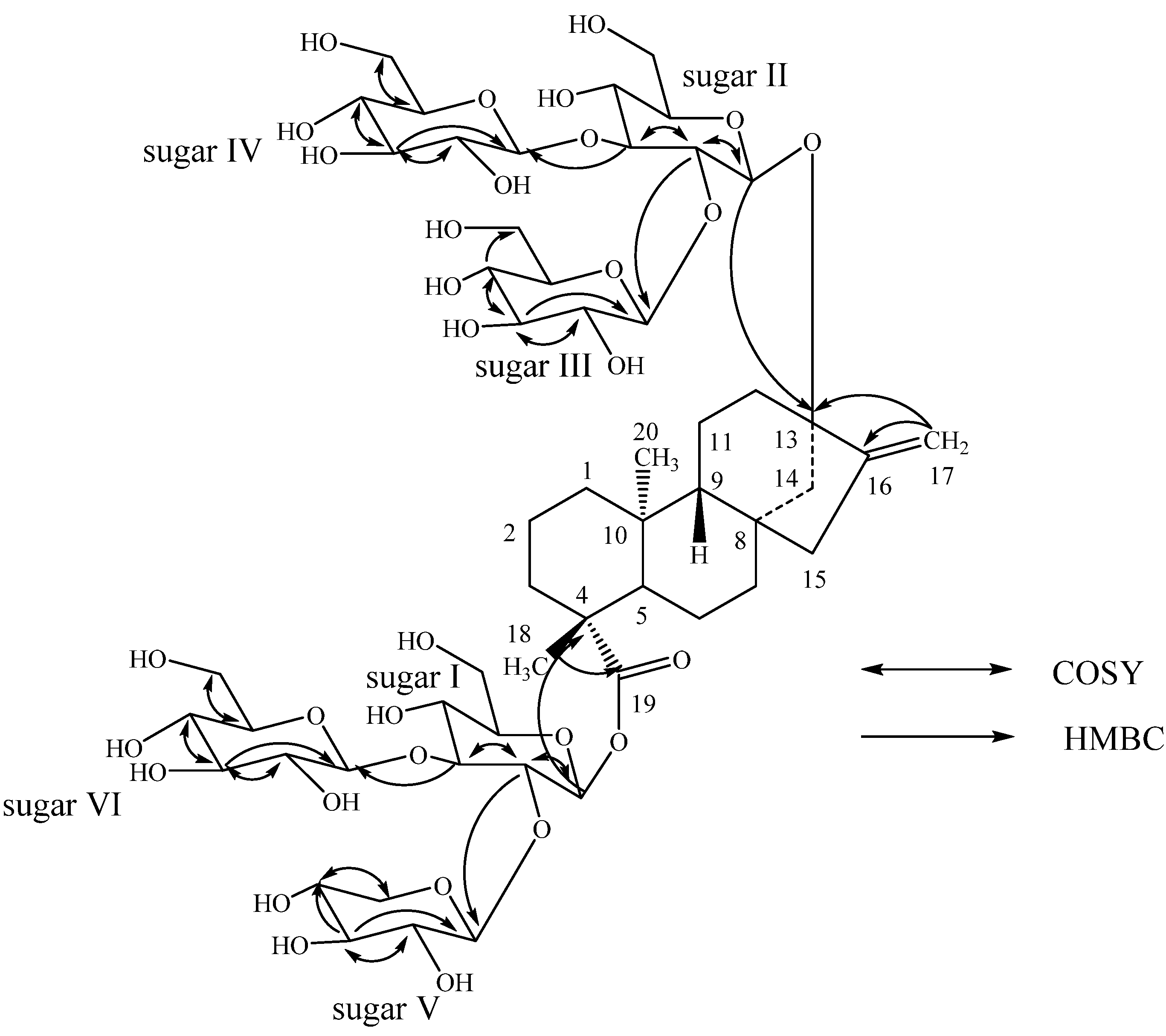
2. Results and Discussion

| Position | 1 | 2 | ||
|---|---|---|---|---|
| 1H-NMR | 13C-NMR | 1H-NMR | 13C-NMR | |
| 1 | 0.75 br t (13.2), 1.76 m | 40.0 | 0.77 br t (12.3), 1.79 m | 39.9 |
| 2 | 1.35 m, 2.23 m | 19.4 | 1.37 m, 2.28 m | 19.3 |
| 3 | 1.01 m, 2.29 m | 38.0 | 1.02 m, 2.29 m | 38.1 |
| 4 | − | 43.9 | − | 44.0 |
| 5 | 1.02 br d (13.0) | 57.0 | 1.06 br d (12.9) | 57.0 |
| 6 | 2.07 m, 2.42 q (13.5) | 23.1 | 2.24 m, 2.41 m | 23.2 |
| 7 | 1.37 m, 1.73 m | 42.2 | 1.42 m, 1.81 m | 42.3 |
| 8 | − | 39.2 | − | 39.7 |
| 9 | 0.90 br d (8.1) | 54.0 | 0.92 br d (7.7) | 54.0 |
| 10 | − | 41.3 | − | 41.4 |
| 11 | 1.65 m, 1.75 m | 19.9 | 1.67 m, 1.76 m | 19.8 |
| 12 | 1.86 m, 2.70 m | 38.2 | 1.81 m, 2.74 m | 38.1 |
| 13 | − | 87.5 | − | 87.4 |
| 14 | 2.01 m, 2.72 m | 42.9 | 2.01 m, 2.75 m | 42.9 |
| 15 | 1.88 d (17.0), 2.04 m | 46.3 | 1.88 m, 2.05 m | 46.1 |
| 16 | − | 153.9 | − | 153.7 |
| 17 | 4.89 s, 5.69 s | 104.6 | 4.89 s, 5.72 s | 104.5 |
| 18 | 1.29 s | 27.9 | 1.32 s | 27.9 |
| 19 | − | 176.7 | − | 177.0 |
| 20 | 1.36 s | 16.4 | 1.38 s | 16.6 |
| 1' | 6.38 d (8.4) | 94.4 | 6.41 d (8.2) | 94.5 |
| 2' | 4.38 m | 77.1 | 4.52 t (8.7) | 76.5 |
| 3' | 5.04 m | 88.2 | 5.14 t (8.7) | 88.2 |
| 4' | 4.24 m | 69.8 | 4.20 m | 69.7 |
| 5' | 4.14 m | 78.3 | 4.14 m | 78.0 |
| 6' | 4.20 m 4.33 m | 61.7 | 4.20 m, 4.30 m | 61.7 |
| 1'' | 5.45 d (7.8) | 96.0 | 5.49 d (7.9) | 95.9 |
| 2'' | 4.13 m | 81.0 | 4.08 m | 81.1 |
| 3'' | 4.98 t (9.1) | 87.6 | 5.01 m | 87.7 |
| 4'' | 4.08 t (9.1) | 70.1 | 4.09 m | 70.3 |
| 5'' | 3.95 m | 77.4 | 3.96 m | 77.4 |
| 6'' | 4.21 m, 4.35 m | 62.4 | 4.20 m, 4.32 m | 62.2 |
| 1''' | 5.48 d (7.9) | 104.5 | 5.39 d (8.0) | 104.3 |
| 2''' | 4.16 m | 75.3 | 4.14 m | 75.5 |
| 3''' | 4.13 m | 78.2 | 4.04 m | 78.1 |
| 4''' | 3.99 m | 72.9 | 3.69 t (8.9) | 76.7 |
| 5''' | 3.75 ddd (3.1, 6.5, 9.7) | 77.3 | 3.47 dq (6.0, 8.9) | 72.4 |
| 6''' | 4.28 m, 4.51 dd (1.1, 11.6) | 63.6 | 1.63 d (6.1) | 18.3 |
| 1'''' | 5.46 d (7.5) | 103.8 | 5.48 d (7.9) | 103.5 |
| 2'''' | 3.98 m | 75.2 | 4.00 m | 75.2 |
| 3'''' | 4.47 t (8.6) | 77.6 | 4.55 t (9.2) | 77.4 |
| 4'''' | 4.14 m | 70.9 | 4.18 m | 71.0 |
| 5'''' | 3.99 m | 77.7 | 4.01 m | 77.6 |
| 6'''' | 4.20 m, 4.33 m | 61.7 | 4.20 m, 4.30 m | 61.7 |
| 1''''' | 5.62 d (7.8) | 104.7 | 5.81 d (7.5) | 103.9 |
| 2''''' | 4.17 m | 75.2 | 4.25 m | 75.0 |
| 3''''' | 4.12 m | 78.3 | 4.18 m | 78.0 |
| 4''''' | 4.32 m | 71.3 | 4.10 m | 73.3 |
| 5''''' | 3.54 t (11.0), 4.32 m | 66.6 | 3.90 ddd (3.3, 6.8, 9.3) | 77.4 |
| 6''''' | − | − | 4.32 m, 4.66 d (11.3) | 63.6 |
| 1'''''' | 5.33 d (8.1) | 103.9 | 5.30 d (8.0) | 103.9 |
| 2'''''' | 3.97 m | 75.2 | 3.95 m | 75.1 |
| 3'''''' | 4.38 m | 77.5 | 4.33 m | 77.6 |
| 4'''''' | 4.11 m | 70.9 | 4.09 m | 70.6 |
| 5'''''' | 3.87 m | 77.8 | 3.82 m | 77.7 |
| 6'''''' | 4.10 m, 4.31 m | 61.8 | 4.10 m, 4.32 m | 61.7 |

3. Experimental
3.1. General
3.2. Plant Material
3.3. Isolation

4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Mosettig, E.; Nes, W.R. Stevioside. II. The structure of the aglucon. J. Org. Chem. 1955, 20, 884–899. [Google Scholar] [CrossRef]
- Mosettig, E.; Beglinger, U.; Dolder, F.; Lichiti, H.; Quitt, P.; Waters, J.A. The absolute configuration of steviol and isosteviol. J. Am. Chem. Soc. 1963, 85, 2305–2309. [Google Scholar] [CrossRef]
- Brandle, J.E.; Starrratt, A.N.; Gijen, M. Stevia rebaudiana: Its agricultural, biological and chemical properties. Can. J. Plant Sci. 1998, 78, 527–536. [Google Scholar] [CrossRef]
- Prakash, I.; Chaturvedula, V.S.P.; Markosyan, A. Isolation, characterization and sensory evaluation of a hexa β-D-glucopyranosyl diterpene from Stevia rebaudiana. Nat. Prod. Commun. 2013, in press. [Google Scholar]
- Chaturvedula, V.S.P.; Prakash, I. A new diterpenoid glycoside from Stevia rebaudiana. Molecules 2011, 16, 2937–2943. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Mani, U.; Prakash, I. Diterpene glycosides from Stevia rebaudiana. Molecules 2011, 16, 3552–3562. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Structures of the novel diterpene glycosides from Stevia rebaudiana. Carbohydr. Res. 2011, 346, 1057–1060. [Google Scholar] [CrossRef]
- Prakash, I.; Clos, J.F.; Chaturvedula, V.S.P. Stability of rebaudioside A under acidic conditions and its degradation products. Food Res. Int. 2012, 48, 65–75. [Google Scholar] [CrossRef]
- Prakash, I.; Campbell, M.; Miguel, R.I.S.; Chaturvedula, V.S.P. Synthesis and sensory evaluation of ent-kaurane diterpene glycosides. Molecules 2012, 17, 8908–8916. [Google Scholar] [CrossRef]
- Prakash, I.; Campbell, M.; Chaturvedula, V.S.P. Catalytic hydrogenation of the sweet principles of Stevia rebaudiana, rebaudioside B, rebaudioside C, and rebaudioside D and sensory evaluation of their reduced derivatives. Int. J. Mol. Sci. 2012, 13, 15126–15136. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Klucik, J.; Mani, U.; Prakash, I. Synthesis of ent-kaurane diterpene glycosides. Molecules 2011, 16, 8402–8409. [Google Scholar] [CrossRef]
- Bedir, E.; Toyang, N.J.; Khan, I.A.; Walker, L.A.; Clark, A.M. A new dammarane type triterpene glycoside from Polyscias fulva. J. Nat. Prod. 2001, 64, 95–97. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Schilling, J.K.; Miller, J.S.; Andriantsiferana, R.; Rasamison, V.E.; Kingston, D.G.I. New cytotoxic oleanane saponis from the infructescences of Polyscias amplifolia from the Madagascar rainforest. Planta Med. 2003, 69, 440–444. [Google Scholar] [CrossRef]
- Huan, V.D.; Yamamura, S.; Ohtani, K.; Kasai, R.; Yamasaki, K.; Nham, N.T. Oleanane saponins from Polyscias fructicosa. Phytochemistry 1998, 47, 451–457. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, T.; Ueda, T.; Tomii, K.; Kouno, I. Facile discrimination of aldose enantiomers by reversed-phase HPLC. Chem. Pharm. Bull. 2007, 55, 899–901. [Google Scholar] [CrossRef]
- Ohtani, K.; Aikawa, Y.; Kasai, R.; Chou, W.; Yamasaki, K.; Tanaka, O. Minor diterpene glycosides from sweet leaves of Rubus suavissimus. Phytochemistry 1992, 31, 1553–1559. [Google Scholar] [CrossRef]
- Ohta, M.; Sasa, S.; Inoue, A.; Tamai, T.; Fujita, I.; Morita, K.; Matsuura, F. Characterization of novel steviol glycosides from leaves of Stevia rebaudiana Morita. J. Appl. Glycosci. 2010, 57, 199–209. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–3 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Prakash, I.; Chaturvedula, V.S.P. Additional Minor Diterpene Glycosides from Stevia rebaudiana Bertoni. Molecules 2013, 18, 13510-13519. https://doi.org/10.3390/molecules181113510
Prakash I, Chaturvedula VSP. Additional Minor Diterpene Glycosides from Stevia rebaudiana Bertoni. Molecules. 2013; 18(11):13510-13519. https://doi.org/10.3390/molecules181113510
Chicago/Turabian StylePrakash, Indra, and Venkata Sai Prakash Chaturvedula. 2013. "Additional Minor Diterpene Glycosides from Stevia rebaudiana Bertoni" Molecules 18, no. 11: 13510-13519. https://doi.org/10.3390/molecules181113510
APA StylePrakash, I., & Chaturvedula, V. S. P. (2013). Additional Minor Diterpene Glycosides from Stevia rebaudiana Bertoni. Molecules, 18(11), 13510-13519. https://doi.org/10.3390/molecules181113510




