C-4 Gem-Dimethylated Oleanes of Gymnema sylvestre and Their Pharmacological Activities
Abstract
:1. Introduction
| Scientific names | |
|---|---|
| Gymnema sylvestre, Asclepias geminata, Asclepias geminata, Periploca sylvestris, Gymnema melicida | |
| Language | Common names |
| English | Gymnema, Cowplant, Australian cowplant, Gurmari, Gurmarbooti, Gurmar, Periploca of the woods, Meshasringa, Gemnema Melicida, Gimnema, Gur-Mar, Gymnema montanum, Gymnéma, Gymnéma Sylvestre, Miracle plant, Periploca sylvestris, Shardunika, Vishani, Ram’s horn, Miracle fruit, Merasingi, Small Indian ipecac, Sugar destroyer |
| Sanskrit | Meshashringi, Madhunashini, Ajaballi, Ajagandini, Bahalchakshu, Karnika, Chakshurabahala, Kshinavartta |
| Marathi | Kavali, Kalikardori, Vakundi |
| Hindi | Gurmar, Merasingi |
| Marathi | Kavali, Kalikardori, Vakundi |
| Gujrathi | Dhuleti, Mardashingi |
| Telugu | Podapatri |
| Tamil | Adigam, Cherukurinja, Sarkarikolli |
| Kannada | Sannager-asehambu |
| Malayalam | Chakkarakolli, Madhunashini |
| Bengali | Mera-Singi |
| Kingdom | Plantae |
|---|---|
| Subkingdom | Tracheobionta |
| Superdivision | Spermatophyta |
| Division | Magnoliophyta |
| Class | Magnoliopsida |
| Subclass | Asteridae |
| Order | Gentianales |
| Family | Asclepiadaceae |
| Genus | Gymnema R. Br. |
| Species | sylvestre |
2. Plant Description
3. Chemical Composition
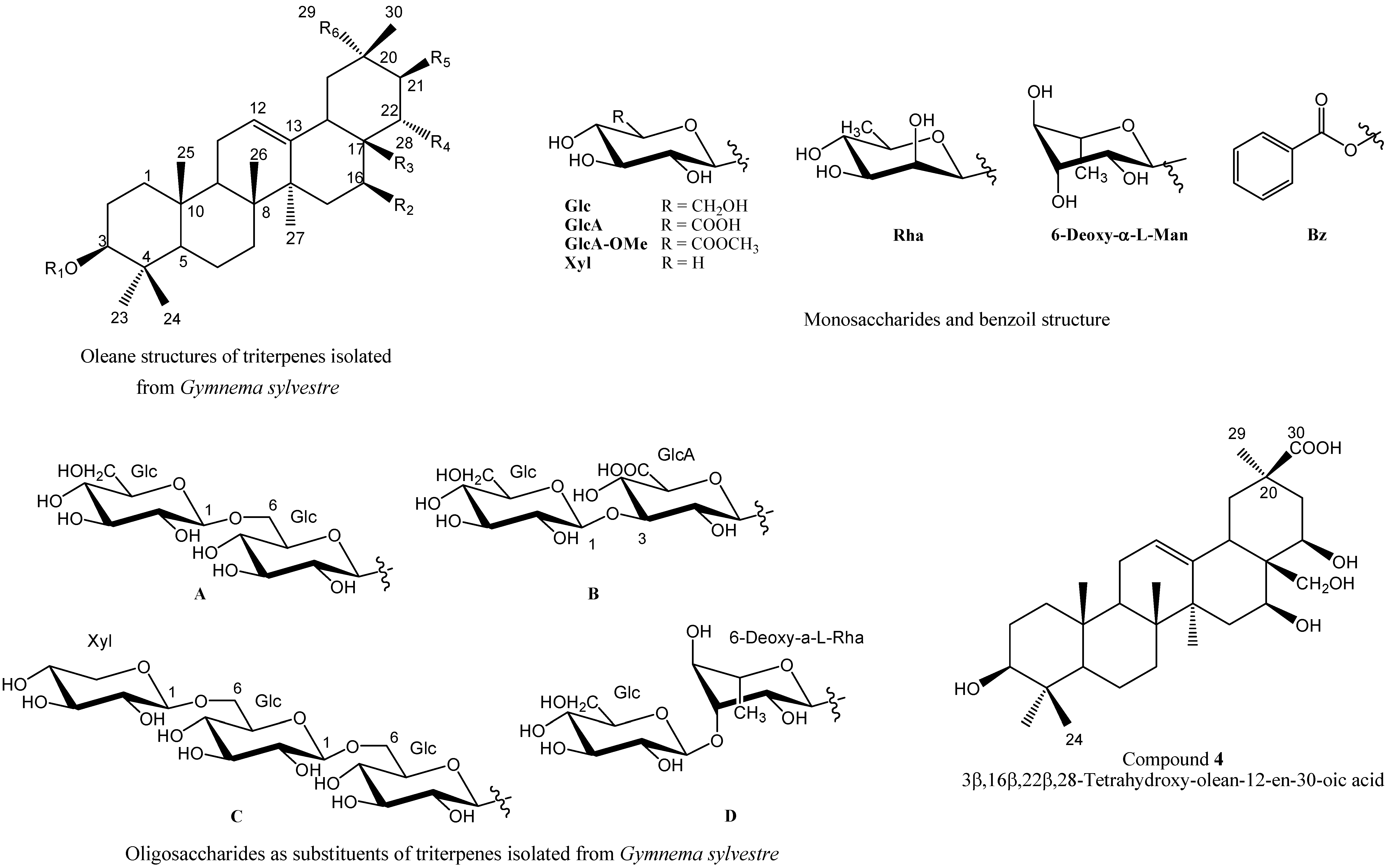
4. Pharmacological Studies
4.1. Anti-Diabetic Activity and Mechanism of Action
4.2. Inhibitory Effects on Palatal Taste Response
4.3. Hypolipidemic Activity
4.4. Anti-Obesity Activity
4.5. Anti-Cancer Activity
4.6. Haemolytic Activity and Mechanism of Action
4.7. Antimicrobial Activity
4.8. Anti-Inflammatory Activity
4.9. Anti-Larvicidal Activity
4.10. Antioxidant Activity
4.11. Side-Effects
4.12. Toxicity
5. Discussion of the Specific Biological Activity of Tested Molecules
| No. | CAS | Common Name | R1 | R2 | R3 | R4 | R5 | R6 |
|---|---|---|---|---|---|---|---|---|
| 1 | 465-94-1 | Longispinogenin | H | OH | CH2OH | H | H | CH3 |
| 2 | 474-15-7 | Chichipegenin | H | OH | CH2OH | OH | H | CH3 |
| 3 | 53187-93-2 | Sitakisogenin | H | OH | CH2OH | H | OH | CH3 |
| 4 | 862377-55-7 | 3β,16β,22β,28-tetrahydroxy-olean-12-en-30-oic acid | See Figure 2 | |||||
| 5 | 287390-11-8 | Longispinogenin 3-O-β-d-glucuronopyranoside | GlcA | OH | CH2OH | H | H | CH3 |
| 6 | 287389-94-0 | 21β-O-Benzoylsitakisogenin 3-O-β-d-glucuronopyranoside | GlcA | OH | CH2OH | H | Bz | CH3 |
| 7 | 873799-50-9 | Gymnemic acid A | GlcA | OH | CH2OH | H | H | COOH |
| 8 | 1096581-47-3 | Gymnemoside W2 | GlcA-OMe | OH | CH2OH | H | OH | CH3 |
| 9 | 330595-34-1 | Longispinogenin 3-O-β-d-glucopyranosyl-(1→3)-β-d-glucuronopyranoside potassium salt | B | OH | CH2OH | H | H | CH3 |
| 10 | 212775-47-8 | Alternoside VII | B | OH | CH2OH | OH | H | CH3 |
| 11 | 330595-32-9 | 21β-O-Benzoylsitakisogenin 3-O-β-d-glucopyranosyl-(1→3)-β-d-glucuronopyranoside | B | OH | CH2OH | H | Bz | CH3 |
| 12 | 330595-36-3 | 29-Hydroxylongispinogenin 3-O-β-d-glucopyranosyl-(1→3)-β-d-glucuronopyranoside potassium salt | B | OH | CH2OH | H | H | CH2OH |
| 13 | 1096581-44-0 | Gymnemoside W1 | A | OH | CH2OGlc | H | H | CH3 |
| 14 | 256510-01-7 | Alternoside XIX | C | OH | CH2OGlc | H | H | CH3 |
| 15 | 1422031-89-7 | 6-Deoxy-α-l-Rhamnopyranoside, (3β,16β,22α)-16-(hydroxy)-28-[(6-deoxy-α-L-mannopyranosyl)oxy]-22-hydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl | D | OH | CH2O(6-Deoxy-α-l)Man | OH | H | CH3 |
| 16 | 212775-23-0 | Alternoside II | B | OAc | CH2O(6-Deoxy-α-l)Man | OH | H | CH3 |
| 17 | 508-02-1 | Oleanolic acid | H | H | H | H | H | CH3 |
| 18 | 240140-86-7 | Oleanolic acid 3-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside | A | H | COOH | H | H | CH3 |
| 19 | 287389-96-2 | Oleanolic acid 3-O-β-d-xylopyranosyl(1→6)-β-d-glucopyranosyl(1→6)-β-d-glucopyranoside | C | H | COOH | H | H | CH3 |
| 20 | 14162-53-9 | Oleanolic acid 28-O-β-d-glucopyranoside | H | H | COOGlc | H | H | CH3 |
| 21 | 78454-20-3 | Silphioside B | Glc | H | COOGlc | H | H | CH3 |
| 22 | 287389-95-1 | 3-O-β-d-Glucopyranosyl(1→6)-β-d-glucopyranosyl oleanolic acid 28-O-β-d-glucopyranosyl ester | A | H | COOGlc | H | H | CH3 |
| 23 | 287389-97-3 | 3-O-β-d-Xylopyranosyl(1→6)-β-d-glucopyranosyl(1→6)-β-d-glucopyranosyl oleanolic acid 28-β-d-glucopyranosyl ester | C | H | COOGlc | H | H | CH3 |
| 24 | 287389-98-4 | 3-O-β-d-Glucopyranosyl(1→6)-β-d-glucopyranosyl oleanolic acid 28-β-d-glucopyranosyl(1→6)-β-d-glucopyranosyl ester | A | H | COO-A | H | H | CH3 |
| 25 | 1422031-87-5 | 3β,16β,22α-Trihydroxy-olean-12-ene 3-O-β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside | C | OH | CH3 | OH | H | CH3 |
| No. | Chromatographic conditions | Melting point (°C) | IR analysis (cm−1) | Mass analysis | [α]D (Concentration, Solvent) | Reference |
|---|---|---|---|---|---|---|
| 1 | - | 247–249 | - | - | +53 (Acetone) | [80] |
| Preparative TLC (CH2Cl2/Me2CO, 17:3) | - | - | - | - | [81] | |
| - | 216–218 | - | EI: [M]+ 458 | +38.7 (c2.5, CHCl3) | [82] | |
| HR-ESI: [M]+ 458.3755 | ||||||
| - | 218–220 | - | - | +51.0 (CHCl3) | [83] | |
| - | 244–245 | - | - | - | [84] | |
| 2 | By synthesis | 315–317 | - | HR-ESI: [M-H2O]+ 456.3580 | +35.4 (c1.2, CHCl3) | [85] |
| - | 321–323 | - | - | +43 (c1, CHCl3) | [86] | |
| HPLC (RP-C18; CH3OH/CH3CN/H2O, 2:7:1) | - | - | - | - | [81] | |
| 3 | HPLC (RP-C18; CH3OH/CH3CN/H2O, 2:5:3) | - | - | - | - | [81] |
| HPLC (S-5; H2O/CH3CN, 63:37) | 333–335 | - | HR-ESI: [M-H2O]+ 456.3628 | +57.0 (c0.9, CHCl3:CH3OH 1:1) | [82,87] | |
| 4 | - | - | - | ESI: [M+H]+ 505, [M+Na]+ 527 | - | [88] |
| 5 | Silica gel column chromatography using as eluent CHCl3/CH3OH | 198–202 | 3414 (OH), 1724 | FAB: [M+Na]+ 657 | +16.08 (c0.10, CH3OH). | [89] |
| (COOH), 1636 (C=C), 1458, 1380, 1054. | ||||||
| 6 | Silica gel column chromatography using as eluent CHCl3/CH3OH | 192–195 | 3444 (OH), 1724, 1700, 1635 (C=C), 1457, 1388, 1280, 1074, 720. | FAB: [M+Na]+ 777 | +27.2 (c0.15, CH3OH). | [89] |
| 7 | - | - | - | - | - | [90] |
| 8 | HPLC (RP-C18; CH3OH/H2O, 4:1) and Si-60 (CHCl3/CH3OH, 10:1 to 5:2) | - | - | HR-ESI: [M+Na]+ 687.4085 | −6.5 (c0.01, CH3OH) | [91] |
| 9 | HPLC (RP-C18; CH3OH/H2O, 1:4 to 7:3) | 305–310 | 3440, 2948, 1636, 1420, 1078, 1028 | HR-ESI: [(M.K)+H]+ 835.4065 | +18.1 (c0.08, CH3OH) | [92] |
| 10 | HPLC (ODS S-5, H2O/CH3CN, 4:1) | 213–215 | 3460, 1720, 1655, 1155 | FAB: [M−H]− 811 | +9.1 (c4.5, CH3OH) | [93] |
| 11 | HPLC (RP-C18; CH3OH/H2O, 1:4 to 7:3) | 226–228 | 3422, 2948, 1702, 1636, 1460, 1388, 1282, 1160, 1076, 1026 | FAB: [M+H]+ 917, [M+Na]+ 939 | +15.4 (c0.16, CH3OH) | [92] |
| 12 | HPLC (RP-C18; CH3OH/H2O, 1:4 to 7:3) | 290–293 | 3422, 2928, 1618, 1430, 1028 | FAB: [(M.K)+H]+ 851 | +10.3 (c0.12, CH3OH) | [92] |
| 13 | HPLC (RP-C18; CH3OH/H2O, 4:1) and Si-60 (CHCl3/CH3OH, 10:1 to 5:2) | - | - | ESI: [M-H]− 943 | −26.6 (c0.004, CH3OH) | [91] |
| [M-Glc]− 781, [M-2×Glc]− 619 | ||||||
| [M-3×Glc]− 457, [M+Na]+ 967 | ||||||
| HR-ESI: [M]+ 944.5290 | ||||||
| 14 | HPLC (YMC; S-5; H2O/CH3CN 3:1 to 13:7) | 187–189 | 3450, 1155 | FAB: [M-H]− 1075 | −23.4 (c3.1, CH3OH) | [94] |
| 15 | - | - | - | - | - | [95] |
| 16 | HPLC (RP-C18; CH3OH/H2O, 1:4 to 7:3) | 294–296 | 3418, 2948, 1738, 1713, 1621, 1430, 1374, 1266, 1076, 1031 | FAB: [(M.K)+H]+ 1023 | +1.5 (c0.19, CH3OH) | [92] |
| HPLC (ODS S-5; H2O/CH3CN, 4:1) | 230–232 | 3400, 1730, 1665, 1240, 1160 | FAB: [M-H]− 999 | +2.3 (2.4, CH3OH) | [93] |
| No. | Chromatographic conditions | Melting point (°C) | IR analysis (cm−1) | Mass analysis | [α]D (Concentration, Solvent) | Reference |
|---|---|---|---|---|---|---|
| 17 | HPLC (RP-C18; CH3OH/H20, 30:1) | 296–298 | 3420, 2930, 1680 | EI: 456, 438, 248, 207, 203, 189 | +70.0 (c0.4, CHCl3) | [96] |
| HPLC (RP-C18; CH3OH/H20, 4:1) | - | - | ESI: [M+CH3OH+ Na]+ 511, [M+Na]+ 479, 203, 191. | - | [97] | |
| HPLC (RP-C18; CH3OH/Acetic acid/Triethylamine, 99.55:0.30:0.15) | - | - | ESI: [M−H]− 455 | - | [98] | |
| 18 | Preparative TLC (CH2Cl2/CH3OH, 4:1) | 220 (decomp.) | 3375, 2943, 1559, 1459, 1385, 1311, 1074, 1032, 913, 630 | LSI (%): [M+−H] 779 (18.4), 777(2.5), 733(0.5), 645(0.6), 617(3.0), 551(0.8), 497(0.5), 483(1.3), 455(2.8), 437(1.3), 367(6.0), 331(0.8), 275(24.2), 273(5.3), 183(100.0), 151(6.0), 91(100.0), 71(12.1), 45 (1.5) | +4.2 (c0.24, CH3OH) | [99] |
| 19 | HPLC (RP-C18; CH3OH/H2O, 3:7 to 7:3) | 202–204 | 3410 (OH), 1710 (COOH), 1638 (C=C), 1458, 1036. | FAB: [M+Na]+ 935 | −3.28 (c0.15, CH3OH) | [89] |
| 20 | Silica gel column chromatography using as eluent CHCl3/CH3OH/H2O, 10:3:1 (lower layer) | - | - | ESI: [M+Na]+ 641.2 | - | [100,101] |
| CC silica gel, using EtOAc | [102] | |||||
| Flash chromatography (CH2Cl2/CH3OH, 20:1) | 224–226 | 3435, 2944, 2873, 1735, 1460, 1385, 1072, 1029 | ESI: [M+Na]+ 641.4 | - | [103] | |
| 21 | HPLC (RP-C18; CH3OH/H2O, 6:4) | - | - | FAB: [M−H]− 779, | - | [104] |
| [(M−H)−162]− 617, | ||||||
| [(M−H)−178]− 601 | ||||||
| HPLC (RP-C8; CH3OH/H2O) | - | - | HR-ESI: [M+Na]+ 803.4509 | - | [105] | |
| HPLC (RP-C18; CH3OH/H2O, 13:7) | 230 | - | Incorrect data | −6.3 (c0.17, MeOH) | [103] | |
| 22 | HPLC (RP-C18; CH3OH/H2O, 3:7 to 7:3) | 206–209 | 3424 (OH), 1735 (COOH), 1636 (C=C), 1457, 1034 | FAB: [M+Na]+ 943 | −6.5 (0.11, MeOH) | [89] |
| 23 | HPLC (RP-C18; CH3OH/H2O, 3:7 to 7:3) | 212–215 | 3414 (OH), 1740 (COOR), 1636 (C=C), 1460, 1364, 1044, 896 | FAB: [M+Na]+ 1097 | −9.68 (c0.20, MeOH). | [89] |
| 24 | HPLC (RP-C18; CH3OH/H2O, 3:7 to 7:3) | 209–211 | 3424 (OH), 1734 (COOR), 1636 (C=C), 1458, 1074. | FAB: [M+Na]+ 1127 | −12.18 (c0.12, MeOH) | [89] |
| 25 | - | - | - | - | - | [95] |
| No. | Mol. formula | Mol. weight | Aspect | 1H-NMR | 13C-NMR | Systematic Name | Reference |
|---|---|---|---|---|---|---|---|
| 1 | C30H50O3 | 458.72 | Amorphous powder | CDCl3 | CDCl3 | Olean-12-ene-3β,16β,28-triol | [81,82,83] |
| - | C5D5N | [93] | |||||
| 2 | C30H50O4 | 474.72 | Amorphous powder | C5D5N | C5D5N | Olean-12-ene-3β,16β,22α,28-tetrol | [81,82] |
| 3 | C30H50O4 | 474.72 | Amorphous powder | C5D5N | C5D5N | Olean-12-ene-3β,16β,21β,28-tetrol | [81,83] |
| 4 | C30H48O6 | 504.70 | White amorphous powder | C5D5N | C5D5N | Olean-12-en-30-oic acid, 3,16,22,28-tetrahydroxy-, (3β,16β,20β,22β) a | [88] |
| 5 | C36H58O9 | 834.84 | Amorphous powder | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β)-16,28-dihydroxyolean-12-en-3-yl | [89] |
| 6 | C43H62O11 | 754.95 | Amorphous powder | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β,21β)-21-benzoyloxy-16,28-dihydroxyolean-12-en-3-yl | [89] |
| 7 | C36H56O11 | 664.82 | N. a. | N. a. | N. a. | β-d-Glucopyranosiduronic acid, (3β,16β,20α)-20-carboxy-16,28-dihydroxy-30-norolean-12-en-3-yl | [103] |
| 8 | C37H60O10 | 664.87 | White powder | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β,21β)-16,21,28-trihydroxyolean-12-en-3-yl, methyl ester | [91] |
| 9 | C42H68O14K | 836.08 | Amorphous powder | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β)-16,28-dihydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl-, monopotassium salt | [92] |
| 10 | C42H68O15 | 812.98 | Colorless needles | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β,22α)-16,22,28-trihydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl | [93] |
| 11 | C49H72O16 | 917.09 | Amorphous powder | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β,21β)-21-benzoyloxy-16,28-dihydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl | [92] |
| 12 | C42H68O15K | 852.08 | Amorphous powder | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β,20α)-16,28,29-trihydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl-, monopotassium salt | [92] |
| 13 | C48H80O18 | 945.14 | White powder | C5D5N | C5D5N | β-d-Glucopyranoside, (3β,16β)-28-β-d-glucopyranosyloxy-16-hydroxyolean-12-en-3-yl 6-O-β-d-glucopyranosyl | [91] |
| 14 | C53H88O22 | 1077.25 | Colorless needles | C5D5N | C5D5N | β-d-Glucopyranoside, (3β,16β)-28-β-d-glucopyranosyloxy-16-hydroxyolean-12-en-3-yl 6-[β-d-xylopyranosyl-(1→6)-O-β-d-glucopyranosyl] | [94] |
| 15 | C48H80O17 | 929.14 | White amorphous powder | N. a. | N. a. | 6-Deoxy-α-l-Rhamnopyranoside, (3β,16β,22α)-16-(hydroxy)-28-[(6-deoxy-α-L-mannopyranosyl)oxy]-22-hydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl | [95] |
| 16 | C50H80O20 | 1001.16 | Colorless needles | C5D5N | C5D5N | β-d-Glucopyranosiduronic acid, (3β,16β,22α)-16-acetyloxy-28-[(6-deoxy-α-L-mannopyranosyl)oxy]-22-hydroxyolean-12-en-3-yl 3-O-β-d-glucopyranosyl | [92,93] |
| No. | Mol. formula | Mol. weight | Aspect | 1H-NMR | 13C-NMR | Systematic Name | Reference |
|---|---|---|---|---|---|---|---|
| 17 | C30H48O3 | 456.70 | White solid | C5D5N | C5D5N | 3β-Hydroxyolean-12-en-28-oic acid | [107] |
| CD3OD | CD3OD | [106] | |||||
| 18 | C42H68O13 | 780.98 | White solid | CD3OD | CD3OD | Olean-12-en-28-oic acid, 3-[(6-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-, (3β) | [99] |
| C5D5N | C5D5N | [51] | |||||
| 19 | C47H76O17 | 913.10 | Amorphous powder | C5D5N | C5D5N | Olean-12-en-28-oic acid, 3-[(O-β-d-xylopyranosyl-(1→6)-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]-, (3β) | [89] |
| 20 | C36H58O8 | 618.84 | Colorless powder | C5D5N | C5D5N | Oleanolic acid 28-O-β-d-glucopyranoside | [100] |
| 21 | C42H68O13 | 780.98 | White amorphous powder | - | CDCl3 | 3-O-(β-d-Glucopyranosyl)-oleanolic acid-28-O-β-d-glucopyranoside | [108] |
| - | C5D5N | [105] | |||||
| CD3OD | CD3OD | [104] | |||||
| 22 | C48H78O18 | 943.12 | Amorphous powder | C5D5N | C5D5N | Olean-12-en-28-oic acid, 3-[(6-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-, β-d-glucopyranosyl ester, (3β)- | [89,109] |
| 23 | C53H86O22 | 1075.24 | Amorphous powder | C5D5N | C5D5N | Olean-12-en-28-oic acid, 3-[(O-β-d-xylopyranosyl-(1→6)-O-β-d-glucopyranosyl-(1→6)-β-d-glucopyranosyl)oxy]-, β-d-glucopyranosyl ester, (3β) | [89,95] |
| 24 | C54H88O23 | 1105.26 | Amorphous powder | C5D5N | C5D5N | Olean-12-en-28-oic acid, 3-[(6-O-β-d-glucopyranosyl-β-d-glucopyranosyl)oxy]-, 6-O-β-d-glucopyranosyl-β-d-glucopyranosyl ester, (3β) | [89] |
| 25 | C47H78O17 | 915.11 | N. a. | N. a. | N. a. | 3β,16β,22α-Trihydroxy-olean-12-ene 3-O-β-d-xylopyranosyl-(1→6)-β-d-glucopyranosyl-(1→6)-β-d-glucopyranoside | [95] |
| No. | Part of the plant | Extract | Reference | Activity | Reference |
|---|---|---|---|---|---|
| 1 | Stems | EtOH | [110] | Antitubercular activity against Mycobacterium tuberculosis strain H37Rv | [110] |
| Leaves | EtOH/H2O (19:1) | [111] | Inhibition on human immunodeficiency virus type 1 (HIV-1) reverse transcriptase | [112] | |
| Aerial parts | CH2Cl2 | [81] | Anti-inflammatory | [113] | |
| 2 | Aerial parts | CH2Cl2 | [81] | Insect growth regulatory against Spodoptera frugiperda and Tenebrio molitor | [114] |
| Leaves | EtOH/H2O (19:1) | [111] | |||
| Stems | EtOH | [110] | |||
| Entire part | MeOH/CH2Cl2 (1:1) | [114] | |||
| 3 | Aerial parts | CH2Cl2 | [81] | - | - |
| Leaves | EtOH/H2O (19:1) | [111] | |||
| Leaves | EtOH | [108] | |||
| Stems | N. a. | [82] | |||
| 4 | Leaves | Not specified | [88] | - | - |
| 5 | Leaves | EtOH | [89] | Prevention or treatment of disorders related to high blood sugar, high blood lipids, or blood clotting | [115] |
| 6 | Leaves | EtOH | [89] | Prevention or treatment of disorders related to high blood sugar, high blood lipids, or blood | [115] |
| 7 | N. a. | N. a. | [103] | - | - |
| 8 | Leaves | EtOH/H2O (4:1) | [91] | - | - |
| 9 | Leaves | EtOH/H2O (3:2) | [92] | Antisweet activity | [92] |
| 10 | Roots | EtOH/H2O (7:3) | [93] | Antisweet activity | [93] |
| 11 | Leaves | EtOH/H2O (3:2) | [92] | Antisweet activity | [92] |
| 12 | Leaves | EtOH/H2O (3:2) | [92] | Antisweet activity | [92] |
| 13 | Leaves | EtOH/H2O (4:1) | [91] | - | - |
| 14 | Roots | EtOH | [94] | Antisweet activity | [94] |
| 15 | Stems | N. a. | [95] | - | - |
| 16 | Roots | EtOH/H2O (7:3) | [93] | Antisweet activity | [93] |
| Leaves | EtOH/H2O (3:2) | [92] | Antisweet activity | [92] |
| No. | Part of the plant | Extract | Reference | Activity | Reference |
|---|---|---|---|---|---|
| 17 | Leaves Leaves | EtOH/H2O (19:1) H2O+microwave | [111] [116,117] | Inhibition on human immunodeficiency virus type 1 (HIV-1) reverse transcriptase | [112] |
| Glucose uptake and gastric emptying | [101,118] | ||||
| Inhibition of Glycogen Phosphorylase | [108] | ||||
| 18 | Stems | N. a. | [95] | Haemolytic Activity | [99] |
| Obtained by synthesis | [99] | ||||
| 19 | Leaves | EtOH | [89] | Prevention or treatment of disorders related to high blood sugar, high blood lipids, or blood | [115] |
| 20 | Leaves | N. a. | [108] | Glucose uptake and gastric emptying | [101,118] |
| Haemolytic Activity | [51] | ||||
| Inhibition of Glycogen Phosphorylase | [103] | ||||
| Antimicrobial Activities | [119] | ||||
| 21 | Leaves | N. a. | [108] | Inhibition of the growth of HL60, A549 and | [105] [119] |
| Leaves | EtOH/H2O (3:2) | [105] | DU145 cancer cells | ||
| Leaves | MeOH/CH2Cl2 (1:1) | [104] | Amylase activity, total protein content and seedling growth | ||
| 22 | Leaves | EtOH | [89] | Prevention or treatment of disorders related to high blood sugar, high blood lipids, or blood | [115] |
| 23 | Leaves | EtOH | [89] | Prevention or treatment of disorders related to high blood sugar, high blood lipids, or blood | [115] |
| 24 | Leaves | EtOH | [89] | Prevention or treatment of disorders related to high blood sugar, high blood lipids, or blood | [115] |
| 25 | Stems | N. a. | [95] | - | - |
6. Conclusions
Acknowledgments
Conflicts of Interest
References
- Kanetkar, P.; Rekha, S.; Madhusudan, K. Gymnema sylvestre: A memoir. J. Clin. Biochem. Nutr. 2007, 41, 77–81. [Google Scholar] [CrossRef]
- Paliwal, R.; Kathori, S.; Upadhyay, B. Effect of Gurmar (Gymnema sylvestre) powder intervention on the blood glucose levels among diabetics. Ethno. Med. 2009, 3, 133–135. [Google Scholar]
- Rachh, P.R.; Rachh, M.R.; Ghadiya, N.R.; Modi, D.C.; Modi, K.P.; Patel, N.M.; Rupareliya, M.T. Antihyperlipidemic activity of Gymnema sylvestre R. Br. leaf extract on rats fed with high cholesterol diet. Int. J. Pharmacol. 2010, 6, 138–141. [Google Scholar] [CrossRef]
- Yeh, G.Y.; Eisenberg, D.M.; Kaptchuk, T.J.; Phillips, R.S. Systematic review of herbs and dietary supplements for glycemic control in diabetes. Diabetes Care 2003, 26, 1277–1294. [Google Scholar] [CrossRef]
- Kurihara, Y. Characteristics of antisweet substances, sweet proteins, and sweetness inducing proteins. Crit. Rev. Food. Sci. Nutr. 1992, 32, 231–252. [Google Scholar] [CrossRef]
- Duke, J.A.; Jones, P.M.; Danny, B.; Jony, D.; Bully, P. The Green Pharmacy; Rodale Press, Inc.: Emmaus, PA, USA, 1997. [Google Scholar]
- Chodisetti, B.; Rao, K.; Giri, A. Phytochemical analysis of Gymnema sylvestre and evaluation of its antimicrobial activity. Nat. Prod. Res. 2013, 27, 583–587. [Google Scholar] [CrossRef]
- Vediyappan, G.; Dumontet, V.; Pelissier, F.; d’Enfert, C. Gymnemic acids inhibit hyphal growth and virulence in Candida albicans. PLoS One 2013, 8, e74189. [Google Scholar]
- Bishayee, A.; Malay, C. Hypolipidaemic and antiatherosclerotic effects of oral Gymnema sylvestre R. Br. leaf extract in albino rats fed on a high fat diet. Phytother. Res. 1994, 8, 118–120. [Google Scholar] [CrossRef]
- Rana, A.C.; Avadhoot, Y. Experimental evaluation of hepatoprotective activity of Gymnema sylvestre and Curcuma zedoaria. Fitoterapia 1992, 63, 60. [Google Scholar]
- Granich, M.S.; Halpern, B.P.; Eisner, T. Gymnemic acids: Secondary plant substances of dual defensive action. J. Insect Physiol. 1974, 20, 435–439. [Google Scholar]
- Hiji, Y. Cariostatic Materials and Foods, and Method for Preventing Dental Caries. U.S. Patent 4912089A, 27 March 1990. [Google Scholar]
- Komalavalli, N.; Rao, M.V. In vitro micropropagation of Gymnema sylvestre–A multipurpose medicinal plant. Plant Cell Tiss. Org. 2000, 61, 97–105. [Google Scholar]
- Kini, R.M.; Gowda, T.V. Studies on snake venom enzymes: Part I Purification of ATPase, a toxic component of Naja naja venom and its inhibition by potassium gymnemate. Ind. J. Biochem. Biophys. 1982, 22, 152–154. [Google Scholar]
- Kini, R.M.; Gowda, T.V. Studies on snake venom enzymes: Part II Partial characterization of ATPases from Russell’s Viper (Vipera russelli) venom & their Interaction with potassium gymnemate. Ind. J. Biochem. Biophys. 1982, 19, 342–346. [Google Scholar]
- Kritikar, K.; Basu, B. Indian Medicinal Plants; International Book Distributors: Dehradun, India, 1998; p. 1625. [Google Scholar]
- Keshavamurthy, K.R.; Yoganarasimhan, S.N. Flora of Coorg-Karnataka; Vimsat Publishers: Banglore, India, 1990; p. 282. [Google Scholar]
- Saneja, A.; Chetan, S.; Aneja, K.R.; Rakesh, P. Gymnema sylvestre (Gurmar): A review. Der Pharm. Lett. 2010, 2, 275–284. [Google Scholar]
- Ninomiya, Y.; Imoto, T. Gurmarin inhibition of sweet taste responses in mice. Am. J. Physiol. 1995, 268, 1019–1025. [Google Scholar]
- Madhurima, S.H.; Ansari, P.; Alam, S.; Ahmad, M.S.; Akhtar, S. Pharmacognostic and phytochemical analysis of Gymnema sylvestre R. (Br.) leaves. J. Herb. Med. Toxicol. 2009, 3, 73–80. [Google Scholar]
- Potawale, S.E.; Shinde, V.M.; Anandi, L.; Borade, L.; Dhalawat, L.; Deshmukh, R.S. Development and validation of a HPTLC method for simultaneous densitometric analysis of gymnemagenin and 18β-glycyrrhetinic acid in herbal drug formulation. Pharmacologyonline 2008, 2, 144–157. [Google Scholar]
- Zhen, H.; Xu, S.; Pan, S. Research on chemical constituents from stem of Gymnema sylvestre. Zhong Yao Cai 2001, 24, 95–97. [Google Scholar]
- Gurav, S.; Gulkari, V.; Durgkar, N.; Patil, A. Systematic review: Pharmacognosy, phytochemistry, pharmacology and clinical applications of Gymnema sylvestre R. Br. Pharmacogn. Rev. 2007, 1, 338–343. [Google Scholar]
- Yackzan, K.S. Biological effects of Gymnema sylvestre fractions. Alabama J. Med. Sci. 1966, 3, 1–9. [Google Scholar]
- Agnihotri, A.K.; Khatoon, S.; Agarwal, M.; Rawat, A.K.S.; Mehrotra, S.; Pushpangadan, P. Pharmacognostical evaluation of Gymnema sylvestre R. Br. Nat. Prod. Sci. 2004, 10, 168–172. [Google Scholar]
- Joshi, D.D. A method to prepare standardized extract of Gymnema sylvestre leaves for 75% gymnemic acids. Indian Patent IN2009DE00996 A, 19 November 2009. [Google Scholar]
- Sinsheimer, J.E.; Manni, P.E. Constituents from Gymnema sylvestre leaves. J. Pharm. Sci. 1965, 54, 1541–1544. [Google Scholar] [CrossRef]
- Charpurey, K.G. Indian Medical Gazette; Spink&co: Calcutta, India, 1926; p. 155. [Google Scholar]
- Hardy, M.L.; Coulter, I.; Venuturupalli, S.; Roth, E.A.; Favreau, J.; Morton, S.C.; Shekelle, P. Ayurvedic interventions for diabetes mellitus: A systematic review. J. Altern. Complement. Med. 2001, 41, 977–983. [Google Scholar]
- Baskaran, K.; Kizar, A.B.K.; Rhada, S.K.R. Antidiabetic effect of a lead extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. J. Ethnopharmacol. 1990, 30, 295–300. [Google Scholar] [CrossRef]
- Shanmugasundaram, E.R.; Rajeswari, G.; Baskaran, K.; Rajesh, K.B.R.; Radha, S.K.; Kizar, A.B. Use of Gymnema sylvestre leaf extract in the control of blood glucose in insulin-dependent diabetes mellitus. J. Ethnopharmacol. 1990, 30, 281–294. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied. Sci. 2011, 3, 504–512. [Google Scholar] [CrossRef]
- Bhatt, H.V.; Mohan, R.N.; Panchal, G.M. Differential diagnosis of byssinosis by blood histamine and pulmonary function test: A review and an appraisal. Int. J. Toxicol. 2001, 20, 321–327. [Google Scholar] [CrossRef]
- Srivastava, Y.; Nigam, S.K.; Bhatt, H.V.; Verma, Y.; Prem, A.S. Hypoglycemic and lifeprolonging properties of Gymnema sylvestre leaf extract in diabetic rats. Isr. J. Med. Sci. 1985, 21, 540–542. [Google Scholar]
- Snigur, G.L.; Samokhina, M.P.; Pisarev, V.B.; Spasov, A.A.; Bulanov, A.E. Structural alterations in pancreatic islets in streptozotocin-induced diabetic rats treated with of bioactive additive on the basis of Gymnema sylvestre. Morfologiia 2008, 133, 60–64. [Google Scholar]
- Chattopadhyay, R.R. Possible mechanism of antihyperglycemic effect of Gymnema sylvestre leaf extract, Part I. Gen. Pharm. 1998, 3, 495–496. [Google Scholar] [CrossRef]
- Nash, R.J.; Wilson, F.X.; Horne, G. Compositions Comprising Imino Sugar Acids for Treatment of Energy Utilization Disease such as Metabolic Syndrome. PCT Int. Appl. WO 2009103953A1, 27 August 2009. [Google Scholar]
- Shane-McWhorter, L. Biological complementary therapies: A focus on botanical products in diabetes. Diabetes Spectr. 2001, 14, 199–208. [Google Scholar] [CrossRef]
- Joffe, D.J.; Freed, S.H. Effect of extended release Gymnema sylvestre leaf extract (Beta Fast G-XR) alone or in combination with oral hypoglycemics or insulin regimens for type 1 and type 2 diabetes. Diabetes Control Newslett. 2001, 76, 23–24. [Google Scholar]
- Shapiro, K.; Gong, W.C. Natural products used for diabetes. J. Am. Pharm. Assoc. 2002, 2, 217–226. [Google Scholar]
- Nakamura, Y.; Tsumura, Y.; Tonogai, Y.; Shibata, T. Fecal steroid excretion is increased in rats by oral administration of gymnemic acids contained in Gymnema sylvestre leaves. J. Nutr. 1999, 129, 1214–1222. [Google Scholar]
- Pothuraju, R.; Sharma, R.K.; Jayasimha, C.; Jangr, S.; Kumar, P. A systematic review on Gymnema sylvestre in the obesity and diabetes management. J. Sci. Food Agric. 2013. [Google Scholar] [CrossRef]
- Starcevic, J.N.; Petrovic, D. Carotid intima media-thickness and genes involved in lipid metabolism in diabetic patients using statins-a pathway toward personalized medicine. Cardiovasc. Hematol. Agents Med. Chem. 2013, 11, 3–8. [Google Scholar] [CrossRef]
- Shigematsu, N.; Ryuji, A.; Makoto, S.; Mitsuo, O. Effect of administration with the extract of Gymnema sylvestre R. Br leaves on lipid metabolism in rats. Biol. Pharm. Bull. 2001, 24, 713–717. [Google Scholar] [CrossRef]
- Preuss, H.G.; Jarrell, S.; Taylor, S.; Rich, L.; Shari, A.; Richard, A. Comparative effects of chromium, vanadium and Gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J. Am. Coll. Nutr. 1998, 17, 116–123. [Google Scholar] [CrossRef]
- Pierce, A. Gymnema Monograph: Practical Guide to Natural Medicine; Stonesong Press Book: New York, NY, USA, 1999; pp. 324–326. [Google Scholar]
- Srikanth, A.V.; Sayeeda, M.; Lakshmi, N.; Ravi, M.; Kumar, P.; Madhava, R.B. Anticancer activity of Gymnema sylvestre R. Br. Int. J. Pharm. Sci. Nanotech. 2010, 3, 897–899. [Google Scholar]
- Tamaki, H.; Satoh, H.; Satoko, H.; Hisakazu, O.; Yasufumi, S. Inhibitory effects of herbal extracts on Breast Cancer Resistance Protein (BCRP) and structure-inhibitory potency relationship of isoflavonoids. Drug Metab. Pharm. 2010, 25, 170–179. [Google Scholar] [CrossRef]
- Qingcheng, M.; Jashvant, D.U. Role of the Breast Cancer Resistance Protein (ABCG2) in drug transport. AAPS J. 2005, 7, 118–133. [Google Scholar] [CrossRef]
- Romussi, G.; Cafaggi, S.; Bignardi, G. Hemolytic action and surface activity of triterpene saponins from Anchusa officinalis L. Part 21: On the costituents of boraginaceae. Pharmazie 1980, 35, 498–499. [Google Scholar]
- Hase, J.; Kobashi, K.; Mitsui, K.; Namba, T.; Yoshizaki, M.; Tomimori, T. The structure-hemolysis relationship of oleanolic acid derivatives and inhibition of the saponin-induced hemolysis with sapogenins. J. Pharmacobiodyn. 1981, 4, 833–837. [Google Scholar] [CrossRef]
- Schloesser, E.; Wulff, G.Z. Uber die strukturspezifität des saponinhämolyse. I. Triterpensaponine und aglykone. Z. Naturforsch. B 1969, 24, 1284–1290. [Google Scholar]
- Segal, R.; Milo-Goldzweig, I. On the mechanism of saponins hemolysis. II. Inhibition of hemolysis by aldonolactones. Biochem. Pharmacol. 1975, 24, 77–81. [Google Scholar] [CrossRef]
- Segal, R.; Milo-Goldzweig, I.; Seiffe, M. The hemolytic properties of non-ionic hemolysins. Life Sci. 1972, 11, 61–70. [Google Scholar] [CrossRef]
- Segal, R.; Schloesser, E. Role of glycosidases in the membranlytic, antifungal action of Saponins. Arch. Microbiol. 1975, 104, 147. [Google Scholar] [CrossRef]
- Segal, R.; Shatovski, P.; Milo-Goldzweig, I. Mechanism of saponin hemolysis. I. Hydrolysis of the glycosidic bond. Biochem. Pharmacol. 1974, 23, 973–981. [Google Scholar] [CrossRef]
- Hostettmann, K.; Marston, A. Saponins; Cambridge University Press: New York, NY, USA, 1995; p. 232. [Google Scholar]
- Gauthier, C.; Legault, J.; Girard-Lalancette, K.; Mshvildadze, V.; Pichette, A. Haemolytic activity, cytotoxicity and membrane cell permeabilization of semi-synthetic and natural lupane- and oleanane-type saponins. Bioorgan. Med. Chem. 2009, 17, 2002–2008. [Google Scholar]
- Satdive, R.K.; Abhilash, P.; Devanand, P.F. Antimicrobial activity of Gymnema sylvestre leaf extract. Fitoterapia 2003, 74, 699–701. [Google Scholar] [CrossRef]
- Chand, P.; Shaik, S.; Sadath, A.; Ziaullah, K. Anti salmonella activity of selected medicinal plants. Turk. J. Biol. 2008, 33, 59–64. [Google Scholar]
- Paul, J.P.; Jayapriya, K. Screening of antibacterial effects of Gymnema sylvestre (L.) R.Br. Pharmacologyonline 2009, 3, 832–836. [Google Scholar]
- Malik, J.K.; Manvi, F.V.; Alagawadi, K.R.; Noolvi, M. Evaluation of anti-inflammatory activity of Gymnema sylvestre leaves extract in rats. Int. J. Green Pharm. 2007, 2, 114–115. [Google Scholar]
- Diwan, P.V.; Margaret, J.; Rama Krishna, S. Influence of Gymnema sylvestre on inflammation. Inflammopharmacology 1995, 3, 271–277. [Google Scholar] [CrossRef]
- Kubo, I. Tyrosinase Inhibitors from Plants. In Phytochemicals for Pest Control; Hedin, P., Hollingworth, R., Masler, E., Miyamoto, J., Thompson, D., Eds.; American Chemical Society: Washington, DC, USA, 1997; Volume 685, pp. 311–326. [Google Scholar]
- Cespedes, C.L.; Martınez-Vazquez, M.; Calderon, J.S.; Salazar, J.R.; Aranda, E. Insect growth regulatory activity of some extracts and compounds from Parthenium argentatum on fall armyworm Spodoptera frugiperda. Z. Naturforsch C. 2001, 56, 95–105. [Google Scholar]
- Torres, P.; Avila, J.G.; Romo de Vivar, A.; Garcı´a, A.M.; Marı´n, J.C.; Aranda, E.; Ce´spedes, C.L. Antioxidant and insect growth regulatory activities of stilbenes and extracts from Yucca periculosa. Phytochemistry 2003, 64, 463–473. [Google Scholar] [CrossRef]
- Ikekawa, N.; Morisaki, M.; Fujimoto, Y. Sterol metabolism in insects: Dealkylation of phytosterol to cholesterol. Acc. Chem. Res. 1993, 26, 139–146. [Google Scholar] [CrossRef]
- Kubo, I.; Klocke, J.A. Isolation of Phytoecdysones as Insect Ecdysis Inhibitors and Feeding Deterrents. In Plant Resistance to Insects; Hedin, P.A., Ed.; American Chemical Society: Washington, DC, USA, 1983; Volume 208, pp. 329–346. [Google Scholar]
- Caldero´n, J.S.; Ce´spedes, C.L.; Rosas, R.; Go´mez-Garibay, F.; Salazar, J.R.; Lina, L.; Aranda, E.; Kubo, I. Acetylcholinesterase and insect growth inhibitory activities of Gutierrezia microcephala on fall armyworm Spodoptera frugiperda J.E. Smith. Z. Naturforsch. C 2001, 56, 382–394. [Google Scholar]
- Swain, T. Tannins and Lignins. In Herbivores: Their Interactions with Secondary Plant Metabolites; Rosenthal, G.A., Janzen, D.H., Eds.; Academic Press: New York, NY, USA, 1979; pp. 657–682. [Google Scholar]
- Tandon, P.; Sirohi, A. Assessment of larvicidal properties of aqueous extracts of four plants against Culex quinquefasciatus larvae. Jordan J. Biol. Sci. 2010, 3, 1–6. [Google Scholar]
- Khanna, V.G.; Kannabiran, K.; Rajakumar, G.; Rahuman, A.A.; Santhosh Kumar, T. Biolarvicidal compound gymnemagenol isolated from leaf extract of miracle fruit plant, Gymnema sylvestre (Retz) Schult against malaria and filariasis vectors. Parasitol. Res. 2011, 109, 1373–1386. [Google Scholar] [CrossRef]
- Sharma, K.; Singh, U.; Vats, S.; Priyadarsini, K.; Bhatia, A.; Kamal, R. Evaluation of evidenced-based radioprotective efficacy of Gymnema sylvestre leaves in mice brain. J. Environ. Pathol. Toxicol. Oncol. 2009, 28, 311–323. [Google Scholar] [CrossRef]
- Babu, P.S.; Stanely, M.P.P. Antihyperglycaemic and antioxidant effect of hyponidd, an ayurvedic herbomineral formulation in streptozotocin-induced diabetic rats. J. Pharm. Pharmacol. 2004, 56, 1435–1442. [Google Scholar] [CrossRef]
- Patel, S.S.; Shah, R.S.; Goyal, R.K. Antihyperglycemic, antihyperlipidemic and antioxidant effects of dihar, a polyherbal ayurvedic formulation in streptozotocin induced diabetic rats. Indian J. Exp. Biol. 2009, 47, 564–570. [Google Scholar]
- Dodson, D.; Mitchell, D.R.; Dodson, D.C. The Diet Pill Guide: The Consumer’S Book of Over-the-Counter and Prescription Weight-Loss Pills and Supplements; St. Martin’s Press: 175 Fifth Avenue, New York, NY, USA, 2001; p. 76. [Google Scholar]
- Ogawa, Y.; Sekita, K.; Umemura, T.; Saito, M.; Ono, A.; Kawasaki, Y.; Uchida, O.; Matsushima, Y.; Inoue, T.; Kanno, J. Gymnema sylvestre leaf extract: A 52-week dietary toxicity study in wistar rats. Shōni Shikagaku Zasshi 2004, 45, 8–18. [Google Scholar]
- Russell, F.E. Snake venom poisoning in the United States. Annu. Rev. Med. 1980, 31, 247–259. [Google Scholar] [CrossRef]
- Bhakuni, D.S.; Dhar, M.L.; Dhar, M.M.; Dhawan, B.N.; Gupta, B.; Srimal, R.C. Screening of Indian plats for biological activity. Ind. J. Exp. Biol. 1971, 9, 91–102. [Google Scholar]
- Djerassi, C.; McDonald, R.M.; Lemin, A.J. Terpenoids. III. 1 The isolation of erythrodiol, oleanolic acid and a new triterpene triol, longispinogenin, from the cactus Lemaiveocevcus longispinus. J. Am. Chem. Soc. 1953, 5940–5942. [Google Scholar] [CrossRef]
- Zarrelli, A.; Della Greca, M.; Ladhari, A.; Haouala, R.; Previtera, L. New triterpenes from Gymnema sylvestre. Helv. Chim. Acta 2013, 96, 1036–1045. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, K.; Taninaka, H.; Kan, Y.; Arihara, S. Antisweet natural products. XI. Structures of sitakisosides VI–X from Stephanotis lutchuensis Koidz. var. Japonica. Chem. Pharm. Bull. 1994, 42, 2455–2460. [Google Scholar] [CrossRef]
- Tori, K.; Yoshimura, Y.; Seo, S.; Sakurawi, K.; Tomita, Y.; Ishii, H. Carbon-13 NMR spectra of saikogenins. Stereochemical dependence in hydroxylation effects upon carbon-13 chemical shifts of oleanene-type triterpenoids. Tetrahedron Lett. 1976, 46, 4163–4166. [Google Scholar]
- Yasukawa, K.; Akihisa, T.; Oinuma, H.; Kasahara, Y.; Kimura, Y.; Yamanouchi, S.; Kumaki, K.; Tamura, T.; Takido, M. Inhibitory effect of di- and trihydroxy triterpenes from the flowers of compositae on 12-O-tetradecanoylphorbol-13-acetate-induced inflammation in mice. Biol. Pharm. Bull. 1996, 19, 1329–1331. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Taninaka, H.; Kan, Y.; Arihara, S. Antisweet natural products. X. Structures of sitakisoside I–V from Stephanotis lutchuensis Koidz. var. japonica. Chem. Pharm. Bull. 1994, 42, 2023–2027. [Google Scholar] [CrossRef]
- Khong, P.W.; Lewis, K.G. New triterpenoid extractives from Lemaireocereus chichipe. Aust. J. Chem. 1975, 28, 165–172. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Mizutani, A.; Kan, Y.; Arihara, S. Antisweet natural products. XII. Structures of sitakisosides XI–XX from Stephanotis lutchuensis Koidz. var. japonica. Chem. Pharm. Bull. 1997, 45, 62–67. [Google Scholar]
- Peng, S.L.; Zhu, X.M.; Wang, M.K.; Ding, L.S. A Novel triterpenic acid from Gymnema sylvestre. Chin. Chem. Lett. 2005, 16, 223–224. [Google Scholar]
- Ye, W.C.; Zhang, Q.W.; Liu, Xin; Che, C.T.; Zhao, S.X. Oleanane saponins from Gymnema sylvestre. Phytochemistry 2000, 53, 893–899. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Y.; Wang, X.; Xu, H. Isolation and identification of a new component from Gymnema sylvestre. Huaxi Yaoxue Zazhi 2004, 19, 336–338. [Google Scholar]
- Zhu, X.M.; Xie, P.; di, Y.T.; Peng, S.L.; Ding, L.S.; Wang, M.K. Two new triterpenoid saponins from Gymnema sylvestre. J. Integ. Plant Biol. 2008, 50, 589–592. [Google Scholar] [CrossRef]
- Ye, W.; Liu, X.; Zhang, Q.; Che, C.T.; Zhao, S. Antisweet saponins from Gymnema sylvestre. J. Nat. Prod. 2001, 64, 232–235. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Ogata, H.; Arihara, S.; Chang, H.C.; Wang, R.R. Antisweet natural products. XIII. Structures of alternosides I-X from Gymnema alternifolium. Jen-Der. Chem. Pharm. Bull. 1998, 46, 1102–1107. [Google Scholar] [CrossRef]
- Yoshikawa, K.; Takahashi, K.; Matsuchika, K.; Arihara, S.; Chang, H.C.; Wang, J.D. Antisweet natural products. XIV. Structures of alternosides XI–XIX from Gymnema alternifolium. Chem. Pharm. Bull. 1999, 47, 1598–1603. [Google Scholar] [CrossRef]
- Zhang, M.Q.; Liu, Y.; Xie, S.X.; Xu, T.H.; Liu, T.H.; Xu, Y.J.; Xu, D.M. A new triterpenoid saponin from Gymnema sylvestre. J. Asian Nat. Prod. Res. 2012, 14, 1186–1190. [Google Scholar] [CrossRef]
- Kim, D.H.; Han, K.M.; Chung, I.S.; Kim, D.K.; Kim, S.H.; Kwon, B.M.; Jeong, T.S.; Park, M.H.; Ahn, E.M.; Baek, N.I. Triterpenoids from the flower of Campsis grandiflora K. Schum. as human AcyI-CoA: Cholesterol Acyltransferase inhibitors. Arch. Pharm. Res. 2005, 2, 550–556. [Google Scholar]
- Diandian, S.; Min-Hsiung, P.; Qing-Li, W.; Chung-Heon, P.; Rodolfo, J.; Chi-Tang, H.; James, E.S. LC-MS method for the simultaneous quantitation of the anti-inflammatory constituents in oregano (Origanum species). J. Agric. Food Chem. 2010, 58, 7119–7125. [Google Scholar] [CrossRef]
- Guo, S.; Duan, J.A.; Tang, Y.P.; Yang, N.Y.; Qian, D.W.; Su, S.L.; Shang, E.X. Characterization of triterpenic acids in fruits of Ziziphus species by HPLC-ELSD-MS. J. Agric. Food Chem. 2010, 58, 6285–6289. [Google Scholar]
- Seebacher, W.; Haslinger, E.; Rauchensteiner, K.; Jurenitsch, J.; Presser, A.; Weis, R. Synthesis and haemolytic activity of randianin isomers. Monats. Chem. 1999, 130, 887–897. [Google Scholar]
- Zhu, Y.-Y.; Qian, L.-W.; Zhang, J.; Liu, J.-H.; Yu, B.-Y. New approaches to the structural modification of olean-type pentacylic triterpenes via microbial oxidation and glycosylation. Tetrahedron 2011, 67, 4206–4211. [Google Scholar]
- Matsuda, H.; Li, Y.; Murakami, T.; Matsumura, N.; Yamahara, J.; Yoshikawa, M. Antidiabetic principles of natural medicines. III. Structure-related inhibitory activity and action mode of oleanolic acid glycosides on hypoglycemic activity. Chem. Pharm. Bull. 1998, 46, 1399–1403. [Google Scholar] [CrossRef]
- Chiozem, D.D.; Trinh-Van-Dufat, H.; Wansi, J.D.; Djama, C.M.; Fannang, V.S.; Seguin, S.; Tillequin, F.; Wandji, J. New friedelane triterpenoids with antimicrobial activity from the stems of Drypetes paxii. Chem. Pharm. Bull. 2009, 57, 1119–1122. [Google Scholar] [CrossRef]
- Xiaoan, W.; Hongbin, S.; Jun, L.; Keguang, C.; Pu, Z.; Liying, Z.; Jia, H.; Luyong, Z.; Peizhou, N.; Spyros, E.Z.; et al. Naturally occurring pentacyclic triterpenes as inhibitors of glycogen phosphorylase: Synthesis, structure-activity relationships, and x-ray crystallographic studies. J. Med. Chem. 2008, 51, 3540–3554. [Google Scholar] [CrossRef]
- Rastrelli, L.; Aquino, R.; Abdo, S.; Proto, M.; De Simone, F.; de Tommasi, N. Studies on the constituents of Amaranthus caudatus leaves: Isolation and structure elucidation of new triterpenoid saponins and ionol-derived glycosides. J. Agric. Food Chem. 1998, 46, 1797–1804. [Google Scholar]
- Zhang, Y.; Peng, Y.; Li, L.; Zhao, L.; Hua, Y.; Hu, C.; Song, S. Studies on cytotoxic triterpene saponins from the leaves of Aralia elata. Food Chem. 2013, 138, 208–213. [Google Scholar] [CrossRef]
- Mencherini, T.; Picerno, P.; Del Gaudio, P.; Festa, M.; Capasso, A.; Aquino, R. Saponins and polyphenols from Fadogia ancylantha (Makoni tea). J. Nat. Prod. 2010, 73, 247–251. [Google Scholar] [CrossRef]
- Werner, S.; Nebojsa, S.; Robert, W.; Robert, S.; Olaf, K. Spectral assignments and reference data. Complete assignments of 1H and 13C-NMR resonances of oleanolic acid, 18α-oleanolic acid, ursolic acid and their 11-oxo derivatives. Magn. Reson. Chem. 2003, 41, 636–648. [Google Scholar] [CrossRef]
- Liu, X.; Ye, W.; Xu, D.; Zhang, Q.; Che, Z.; Zhao, S. Chemical study on triterpene and saponin from Gymnema sylvestre. Zhongguo Yaoke Daxue Xuebao 1999, 30, 174–176. [Google Scholar]
- Zhang, X.; Huo, L.; Liu, L.; Xu, Z.; Wang, W. Chemical constituents from leaves of Gymnema sylvestre (I). Zhongcaoyao 2011, 42, 866–869. [Google Scholar]
- Toshihiro, A.; Scott, G.; Franzblau, M.U.; Hiroki, O.; Fangqiu, Z.; Ken, Y.; Takashi, S.; Yumiko, K. Antitubercular activity of triterpenoids from asteraceae flowers. Biol. Pharm. Bull. 2005, 28, 158–160. [Google Scholar] [CrossRef]
- Ye, W.-C.; Liu, X.; Zhao, S.-X.; Che, C.-T. Triterpenes from Gymnema sylvestre growing in china. Biochem. Syst. Ecol. 2001, 29, 1193–1195. [Google Scholar] [CrossRef]
- Toshihiro, A.; Jun, O.; Jun, K.; Ken, Y.; Motohiko, U.; Sakae, Y.; Kunio, O. Inhibitory effects of triterpenoids and sterols on human immunodeficiency Virus-1 Reverse Transcriptase. Lipids 2001, 36, 507–512. [Google Scholar] [CrossRef]
- Motohiko, U.; Toshihiro, A.; Ken, Y.; Yoshimasa, K.; Yumiko, K.; Kazuo, K.; Tamotsu, N.; Michio, T. Constituents of compositae plants. 2. Triterpene diols, triols, and their 3-O-fatty acid esters from edible chrysanthemum flower extract and their anti-inflammatory effects. J. Agric. Food Chem. 2001, 49, 3187–3197. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Salazar, J.R.; Martınez, M.; Aranda, E. C24.1. Insect growth regulatory effects of some extracts and sterols from Myrtillocactus geometrizans (Cactaceae) against Spodoptera frugiperda and Tenebrio molitor. Phytochemistry 2005, 66, 2481–2493. [Google Scholar] [CrossRef]
- Ye, W.; Dai, Y.; Cong, X.; Zhu, X.; Zhao, S. Isolation of Novel Gymnemic Acid Derivatives from Gymnema sylvestre R. Br in Prevention or Treatment of Disorders Related to High Blood Sugar, High Blood Lipids, or Blood Clotting. PCT Int. Appl. WO 2000047594A1, 17 August 2000. [Google Scholar]
- Mandal, V.; Dewanjee, S.; Mandal, S.C. Role of modifier in microwave assisted extraction of oleanolic acid from Gymnema sylvestre: Application of green extraction technology for Botanicals. Nat. Prod. Comm. 2009, 4, 1047–1052. [Google Scholar]
- Mandal, V.; Mandal, S.C. Design and performance evaluation of a microwave based low carbon yielding extraction technique for naturally occurring bioactive triterpenoid: Oleanolic acid. Biochem. Eng. J. 2010, 50, 63–70. [Google Scholar] [CrossRef]
- Hisashi, M.; Yuhao, L.; Toshiyuki, M.; Johji, Y.; And Masayuki, Y. Structure-related inhibitory activity of oleanolic acid glycosides on gastric emptying in mice. Bioorg. Med. Chem. 1999, 7, 323–327. [Google Scholar] [CrossRef]
- Davidyans, E.S. Effect of triterpenoid glycosides on α- and β-amylase activity and total protein content in wheat seedlings. Appl. Biochem. Microbiol. 2011, 47, 480–486. [Google Scholar] [CrossRef]
- Maeda, M.; Iwashita, T.; Kurihara, Y. Studies on taste modifiers. Purification and structure determination of gymnemic acids, antisweet active principle from Gymnema sylvestre leaves. Tetrahedron Lett. 1989, 30, 1547–1550. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Di Fabio, G.; Romanucci, V.; Zarrelli, M.; Giordano, M.; Zarrelli, A. C-4 Gem-Dimethylated Oleanes of Gymnema sylvestre and Their Pharmacological Activities. Molecules 2013, 18, 14892-14919. https://doi.org/10.3390/molecules181214892
Di Fabio G, Romanucci V, Zarrelli M, Giordano M, Zarrelli A. C-4 Gem-Dimethylated Oleanes of Gymnema sylvestre and Their Pharmacological Activities. Molecules. 2013; 18(12):14892-14919. https://doi.org/10.3390/molecules181214892
Chicago/Turabian StyleDi Fabio, Giovanni, Valeria Romanucci, Mauro Zarrelli, Michele Giordano, and Armando Zarrelli. 2013. "C-4 Gem-Dimethylated Oleanes of Gymnema sylvestre and Their Pharmacological Activities" Molecules 18, no. 12: 14892-14919. https://doi.org/10.3390/molecules181214892
APA StyleDi Fabio, G., Romanucci, V., Zarrelli, M., Giordano, M., & Zarrelli, A. (2013). C-4 Gem-Dimethylated Oleanes of Gymnema sylvestre and Their Pharmacological Activities. Molecules, 18(12), 14892-14919. https://doi.org/10.3390/molecules181214892









