Synthesis and Biological Activity of 3-[Phenyl(1,3-thiazol-2-yl)-amino]propanoic Acids and Their Derivatives
Abstract
:1. Introduction
2. Results and Discussion
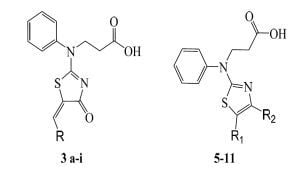
2.1. Synthesis and Structural Peculiarities of New Compounds


2.2. Antimicrobial Evaluation of Synthesized Compounds
| Compound | Staphylococcus aureus | Bacillus cereus | Esherichia coli | Pseudomonas aeruginosa |
|---|---|---|---|---|
| 2 | ni | ni | ni | ni |
| 3a | 250 | 250 | 250 | 250 |
| 3b | 250 | 250 | 250 | 250 |
| 3c | 250 | 250 | 250 | 250 |
| 3d | 250 | 250 | 250 | 250 |
| 3e | 250 | 250 | 250 | 250 |
| 3f | 250 | 250 | 250 | 250 |
| 3g | 250 | 250 | 250 | 250 |
| 3h | ni | ni | ni | ni |
| 3i | 250 | 250 | 250 | 250 |
| 5 | ni | ni | ni | ni |
| 6 | 250 | 250 | 250 | 250 |
| 7 | 250 | 250 | 250 | 250 |
| 8 | 250 | 250 | 250 | 250 |
| 9 | 1000 | 500 | 1000 | ni |
| 10 | 500 | 500 | 500 | 500 |
| 11 | 250 | 250 | 250 | 250 |
| Oxytetracycline | 62.5 | 62.5 | 62.5 | 62.5 |




| Compound | Staphylococcus aureus | Bacillus cereus | Esherichia coli | Pseudomonas aeruginosa |
|---|---|---|---|---|
| 2 | ni | ni | ni | ni |
| 3a | 500 | 1000 | 500 | 500 |
| 3b | 500 | ni | 500 | 1000 |
| 3c | 500 | 1000 | 500 | 500 |
| 3d | 500 | 1000 | 500 | 500 |
| 3e | 350 | 1000 | 500 | 500 |
| 3f | 500 | ni | 500 | 500 |
| 3g | 350 | 350 | 350 | 350 |
| 3h | ni | ni | ni | ni |
| 3i | 250 | 500 | 500 | 500 |
| 4 | ni | 500 | 1000 | 1000 |
| 5 | ni | ni | ni | ni |
| 6 | 500 | ni | 500 | 500 |
| 7 | 500 | ni | 500 | 500 |
| 8 | 250 | ni | 500 | 500 |
| 9 | 1000 | 1000 | 1000 | ni |
| 10 | 1000 | 1000 | 1000 | 1000 |
| 11 | 250 | 250 | 250 | 250 |
| 14 b | ni | 1000 | 1000 | 1000 |
| 14 c | ni | 1000 | 1000 | 1000 |
| 14 i | ni | 1000 | 1000 | 1000 |
| 14 j | ni | 1000 | 1000 | 1000 |
| Oxytetracycline | 62.5 | 62.5 | 250 | 250 |




2.3. Evaluation of Synthesized Compounds on Rapeseed Yield and Biochemical Content
| The concentration of 3-[(4-oxo-4,5-dihydro-1,3-thiazol-2-yl)(phenyl)amino]propanoic acid (2), mg/L | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | 125 | 150 | |
| Yield, t/ha | 1.74 ± 0.11 | 2.07 ± 0.23 | 2.21 ± 0.08 | 2.11 ± 0.28 | 2.20 ± 0.08 | 2.41 ± 0.11 | 2.44 ± 0.20 |
| Seed mass (per 1,000 seeds), g | 3.94 ± 0.16 | 4.17 ± 0.10 | 4.02 ± 0.12 | 3.81 ± 0.16 | 3.96 ± 0.09 | 3.85 ± 0.14 | 3.94 ± 0.21 |
| Oil content, kg/t | 239.9 ± 1.7 | 283.4 ± 8.3 | 271.8 ± 7.5 | 334.3 ± 5.7 | 301.2 ± 4.9 | 281.8 ± 7.9 | 296.7 ± 6.5 |
| Protein content, mg/100g | 15.9 ± 0.1 | 27.1 ± 0.3 | 30.7 ± 0.3 | 31.2 ± 0.3 | 30.3 ± 0.3 | 22.8 ± 0.4 | 22.5 ± 0.2 |
| Ash, % | 4.53 ± 0.02 | 4.53 ± 0.05 | 4.48 ± 0.04 | 4.23 ± 0.10 | 4.16 ± 0.10 | 4.07 ± 0.14 | 4.10 ± 0.13 |
| Fatty acid, % | The concentration of 3-[(4-oxo-4,5-dihydro-1,3-thiazol-2-yl)(phenyl)amino]propanoic acid (2), mg/L | ||||||
|---|---|---|---|---|---|---|---|
| 0 | 25 | 50 | 75 | 100 | 125 | 150 | |
| Palmitic | 5.11 ± 0.05 | 4.57 ± 0.06 | 4.79 ± 0.22 | 4.59 ± 0.07 | 5.23 ± 0.31 | 4.90 ± 0.09 | 4.76 ± 0.03 |
| Stearic | 2.11 ± 0.08 | 2.02 ± 0.02 | 2.07 ± 0.01 | 2.02 ± 0.01 | 2.05 ± 0.01 | 2.04 ± 0.01 | 2.03 ± 0.01 |
| Oleic | 61.04 ± 0.26 | 61.07 ± 0.09 | 61.51 ± 0.03 | 61.28 ± 0.02 | 60.98 ± 0.08 | 61.21 ± 0.18 | 61.41 ± 0.00 |
| Linoleic | 20.55 ± 0.13 | 20.83 ± 0.09 | 20.61 ± 0.01 | 20.96 ± 0.06 | 20.93 ± 0.02 | 20.84 ± 0.05 | 20.85 ± 0.5 |
| Eicosenoic | 1.02 ± 0.08 | 1.09 ± 0.03 | 1.07 ± 0.04 | 1.05 ± 0.01 | 0.93 ± 0.07 | 1.02 ± 0.02 | 0.99 ± 0.01 |
| Linolenic | 8.49 ± 0.03 | 8.75 ± 0.02 | 8.54 ± 0.02 | 8.66 ± 0.02 | 8.67 ± 0.01 | 8.64 ± 0.01 | 8.60 ± 0.02 |
- (a)
- 100 mg/L: the highest number of pods and siliques was obtained;
- (b)
- 150 mg/L: the highest seed yield was obtained;
- (c)
- 25 mg/L: the highest seed mass was obtained.

3. Experimental
3.1. General
3.2. General Procedure for Preparation of 3-{[(5Z)-5-Substituted 4-oxo-4,5-dihydro-1,3-thiazol-2-yl](phenyl)amino}propanoic Acids 3a–i
3.3. General Synthesis of Hydrazones 14 b, c, i, j
3.4. Antimicrobial Activity
3.5. Field Trials and Rapeseed Biochemical Ontent
4. Conclusions
Conflicts of Interest
References
- Juaristi, E.; Soloshonok, V.A. Enantioselective Synthesis of β-amino Acids (E), 2nd ed.; John Wiley & Sons, Inc.: Haboken, NJ, USA, 2005; pp. 477–614. [Google Scholar]
- Kouatly, O.; Geronikaki, A.; Kamoutsis, C.; Hadjipavlou-Litina, D.; Eleftheriou, P. Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem. 2008, 20, 1–7. [Google Scholar]
- Carradori, S.; Secci, D.; Bolasco, A.; De Monte, C.; Yáñez, M. Synthesis and selective inhibitory activity against human COX-1 of novel 1-(4-substituted-thiazol-2-yl)-3,5-di(hetero)aryl-pyrazoline derivatives. Arch. Pharm. 2012, 345, 973–979. [Google Scholar] [CrossRef]
- Holla, B.S.; Malini, K.V.; Rao, B.S.; Sarojini, B.K.; Kumari, N.S. Synthesis of some new 2,4-disubstituted thiazoles as possible antibacterial and anti-inflammatory agents. Eur. J. Med. Chem. 2003, 38, 313–318. [Google Scholar] [CrossRef]
- Toyoshi, K.; Misako, A.; Masako, K.; Katsuya, O.; Shigekatsu, K.; Tamatsu, M.; Takeshi, I. Syntheses and antiinflammatory activity of malonamic acid, malonamate and malonamide derivatives of some heterocyclic compounds. Chem. Pharm. Bull. 1985, 33, 4878–4888. [Google Scholar] [CrossRef]
- Dovlatyan, V.V.; Eliazyan, K.A.; Pivazyan, V.A.; Kazaryan, E.A.; Engoyan, A.P. Thiazolecarboxylic acid derivatives. 1. n-substituted 2-amino-4-methylthiazole-5-carboxylic acid derivatives. Chem. Heterocycl. Compd. 2004, 40, 84–89. [Google Scholar] [CrossRef]
- Zitouni, G.; Demirayak, S.; Ozdemir, A.; Kaplancikli, Z.; Yildiz, M. Synthesis of some 2-[(benzazole-2-yl)thioacetylamino]thiazole derivatives and their antimicrobial activity and toxicity. Eur. J. Med. Chem. 2004, 39, 267–272. [Google Scholar] [CrossRef]
- Argyropoulou, I.; Gerinikaki, A.; Vicini, P.; Zani, F. Synthesis and biological evaluation of sulfonamide thiazole and benzothiazole derivatives as antimicrobial agents. Arkivoc 2009, 18, 89–102. [Google Scholar] [CrossRef]
- Prakasha, K.C.; Raghavendra, G.M.; Harisha, R.; Gowda, D.C. Design, Synthesis and antimicrobial screening of amino acids conjugated 2-amino-4-arylthiazole derivatives. Int. J. Pharm. Sci. 2011, 3, 120–125. [Google Scholar]
- Price, K.E.; Chisholm, D.R.; Godfrey, J.C.; Misiek, M.; Gourevitch, A. Semisynthetic coumermycins: Structure-activity relationships. Appl. Microbiol. 1970, 19, 14–26. [Google Scholar]
- Nair, S.; Garg, S.P.; Sah, P. Synthesis and antibicrobial activity of some new oxazolone derivatives of 4,5-disubstituted-2-aminothiazoles. J. Indian Chem. Soc. 2006, 83, 205–207. [Google Scholar]
- Qin, X.; Yu, H.; Liu, J.; Dai, H.; Bing, G.; Qin, Z.; Zhang, X.; Wang, T.; Fang, J. Synthesis and biological activity of novel N-(5-((1H-1,2,4-triazol-1-yl)methyl)-4-tert-butylthiazol-2-yl)-4-carboxamide derivatives. Arkivoc 2009, 2, 201–210. [Google Scholar]
- Chen, B.C.; Zhao, R.; Wang, B.; Droghini, R.; Lajeunesse, J.; Sirard, P.; Endo, M.; Balasubramanian, B.; Barrish, J.C. A new and efficient preparation of 2-aminothiazole-5-carbamides: applications to the synthesis of the anti-cancer drug dasatinib. Arkivoc 2010, 6, 32–38. [Google Scholar]
- Al-Saadi, M.S.; Faidallah, H.M.; Rostom, S.A. Synthesis and biological evalution of some 2,4,5-trisubstituted thiazole derivatives as potencial antimicrobial and anticancer agents. Arch. Pharm. Chem. Life Sci. 2008, 341, 624–634. [Google Scholar] [CrossRef]
- Wityak, J.; Das, J.; Moquin, R.V.; Shen, Z.; Lin, J.; Chen, P.; Doweyko, A.M.; Pitt, S.; Pang, S.; Shen, D.R.; et al. Discovery and initial SAR of 2-amino-5-carboxamidothiazoles as inhibitors of the Src-family kinase p56Lck. Bioorg. Med. Chem. Lett. 2003, 3, 4007–4010. [Google Scholar]
- Amr, A.E.; Sabrry, N.M.; Abdalla, M.M.; Abdel-Wahab, B.F. Synthesis, Antiarrhytmic and anticoagulant activities of novel thiazolo derivatives from methyl 2-(thiazol-2-ylcarbamoyl)acetate. Eur. J. Med. Chem. 2009, 44, 725–735. [Google Scholar] [CrossRef]
- Harnett, J.J.; Roubert, V.; Dolo, C.; Charnet, C.; Spinnewyn, B.; Cornet, S.; Rolland, A.; Marin, J.G.; Bigg, D.; Chabrier, P.E. Phenolic thiazoles as novel orally-active neuroprotective agents. Bioorg. Med. Chem. Lett. 2004, 14, 157–160. [Google Scholar] [CrossRef]
- Patt, W.C.; Hamilton, H.W.; Taylor, M.D. Structure-activity relationships of a series of 2-amino-4-thiazole-containing rennin inhibitors. Med. Chem. 1992, 35, 2562–2572. [Google Scholar] [CrossRef]
- Kearney, P.C.; Fernandez, M.; Flygare, J.A. Solid-phase synthesis of 2-aminothiazoles. J. Org. Chem. 1998, 63, 196–200. [Google Scholar] [CrossRef]
- Haddad, N.; Tan, J.; Farina, V. Convergent synthesis of the quinolone substructure of BILN 2061 via carbonylative sonogashira coupling/cyclization. J. Org. Chem. 2006, 71, 5031–5034. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Qazi, S.; Samuel, P.; Uckun, F.M. Substituted heterocyclic thiourea compounds as a new class of anti-allergic agents inhibiting IgE/FCeRI receptor mediated mast cell leukotriene release. Bioorg. Med. Chem. 2003, 11, 1095–1105. [Google Scholar] [CrossRef]
- Hargrave, K.D.; Hess, F.K.; Oliver, J.T. N-(4-Substituted-thiazolyl)oxamic acid derivatives, a new series of potent, orally active antiallergy agents. J. Med. Chem. 1983, 26, 1158–1163. [Google Scholar] [CrossRef]
- Venkatachalam, T.K.; Mao, C.; Uckun, F.M. Effect of stereochemistry on the anti-HIV activity of chiral thiourea compounds. Bioorg. Med. Chem. 2004, 12, 4275–4284. [Google Scholar] [CrossRef]
- Bell, F.W.; Cantrell, A.S.; Hogberg, M.; Jaskunas, S.R.; Johanson, N.G.; Zhou, X. Phenethylthiazoletthiourea (PETT) compounds, a new class of HIV-1 reverse transcriptase inhibitors. 1. Synthesis and basic structure-activity relationship studies of PETT analogs. J. Med. Chem. 1995, 38, 4929–4936. [Google Scholar] [CrossRef]
- Barf, T.; Vallgarda, J.; Emond, R.; Ohman, B.; Alberts, P.; Abrahmsen, L. Arylsulfonamidothiazoles as a new class of potencial antidiabetic drugs, discovery of potent and selective inhibitors of the 11β-hydroxysteroid dehydrogenase type 1. J. Med. Chem. Lett. 2002, 45, 3813–3815. [Google Scholar]
- Guannessi, F.; Chiodi, P.; Marzi, M.; Minetti, P.; Pessotto, P.; Tinti, M.; Carminati, P.; Arduini, A. Reversible carnitine palmitoyltransferase inhibitors with broad chemical diversity as potencial antidiabetic agents. J. Med. Chem. 2001, 44, 2383–2386. [Google Scholar] [CrossRef]
- Bienayme, H.; Bouzid, K. A new heterocyclic multicomponent reaction for the combinatorial synthesis of fused 3-aminoimidazoles. Angew. Chem. Int. Ed. 1998, 37, 2234–2237. [Google Scholar] [CrossRef]
- Maradiya, H.; Patel, V.S. Synthesis and dyeing performance of some novel heterocyclic azo disperse dyes. J. Braz. Chem. Soc. 2001, 12, 710–714. [Google Scholar]
- Hu, D.; Liu, S.; Huang, T.; Yang, H.; Zhang, A. Synthesis and herbicidal activities of novel 4-(4-(5-methyl-3-arylisoxazol-4-yl)thiazol-2-yl)piperidyl carboxamides and thiocarboxyamides. Molecules 2009, 14, 1288–1303. [Google Scholar] [CrossRef]
- Geronikaki, A.; Hadjipavlou-Litina, D.; Chatziopoulos, C.; Soloupis, G. Synthesis and biological evaluation of 4,5-disubstituted-thiazolyl amides, derivatives of 4-hydroxy-piperidine or of 4-N-methylpiperazine. Molecules 2003, 8, 472–479. [Google Scholar] [CrossRef]
- Vaickelioniene, R.; Mickevicius, V. Cyclization of N-fluorophenyl-β-alanines and properties of the obtained products. Chem. Heterocycl. Compd. 2006, 6, 862–869. [Google Scholar]
- Mickevicius, V.; Patupaite, A. Cyclization of N-(2,4,6-trimethylphenyl)-β-alanines. Chem. Heterocycl. Compd. 2000, 7, 951–954. [Google Scholar]
- Vaickelionienė, R.; Mickevičius, V.; Mikulskiene, G. Synthesis and cyclizations of N-(2,3-,3,4- and 3,5-dimethylphenyl)-β-alanines. Molecules 2005, 10, 407–416. [Google Scholar] [CrossRef]
- Blėkaitytė, V.; Jonuškienė, I.; Mickevičius, V. The influence of n-substituted β-alanines containing naphthoquinone and thiazole moieties on the growth of st. John’s wort (Hypericum perforatum L.) and its ability to accumulate metabolites. Chem. Tech. 2012, 4, 29–35. [Google Scholar]
- Chowdhry, M.M.; Mingos, D.M.P.; White, A.J.P.; Williams, D.J. Syntheses and characterization of 5-substituted hydantoins and thiazolines—Implications for crystal engineering of hydrogen bonded assemblies. Crystal structures of 5-(2-pyridylmethylene)-hydantoin, 5-(2-pyridylmethylene)-2-thiohydantoin, 5-(2-pyridylmethylene)thiazolidine-2,4-dione, 5-(2-pyridylmethylene)rhodanine and 5-(2-pyridylmethylene)pseudothiohydantoin. Chem. Soc. Perkin Trans. 2000, 1, 3495–3504. [Google Scholar]
- El-Najjar, N.; Gali-Muhtasib, H.; Ketota, R.A.; Vuorela, P.; Urtti, A.; Vuorela, H. The chemical and biological activities of quinones: Overview and implications in analytical detection. Phytochem. Rev. 2011, 10353–103370. [Google Scholar]
- Burg, M.B.; Ferraris, J.D. Intracellular organic osmolytes: Function and regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef]
- Stasevych, M.; Lubenets, V.; Musyanovych, R.; Novikov, V.; Mickevičius, V.; Beresnevičius, Z.J.; Rutkauskas, K. Novel thiazolone derivatives of N-aryl-β-alanines. Chem. Heterocycl. Compd. 2011, 47, 1050–1052. [Google Scholar] [CrossRef]
- Franklin, A.; Acar, J.; Anthony, F.; Gupta, R.; Nicholls, T.; Tamura, Y.; Thompson, S.; Thelfall, E.J.; Vose, D.; van Vuuren, M.; et al. Antimicrobial resistance: Harmonisation of national antimicrobial resistance monitoring and surveillance programmes in animals and in animal-derived food. Rev. Sci. Tech. Off. Int. Epiz. 2001, 20, 859–870. [Google Scholar]
- Goodson, B.; Ehrhardt, A.; Ng, S.; Nuss, J.; Johnson, K.; Giedlin, M.; Yamamoto, R.; Krebber, A.; Ladner, M.; Giacona, M.B.; et al. Characterization of novel antimicrobial peptoids. Antimicrob. Agents Chemother. 1999, 43, 1429–1434. [Google Scholar]
- Petersen, A.; Aarestrup, F.M.; Hofshagen, M.; Sipilä, H.; Franklin, A.; Gunnarsson, E. Harmonization of Antimicrobial Susceptibility Testing among Veterinary Diagnostic Laboratories in the Five Nordic Countries. Microb. Drug Resist. 2003, 9, 381–388. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1–14 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mickevičius, V.; Voskienė, A.; Jonuškienė, I.; Kolosej, R.; Šiugždaitė, J.; Venskutonis, P.R.; Kazernavičiūtė, R.; Brazienė, Z.; Jakienė, E. Synthesis and Biological Activity of 3-[Phenyl(1,3-thiazol-2-yl)-amino]propanoic Acids and Their Derivatives. Molecules 2013, 18, 15000-15018. https://doi.org/10.3390/molecules181215000
Mickevičius V, Voskienė A, Jonuškienė I, Kolosej R, Šiugždaitė J, Venskutonis PR, Kazernavičiūtė R, Brazienė Z, Jakienė E. Synthesis and Biological Activity of 3-[Phenyl(1,3-thiazol-2-yl)-amino]propanoic Acids and Their Derivatives. Molecules. 2013; 18(12):15000-15018. https://doi.org/10.3390/molecules181215000
Chicago/Turabian StyleMickevičius, Vytautas, Aušra Voskienė, Ilona Jonuškienė, Ramūnė Kolosej, Jūratė Šiugždaitė, Petras Rimantas Venskutonis, Rita Kazernavičiūtė, Zita Brazienė, and Elena Jakienė. 2013. "Synthesis and Biological Activity of 3-[Phenyl(1,3-thiazol-2-yl)-amino]propanoic Acids and Their Derivatives" Molecules 18, no. 12: 15000-15018. https://doi.org/10.3390/molecules181215000
APA StyleMickevičius, V., Voskienė, A., Jonuškienė, I., Kolosej, R., Šiugždaitė, J., Venskutonis, P. R., Kazernavičiūtė, R., Brazienė, Z., & Jakienė, E. (2013). Synthesis and Biological Activity of 3-[Phenyl(1,3-thiazol-2-yl)-amino]propanoic Acids and Their Derivatives. Molecules, 18(12), 15000-15018. https://doi.org/10.3390/molecules181215000