Synthesis, Antibacterial and Antifungal Activity of Some New Pyrazoline and Pyrazole Derivatives
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.1.1. Preparation of the Chalcones 2–4
2.1.2. Synthesis of Pyrazoline Derivatives 5–9 and Isoxazoline Derivatives 10–12

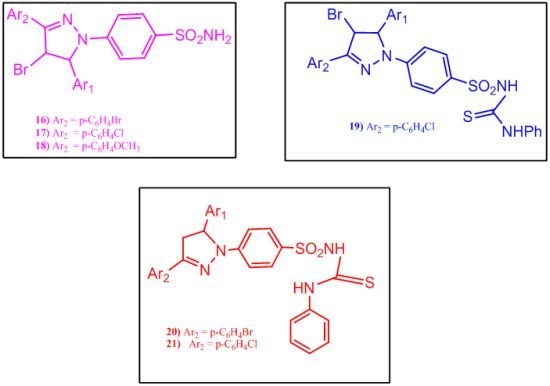
2.1.3. Synthesis of Benzenesulfonamide Derivatives 13–21

2.1.4. Synthesis of [1,2,4]Triazolo[3,4-c][1,2,4]triazino[5,6-b]-5-N-(phenylcarbamothioyl) Ethanoic Acid Hydrazide Derivatives 30, 31 and 3-Methyl-4-(propan-2-ylidene)-1H-pyrazol-5(4H)-one Derivatives 34, 35

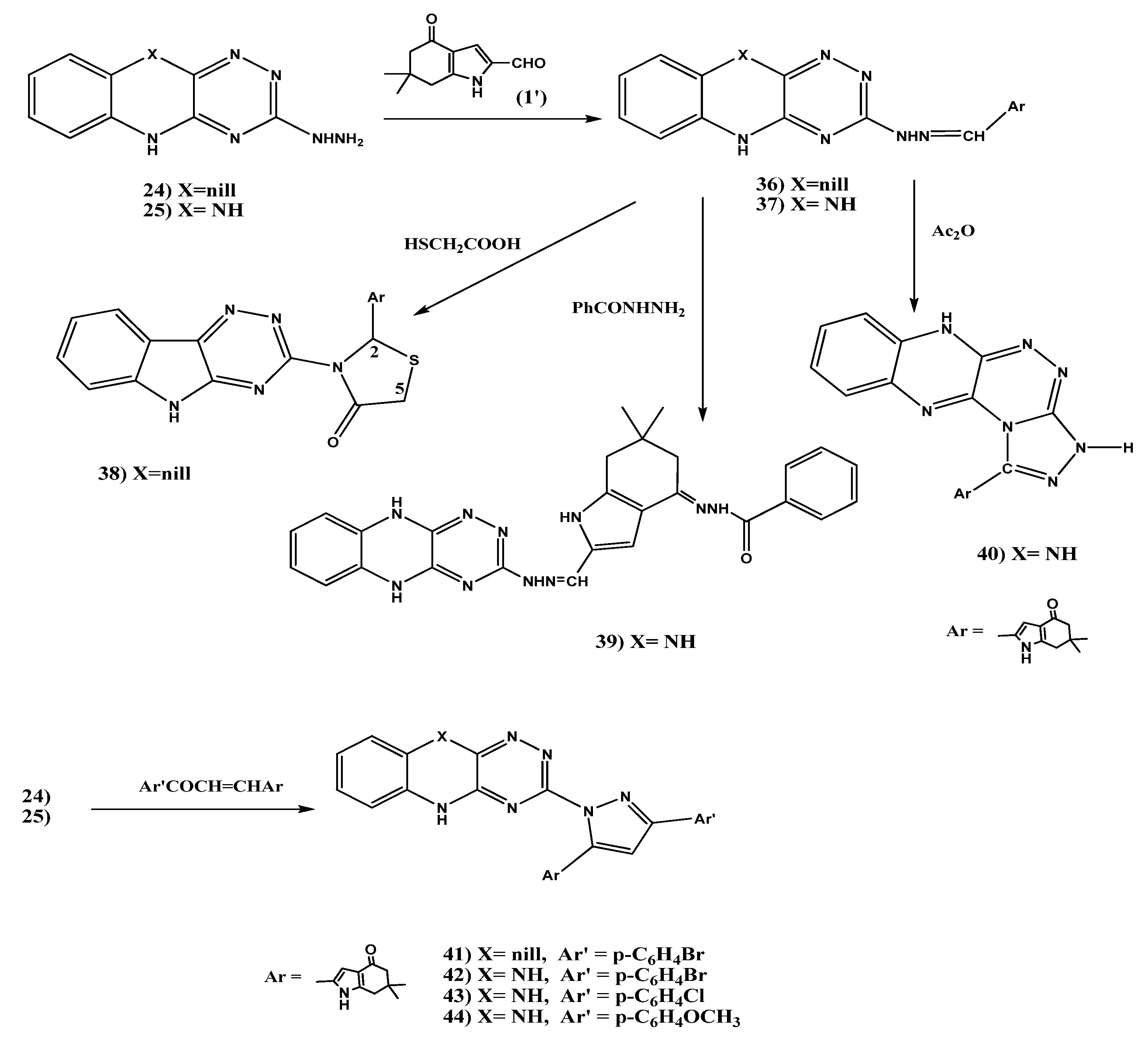
2.1.5. Synthesis of Schiff’s Bases 36, 37, 4-Oxo-4,5,6,7-tetrahydro-1H-indol-2-yl)thiazolidin-4-one (38), Benzohydrazide Derivative 39, 1,2,4]Triazolo[3,4-c]-5,10-dihydro [1,2,4]triazino[5,6-b] quinoxaline (40), and Pyrazole Derivatives 41–44

2.2. Pharmacological Screening
| Microorganism | Escherichia coli | Staphylococcus aureus | Candida albicans | Pseudomonas aeruginosa | ||||
|---|---|---|---|---|---|---|---|---|
| IZ | MIC | IZ | MIC | IZ | MIC | IZ | MIC | |
| ampicillin 10.0 µg/disc | 18 | 25 | 22 | 12.5 | ----- | ------ | ----- | ----- |
| ciprofloxacin 5.0 µg/disc | 28 | 12.5 | 30 | 25 | ----- | ------ | 38 | 25 |
| clotrimazole 100.0 µg/disc | ---- | ----- | ----- | ------ | 40 | 12.5 | ----- | ------ |
| imipenam 10.0 µg/disc | 26 | ----- | 30 | ------ | ----- | ---- | 30 | ------ |
| 2 | 18 | 200 | 17 | 200 | 21 | 200 | 16 | 200 |
| 3 | 19 | 200 | 17 | 200 | 24 | 200 | 18 | 200 |
| 4 | 19 | 200 | 15 | 200 | 23 | 200 | 18 | 200 |
| 5 | 19 | 200 | 15 | 200 | 23 | 200 | 19 | 200 |
| 6 | 19 | 200 | 16 | 200 | 25 | 200 | 20 | 200 |
| 7 | 18 | 200 | 13 | 200 | 21 | 200 | 16 | 200 |
| 8 | 19 | 200 | 17 | 200 | 22 | 200 | 18 | 200 |
| 9 | 18 | 200 | 16 | 200 | 24 | 200 | 18 | 200 |
| 10 | 19 | 200 | 16 | 200 | 24 | 200 | 20 | 200 |
| 11 | 19 | 200 | 18 | 100 | 23 | 200 | 19 | 200 |
| 12 | 19 | 200 | 15 | 200 | 23 | 200 | 18 | 200 |
| 13 | 18 | 200 | 19 | 200 | 23 | 200 | 18 | 200 |
| 14 | 18 | 200 | 15 | 200 | 23 | 200 | 18 | 200 |
| 15 | 18 | 200 | 16 | 100 | 22 | 200 | 19 | 200 |
| 16 | 19 | 200 | 26 | 25 | 27 | 50 | 20 | 200 |
| 17 | 19 | 200 | >50 | 100 | >40 | 50 | 21 | 100 |
| 18 | 19 | 200 | 20 | 100 | 23 | 200 | 19 | 200 |
| 19 | 19 | 200 | 16 | 100 | 25 | 12.5 | 19 | 200 |
| 20 | 18 | 200 | 16 | 200 | 26 | 12.5 | 18 | 200 |
| 21 | 18 | 200 | 16 | 200 | 28 | 50 | 20 | 200 |
| 22 | 19 | 200 | 16 | 200 | 25 | 50 | 18 | 200 |
| 23 | 18 | 200 | 8 | 200 | 24 | 200 | 20 | 200 |
| 24 | 18 | 200 | 16 | 200 | 23 | 200 | 20 | 200 |
| 25 | 19 | 200 | 15 | 200 | 22 | 200 | 18 | 200 |
| 26 | 19 | 200 | 13 | 200 | 23 | 200 | 19 | 200 |
| 27 | 19 | 200 | 17 | 200 | 25 | 200 | 17 | 200 |
| 28 | 19 | 200 | 17 | 200 | 23 | 200 | 18 | 200 |
| 29 | 18 | 200 | 15 | 200 | 21 | 200 | 18 | 200 |
| 30 | 19 | 200 | 17 | 200 | 26 | 200 | 16 | 200 |
| 31 | 19 | 200 | 16 | 200 | 34 | 25 | 20 | 200 |
| 32 | 19 | 200 | 16 | 200 | 23 | 200 | 20 | 200 |
| 33 | 19 | 200 | 17 | 200 | 24 | 200 | 18 | 200 |
| 34 | 19 | 200 | 17 | 200 | 24 | 200 | 17 | 200 |
| 35 | 19 | 200 | 16 | 100 | 23 | 200 | 19 | 200 |
| 36 | 19 | 200 | 17 | 200 | 24 | 200 | 18 | 200 |
| 37 | 17 | 200 | 15 | 200 | 22 | 200 | 18 | 200 |
| 38 | 19 | 200 | 17 | 200 | 21 | 200 | 18 | 200 |
| 39 | 19 | 200 | 17 | 200 | 22 | 200 | 16 | 200 |
| 40 | 19 | 200 | 17 | 200 | 21 | 200 | 18 | 200 |
| 41 | 18 | 200 | 13 | 200 | 21 | 200 | 16 | 200 |
| 42 | 18 | 200 | 16 | 200 | 23 | 200 | 18 | 200 |
| 43 | 18 | 200 | 17 | 100 | 24 | 200 | 19 | 200 |
| 44 | 20 | 200 | 17 | 100 | 23 | 200 | 19 | 200 |
| DMF | 18 | 13 | 21 | 16 | ||||
3. Experimental
3.1. General Methods
3.2. General Procedure for the Preparation of Compounds 2–4
3.3. General Procedure for the Preparation of Compounds 5–7
3.4. General Procedure for the Preparation of Compounds 8 and 9
3.5. General Procedure for the Preparation of Compounds 10–12
3.6. General Procedure for the Preparation of Compounds 13–15
3.7. General Procedure for the Preparation of Compounds 16–18
3.8. 4-(4-Bromo-3-aryl-5-(6,6-dimethyl-4-oxo-4,5,6,7-tetrahydro-1H-indol-2-yl)-1H-pyrazol-1-yl)-N-(phenylcarbamothioyl)benzenesulfonamide (19)
3.9. General Procedure for the Preparation of Compounds 20 and 21
3.10. 5H-[1,2,4]Triazino[5,6-b]indole-3-thiol (22)
3.11. 5,10-Dihydro-[1,2,4]triazino[5,6-b]quinoxaline-3-thiol (23)
3.12. 3-Hydrazinyl-5H-[1,2,4]triazino[5,6-b]indole (24)
3.13. 3-Hydrazinyl-5,10-dihydro-[1,2,4]triazino[5,6-b]quinoxaline (25)
3.14. Ethyl [1,2,4]triazolo[3,4-c][1,2,4]triazino[5,6-b]-5H-indole-5-ethanoate (26)
3.15. Ethyl [1,2,4]triazolo[3,4-c][1,2,4]triazino[5,6-b]-5,10-dihydroquinoxaline-5-ethanoate (27)
3.16. [1,2,4]Triazolo[3,4-c][1,2,4]triazino[5,6-b]-5H-indole-5-ethanoic acid hydrazide (28)
3.17. [1,2,4]Triazolo[3,4-c][1,2,4]triazino[5,6-b]-5,10-dihydroquinoxaline-5-ethanoic acid hydrazide (29)
3.18. [1,2,4]Triazolo[3,4-c][1,2,4]triazino[5,6-b]-5H-indole-5-N-(phenylcarbamothioyl) ethanoic acid hydrazide (30)
3.19. [1,2,4]Triazolo[3,4-c][1,2,4]triazino[5,6-b]-5,10-dihydroquinoxaline-5-N-(phenylcarbamothioyl)-ethanoic acid hydrazide (31)
3.20. 1-(5H-[1,2,4]Triazino[5,6-b]indol-3-yl)-3-methyl-1H-pyrazol-5(4H)-one (32)
3.21. 1-(5,10-Dihydro-[1,2,4]triazino[5,6-b]quinoxalin-3-yl)-3-methyl-1H-pyrazol-5(4H)-one (33)
3.22. 1-(5H-[1,2,4]Triazino[5,6-b]indol-3-yl)-3-methyl-4-(propan-2-ylidene)-1H-pyrazol-5(4H)-one (34)
3.23. 1-(5,10-Dihydro-[1,2,4]triazino[5,6-b]quinoxalin-3-yl)-3-methyl-4-(propan-2-ylidene)-1H-pyrazol-5(4H)-one (35)
3.24. 2-((2-(5H-[1,2,4]Triazino[5,6-b]indol-3-yl)hydrazono)methyl)-6,6-dimethyl-6,7-dihydro-1H-indol-4(5H)-one (36)
3.25. 2-((2-(5,10-Dihydro-[1,2,4]triazino[5,6-b]quinoxalin-3-yl)hydrazono)methyl)-6,6-dimethyl-6,7-dihydro-1H-indol-4(5H)-one (37)
3.26. 3-(5H-[1,2,4]Triazino[5,6-b]indol-3-yl)-2-(6,6-dimethyl-4-oxo-4,5,6,7-tetrahydro-1H-indol-2-yl)thiazolidin-4-one (38)
3.27. N'-2-((2-(5,10-Dihydro-[1,2,4]triazino[5,6-b]quinoxalin-3-yl)hydrazono)methyl)-6,6-dimethyl-6,7-dihydro-1H-indol-4(5H)-ylidene)benzohydrazide (39)
3.28. 3-(6,6-Dimethyl-6,7-dihydro-1H-indol-4(5H)-one)-1,5-dihydro-[1,2,4]triazolo[3,4-c]-5,10-dihydro-[1,2,4]triazino[5,6-b]quinoxaline (40)
3.29. 2-(1-(5H-[1,2,4]Triazino[5,6-b]indol-3-yl)-3-(4-bromophenyl)-1H-pyrazol-5-yl)-(6,6-dimethyl-6,7-dihydro-1H-indol-4(5H)-one (41)
3.30. General Procedure for the Preparation of Compounds 42–44
3.31. Biological Activity Assay
3.31.1. Inhibition Zone Measurement (IZ)
3.31.2. Minimal inhibitory concentration (MIC)
4. Conclusions
Acknowledgments
Supplementary Materials
References
- Vogel, S.; Ohmayer, S.; Brunner, G.; Heilmann, J. Natural and nonnatural prenylated chalcones: Synthesis, cytotoxicity and antioxidative activity. Bioorg. Med. Chem. 2008, 16, 4286–4293. [Google Scholar] [CrossRef]
- Babasaheb, P.B.; Sachin, A.P.; Rajesh, N.G.; Balaji, L.K.; Balwant, S.H.; Santosh, N.K.; Shivkumar, S.J. Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett. 2010, 20, 730–733. [Google Scholar]
- Avila, H.P.; Smania, E.; Monache, F.D.; Smania, A. Structure-activity relationship of antibacterial chalcones. Bioorg. Med. Chem. 2008, 16, 9790–9794. [Google Scholar] [CrossRef]
- Suryawanshi, S.N.; Chandra, N.; Kumar, P.; Porwal, J.; Gupta, S. Chemotherapy of leishmaniasis part-VIII: Synthesis and bioevaluation of novel chalcones. Eur. J. Med. Chem. 2008, 43, 2473–2478. [Google Scholar] [CrossRef]
- Lawrence, N.J.; Patterson, R.P.; Ooi, L.L.; Cook, D.; Ducki, S. Effects of α-substitutions on structure and biological activity of anticancer chalcones. Bioorg. Med. Chem. Lett. 2006, 16, 5844–5848. [Google Scholar] [CrossRef]
- Mojzis, J.; Varinska, L.; Mojzisova, G.; Kostova, I.; Mirossay, L. Antiangiogenic effects of flavonoids and chalcones. Pharmacol. Res. 2008, 57, 259–265. [Google Scholar] [CrossRef]
- Cheng, J.H.; Hung, C.F.; Yang, S.C.; Wang, J.P.; Won, S.J.; Lin, C.N. Synthesis And Cytotoxic, Anti-Inflammatory, and Anti-Oxidant Activities Of 2',5'-Dialkoxyl chalcones As Cancer Chemopreventive Agents. Bioorg. Med. Chem. 2008, 16, 7270–7276. [Google Scholar] [CrossRef]
- Lahtchev, K.L.; Batovska, D.I.; Parushev, S.P.; Ubiyvovk, V.M.; Sibirny, A.A. Antifungal activity of chalcones: A mechanistic study using various yeast strains. Eur. J. Med. Chem. 2008, 43, 2220–2228. [Google Scholar] [CrossRef]
- Liu, M.; Wilairat, P.; Cropft, S.L.; Tan, A.L.C.; Go, M.L. Structure-activity relationships of antileishmanial and antimalarial chalcones. Bioorg. Med. Chem. 2003, 11, 2729–2738. [Google Scholar] [CrossRef]
- Go, M.L.; Wu, X.; Liu, X.L. Chalcones: An update on cytotoxic and chemoprotective properties. Curr. Med. Chem. 2005, 12, 483–499. [Google Scholar] [CrossRef]
- Liu, M.; Wilairat, P.; Go, M.L. Antimalarial alkoxylated and hydroxylated chalcones: Structure activity relationship analysis. J. Med. Chem. 2001, 44, 4443–4452. [Google Scholar] [CrossRef]
- Bhat, B.A.; Dhar, K.L.; Puri, S.C.; Saxena, A.K.; Shanmugavel, M.; Qazi, G.N. Synthesis and biological evaluation of chalcones and their derived pyrazoles as potential cytotoxic agents. Bioorg. Med. Chem. Lett. 2005, 15, 3177–3180. [Google Scholar] [CrossRef]
- Bandgar, B.P.; Gawande, S.S.; Bodade, R.G.; Gawande, N.M.; Khobragade, C.N. Synthesis and biological evaluation of a novel series of pyrazole chalcones as anti-inflammatory, antioxidant and antimicrobial agents. Bioorg. Med. Chem. 2009, 17, 8168–8173. [Google Scholar] [CrossRef]
- Zeba, N.S.; Mohammed, M.T.N.; Anis, A.; Asad, U.K. Thermal solvent-free synthesis of novel pyrazolyl chalcones and pyrazolines as potential antimicrobial agents. Bioorg. Med. Chem. Lett. 2011, 21, 2860–2865. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Manfredini, S.; Romagnoli, R.; Stevanato, L.; Zaid, A.N.; Manservigi, R. Synthesis andAnti-HSV-1 Activity of 6 Substituted Pyrazolo[3,4-d]Pyridazin-7-one Nucleosides’. NucleosidesNucleotides Nucleic Acids 1998, 17, 2165–2173. [Google Scholar] [CrossRef]
- Baraldi, P.G.; Bovero, A.; Fruttarolo, F.; Romagnoli, R.; Tabrizi, M.A.; Preti, D.; Varani, K.; Borea, P.A.; Moorman, A.R. New Strategies for the Synthesis of A3 Adenosine Receptor Antagonists. Bioorg. Med. Chem. 2003, 11, 4161–4169. [Google Scholar] [CrossRef]
- Zitouni, G.T.; Chevallet, P.; Kiliç, F.S.; Erol, K. Synthesis of some thiazolyl-pyrazoline derivatives and preliminary investigation of their hypotensive activity. Eur. J. Med. Chem. 2000, 35, 635–641. [Google Scholar] [CrossRef]
- Palaska, E.; Aytemir, M.; Uzbay, I.T.; Erol, D. Synthesis and antidepressant activities of some 3,5-diphenyl-2-pyrazolines. Eur. J. Med. Chem. 2001, 36, 539–543. [Google Scholar] [CrossRef]
- Johnson, M.; Younglove, B.; Lee, L.; LeBlanc, R.; Holt, H.; Hills, P.; Mackay, H.; Brown, T.; Mooberry, L.S.; Lee, M. Design, synthesis, and biological testing of pyrazoline derivatives of combretastatin-A4. Bioorg. Med. Chem. Lett. 2007, 17, 5897–5901. [Google Scholar]
- Ratkovic, Z.; Juranic, Z.D.; Stanojkovic, T.; Manojlovic, D.; Vukicevic, R.D.; Radulovic, N.; Joksović, M.D. Synthesis, characterization, electrochemical studies and antitumor activity of some new chalcone analogues containing ferrocenyl pyrazole moiety. Bioorg. Chem. 2010, 38, 26–32. [Google Scholar] [CrossRef]
- Rafia, B.; Syed, O.; Shafiya, Y.; Hinna, H.; Alam, M.S.; Mohammad, S.; Surender, S.; Kalim, J. Synthesis of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide as anticancer and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2011, 21, 4301–4305. [Google Scholar]
- Sameena, B.; Kalim, J.; Shamim, A.; Rathish, I.G.; Surender, S.; Alam, M.S. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur. J. Med. Chem. 2011, 46, 5763–5768. [Google Scholar] [CrossRef]
- Rathish, I.G.; Kalim, J.; Shamim, A.; Sameena, B.; Alam, M.S.; Pillai, K.K.; Surender, S.; Vivek, B. Synthesis and antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg. Med. Chem. Lett. 2009, 19, 255–258. [Google Scholar]
- Joshi, S.; Mandhane, P.G.; Diwakar, S.D.; Dabhade, S.K.; Gill, C.H. Synthesis, analgesic and anti-inflammatory activities of some novel pyrazolines derivatives. Bioorg. Med. Chem. Lett. 2010, 20, 3721–3725. [Google Scholar] [CrossRef]
- Mohammad, A.; Harish, K.; Suroor, A.K. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Pawan, K.S.; Satish, K.; Pawan, K.; Pawan, K.; Dhirender, K.; Yogita, D.; Kamal, R.A. Synthesis and biological evaluation of some pyrazolylpyrazolines as anti-inflammatory-antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2650–2655. [Google Scholar] [CrossRef]
- Suresh, K.; Veeresh, M.; Prashant, A.; Mahesh, P.; Pradeep, K.R.; Shivalingarao, M.; Thippeswamy, A.H.M.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted) aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar] [CrossRef]
- Amir, M.; Kumar, H.; Khan, S.A. Synthesis and pharmacological evaluation of pyrazoline derivatives as new anti-inflammatory and analgesic agents. Bioorg. Med. Chem. Lett. 2008, 18, 918–922. [Google Scholar] [CrossRef]
- Rathish, I.G.; Javed, K.; Ahmad, S.; Bano, S. Synthesis, Antiinflammatory activity of some new 1,3,5-trisubstituted pyrazolines bearing benzene sulfonamide. Bioorg. Med. Chem. Lett. 2009, 19, 255–258. [Google Scholar] [CrossRef]
- Barsoum, F.F.; Girgis, A.S. Facile synthesis of bis(4,5-dihydro-1H-pyrazole-1-carboxamides) and their thio-analogues of potential PGE2 inhibitory properties. Eur. J. Med. Chem. 2009, 44, 2172–2177. [Google Scholar] [CrossRef]
- Khode, S.; Maddi, V.; Aragade, P.; Palkar, M.; Ronad, P.K.; Mamledesai, S.; Thippeswamy, A.H.M.; Satyanarayana, D. Synthesis and pharmacological evaluation of a novel series of 5-(substituted)aryl-3-(3-coumarinyl)-1-phenyl-2-pyrazolines as novel anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2009, 44, 1682–1688. [Google Scholar]
- Shoman, M.E.; Abdel-Aziz, M.; Aly, O.M.; Farag, H.H.; Morsy, M.A. Synthesis and investigation of anti-inflammatory activity and gastric ulcerogenicity of novel nitric oxide-donating pyrazoline derivatives. Eur. J. Med. Chem. 2009, 44, 3068–3076. [Google Scholar] [CrossRef]
- Bhat, A.R.; Athar, F.; Azam, A. New Derivatives of 3,5-substituted-1,4,2-dioxazoles; Synthesis and Activity against Entamoeba histolytica. Eur. J. Med. Chem. 2009, 44, 926–936. [Google Scholar] [CrossRef]
- Zuhal Ozdemir, H.; Burak Kandilci, B.G.X.; Unsal, C.; alısx, A.; Altan, B. Synthesis and studies on antidepressant and anticonvulsant activities of some 3-(2-furyl)-pyrazoline derivatives. Eur. J. Med. Chem. 2007, 42, 373–379. [Google Scholar] [CrossRef]
- Manna, K.; Agrawal, Y.K. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity. Bioorg. Med. Chem. Lett. 2009, 19, 2688–2692. [Google Scholar] [CrossRef]
- Abdel-Wahab, B.F.; Abdel-Aziz, H.A.; Ahmed, E.M. Synthesis and antimicrobial evaluation of 1-(benzofuran-2-yl)-4-nitro-3-arylbutan-1-ones and 3-(benzofuran-2 yl)-4,5-dihydro-5-aryl-1-[4-(aryl)-1,3-thiazol-2-yl]-1H-pyrazoles. Eur. J. Med. Chem. 2009, 44, 2632–2635. [Google Scholar] [CrossRef]
- El-Sayed, W.A.; Nassar, I.F.; Abdel-Rahman, A.A.-H. C-Furyl glycosides, II: Synthesis and antimicrobial evaluation of C-furyl glycosides bearing pyrazolines, isoxazolines, and 5,6-dihydropyrimidine-2(1H)-thione. Monatsh. Chem. 2009, 140, 365–370. [Google Scholar] [CrossRef]
- Jadhav, S.B.; Shastri, R.A.; Gaikwad, K.V.; Gaikwad, S.V. Synthesis and antimicrobial studies of some novel pyrazoline and isoxazoline derivatives. Eur. J. Chem. 2009, 6, S183–S188. [Google Scholar]
- Sakthinathan, S.P.; Vanangamudi, G.; Thirunarayanan, G. Synthesis, spectral studies and antimicrobial activities of some 2-naphthyl pyrazoline derivatives. Spectrochim. Acta A 2012, 95, 693–700. [Google Scholar] [CrossRef]
- Kaplancikli, Z.A.; Turan-Zitouni, G.; Ozdemir, A.; Can, O.D.; Chevallet, P. Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur. J. Med. Chem. 2009, 44, 2606–2610. [Google Scholar] [CrossRef]
- Badri, N.; Acharya, D.S.; Mugdha, T.; Asish, K.S.; Ramarao, G.; Saroj, B.; Mahabir, P.K. Synthesis and antimalarial evaluation of 1,3,5-trisubstituted pyrazolines. Eur. J. Med. Chem. 2010, 45, 430–438. [Google Scholar] [CrossRef]
- Carrion, M.D.; Luisa, C.; Lopez, L.C.; Camacho, M.E.; Tapias, V.; Escames, G.; Castroviejo, D.A.; Espinosa, A.; Gallo, M.A.; Entrena, A. Pyrazoles and pyrazolines as neural and inducible nitric oxide synthase (nNOS and iNOS) potential inhibitors (III). Eur. J. Med. Chem. 2008, 43, 2579–2591. [Google Scholar] [CrossRef]
- Gokhan-Kelekc, N.; Koyunoglu, I.S.; Yabanoglu, S. New pyrazoline bearing 4(3H)-quinazolinone inhibitors of monoamine oxidase: Synthesis, biological evaluation, and structural determinants of MAO-A and MAO-B selectivity. Bioorg. Med. Chem. 2009, 17, 675–689. [Google Scholar] [CrossRef]
- Can, O.D.; Ozkay, U.D.; Kaplancikli, Z.A.; Ozturk, Y. Effects of some 1,3,5-trisubstitued-2-pyrazoline derivatives on depression and anxiety parameters of mice. Arch. Pharm. Res. 2009, 32, 1293–1299. [Google Scholar] [CrossRef]
- Havrylyuk, D.; Zimenkovsky, B.; Vasylenko, O.; Zaprutko, L.; Lesyk, R. Synthesis of novel thiazolone-based compounds containing pyrazoline moiety and evaluation of their anticancer activity. Eur. J. Med. Chem. 2009, 44, 1396–1404. [Google Scholar] [CrossRef]
- Braulio, I.; Alexis, T.; Fabian, O.; Jairo, Q.; Rodrigo, A.; Manuel, N.; Adolfo, S.; Justo, C. Synthesis of novel pyrazolic analogues of chalcones and their 3-aryl-4-(3-aryl-4,5-dihydro-1H-pyrazol-5-yl)-1-phenyl-1H-pyrazole derivatives as potential antitumor agents. Bioorg. Med. Chem. 2010, 18, 4965–4974. [Google Scholar]
- Shrey, P.; Dhairya, B.; Mahesh, S.; Shailesh, T.; Abhay, B.; Manisha, P.; Hardevsinh, V.; Ashish, R.; Nilay, P.; Juliana, S.; et al. Snthesis of some novel benzofuran-2-yl(4,5-dihyro-3,5-substituted diphenylpyrazol-1-yl) methanones and studies on the antiproliferative effects and reversal of multidrug resistance of human MDR1-gene transfected mouse lymphoma cells in vitro. Eur. J. Med. Chem. 2011, 46, 1942–1948. [Google Scholar] [CrossRef]
- Kuntal, M.; Yadvendra, K.A. Microwave assisted synthesis of new indophenazine 1,3,5-trisubstruted pyrazoline derivatives of benzofuran and their antimicrobial activity. J. Bioorg. Med. Chem. Lett. 2009, 19, 2688–2692. [Google Scholar] [CrossRef]
- Chandra, T.; Garg, N.; Lata, S.; Saxena, K.K.; Kumar, A. Synthesis of substituted acridinyl pyrazoline derivatives and their evaluation for anti-inflammatory activity. Eur. J. Med. Chem. 2010, 45, 1772–1776. [Google Scholar]
- Jeong, T.-S.; Kim, K.S.; Kim, J.-R.; Cho, K.-Y.; Lee, S.; Lee, W.S. Novel 3,5-diaryl pyrazolines and pyrazole as low-density lipoprotein (LDL) oxidation inhibitor. Bioorg. Med. Chem. Lett. 2004, 14, 2719–2723. [Google Scholar] [CrossRef]
- Abid, M.; Bhat, A.R.; Athar, F.; Azam, A. Synthesis, spectral studies and antiamoebic activity of new 1-N-substituted thiocarbamoyl-3-phenyl-2-pyrazolines. Eur. J. Med. Chem. 2009, 44, 417–425. [Google Scholar] [CrossRef]
- Budakoti, A.; Bhat, A.R.; Azam, A. Synthesis of new 2-(5-substituted-3-phenyl- 2-pyrazolinyl)-1,3-thiazolino[5,4-b]quinoxaline derivatives and evaluation of their antiamoebic activity. Eur. J. Med. Chem. 2009, 44, 1317–1325. [Google Scholar] [CrossRef]
- Faisal, H.; Attar, S.; Sadiq, U.; Amir, A. Synthesis, characterization, antiamoebic activity and cytotoxicity of novel series of pyrazoline derivatives bearing quinoline tail. Eur. J. Med. Chem. 2010, 45, 4669–4675. [Google Scholar] [CrossRef]
- Bekhit, A.A.; Abdel-Aziem, T. Design, synthesis and biological evaluation of some pyrazole derivatives as anti-inflammatory-antimicrobial agents. Bioorg. Med. Chem. 2004, 12, 1935–1945. [Google Scholar] [CrossRef]
- Imran, A.; Waseem, A.W.; Amber, K.; Ashanul, H.; Aijaz, A.; Kishwar, S.; Nikhat, M. Synthesis and synergistic antifungal activities of a pyrazoline based ligand and its copper(II) and nickel(II) complexes with conventional antifungals. Microb. Pathog. 2012, 53, 66–73. [Google Scholar] [CrossRef]
- Kaplancikli, A.; Turan-Zitouni, G.; Ozdemir, A.; Can, O.D.; Chevallet, P. Synthesis and antinociceptive activities of some pyrazoline derivatives. Eur. J. Med. Chem. 2009, 44, 2606–2610. [Google Scholar] [CrossRef]
- Maria, G.M.; Daniele, Z.; Valeria, F.; Luciano, V.; Elena, B. Synthesis and antimycobacterial activity of 5-aryl-1-isonicotinoyl-3-(pyridin-2-yl)-4,5-dihydro-1H-pyrazolederivatives. Farmaco 2001, 56, 593–599. [Google Scholar] [CrossRef]
- Habibullah, K.; Shamshir, K.; Mohamed, J.A.; Bahar, A. Synthesis and antihepatotoxic activity of 5-(2,3-dihydro-1,4-benzodioxane-6-yl)-3-substituted-phenyl-4,5-dihydro-1H-pyrazole derivatives. Bioorg. Med. Chem. Lett. 2011, 21, 7251–7254. [Google Scholar] [CrossRef]
- Berghot, M.A.; Moawad, E.B. Convergent synthesis and antibacterial activity of pyrazole and pyrazoline derivatives of diazepam. Eur. J. Pharm. Sci. 2003, 20, 173–179. [Google Scholar] [CrossRef]
- Geeta, J.N.P.; Pramod, S.; Rawatb, B.S.; Rawata, M.S.M.; Joshi, G.C. Synthesis, characterization and fluorescence studies of 3,5-diaryl substituted 2-pyrazolines. Spectrochim. Acta A 2011, 78, 1075–1079. [Google Scholar]
- El-Sadek, M.M.; Faidalla, H.M.; El Soccary, N.N.; Hassan, S.Y. Synthesis And Spectral Studies Of Some New Pyrazolines And Pyrazoles. Egypt. J. Chem. 1995, 38, 403–418. [Google Scholar]
- Faidallah, H.M.; El-Sadek, M.M.; El-Massry, A.M.I.; Hassan, S.Y. Trisubstituted Pyrazoles of Possible Hypoglycemic Activity. Pak. J. Sci. Ind. Res. 1992, 35, 8–13. [Google Scholar]
- Faidallah, H.M.; Makki, M.S.I.; El-Massry, A.H.I.; Hassan, S.Y. Synthesis And Reactions of Some Ethyl 3-Aroyl-4-Aryl- 2-Pyrazoline-5-Carboxylates. Pharmazie 1997, 5, 101–105. [Google Scholar]
- Basaif, S.A.; Faidallah, H.M.; Hassan, S.Y. Synthesis and Biological Activity Of New Pyrazolines And Pyrazoles. Indian J. Heterocycl. Chem. 1996, 6, 53–58. [Google Scholar]
- Hassan, S.Y.; Basief, S.A.; Faidallah, H.M. Synthesis Of Noval Indan [1,2-c]Pyrazole-benzenesulfonylureas, Thioureas and Their Cyclized Derivatives. Egypt. J. Chem. 1999, 42, 213–220. [Google Scholar]
- Hassan, S.Y. Synthesis and Biological Activity of Some New Pyrazoline and Pyrimidine Derivatives. Braz. Chem. Soc. 2011, 22, 1286–1298. [Google Scholar] [CrossRef]
- González, F.G.; Guillen, M.G.; Perez, J.A.G.; Román, E.G. Reaction of 2-Amino-2-Deoxyheptoses With Cyclic Beta-Dicarbonyl Compounds. Carbohydr. Res. 1980, 78, 17–23. [Google Scholar] [CrossRef]
- Bharat, K.; Vishal, P.; Sushma, R.; Rani, K.; Tewari, I.C. Synthesis and antimicrobial activity of some bromo-benzothiazolo pyrazolines. Int. J. Microbiol. Res. 2009, 1, 20–22. [Google Scholar]
- Kgokong, J.L.; Breytenbach, J.C. Synthesis of novel trifluoromethylquinoline and bis (trifluoromethyl)quinoline derivatives. S. Afr. J. Chem. S. 2000, 53, 100–103. [Google Scholar]
- Joseph, L.K.; Peter, P.S.; Gilbert, M.M. 1,2,4-triazino-[5,6-b]indole derivatives: Effects of the trifluoromethyl group on in vitro antimalarial activity. Bioorg. Med. Chem. 2005, 13, 2935–2942. [Google Scholar] [CrossRef]
- Mohamed, S.K.Y.; Ferial, M.A.; Khairy, M.H.; Mohamed, S.A. Synthesis and some reactions of 7-methyl-5-phenyl-5H-pyrazolo[3,4-e]1,2,4-triazine-3-thiol. J. Heterocycl. Chem. 1984, 21, 923–926. [Google Scholar] [CrossRef]
- Mohsen, A.M.E; Omar, S; El-Din, A.S.; Ibrahim, M.L.; El-Tombary, A.A. Synthesis and evaluation for antimicrobial and antihistaminic properties of new thiosemicarbazide and triazole derivatives of triazolo[4,3-a]quinazolin-5(4H)-ones. Alex. J. Pharm. Sci. 1991, 5, 213–215. [Google Scholar]
- Jack, D.R.; Deborah, A.C.; Wilmer, S.A.; Patick, J.S. Synthesis and reactions of 4-isipropylidene-1-aryl-3-methyl-2-pyrazolin-5-ones. J. Heterocycl. Chem. 1987, 24, 149–153. [Google Scholar] [CrossRef]
- Abou-Elela, G.M.; Ibrahim, H.A.; El-Helow, E.; Sabry, S. Abundance and Antagonistic Interactions among Bacterioplankton in Suez Gulf. World Appl. Sci. J. 2009, 7, 748–755. [Google Scholar]
- Cruickshank, R.; Duguid, J.P.; Marmion, B.P.; Swam, H.A. The Practice of Medical Microbiology, 12th ed; Churchill Livingstone: London, UK, 1975; pp. 544–565. [Google Scholar]
- Sample Availability: Samples of the compounds 2–4, 11, 18, 22, 24, 27, 33–36, 38, and 40 are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hassan, S.Y. Synthesis, Antibacterial and Antifungal Activity of Some New Pyrazoline and Pyrazole Derivatives. Molecules 2013, 18, 2683-2711. https://doi.org/10.3390/molecules18032683
Hassan SY. Synthesis, Antibacterial and Antifungal Activity of Some New Pyrazoline and Pyrazole Derivatives. Molecules. 2013; 18(3):2683-2711. https://doi.org/10.3390/molecules18032683
Chicago/Turabian StyleHassan, Seham Y. 2013. "Synthesis, Antibacterial and Antifungal Activity of Some New Pyrazoline and Pyrazole Derivatives" Molecules 18, no. 3: 2683-2711. https://doi.org/10.3390/molecules18032683
APA StyleHassan, S. Y. (2013). Synthesis, Antibacterial and Antifungal Activity of Some New Pyrazoline and Pyrazole Derivatives. Molecules, 18(3), 2683-2711. https://doi.org/10.3390/molecules18032683