Technologies for the Synthesis of mRNA-Encoding Libraries and Discovery of Bioactive Natural Product-Inspired Non-Traditional Macrocyclic Peptides
Abstract
:1. Introduction
2. Naturally Occurring Peptides and Synthetic Natural Product-Inspired Peptides
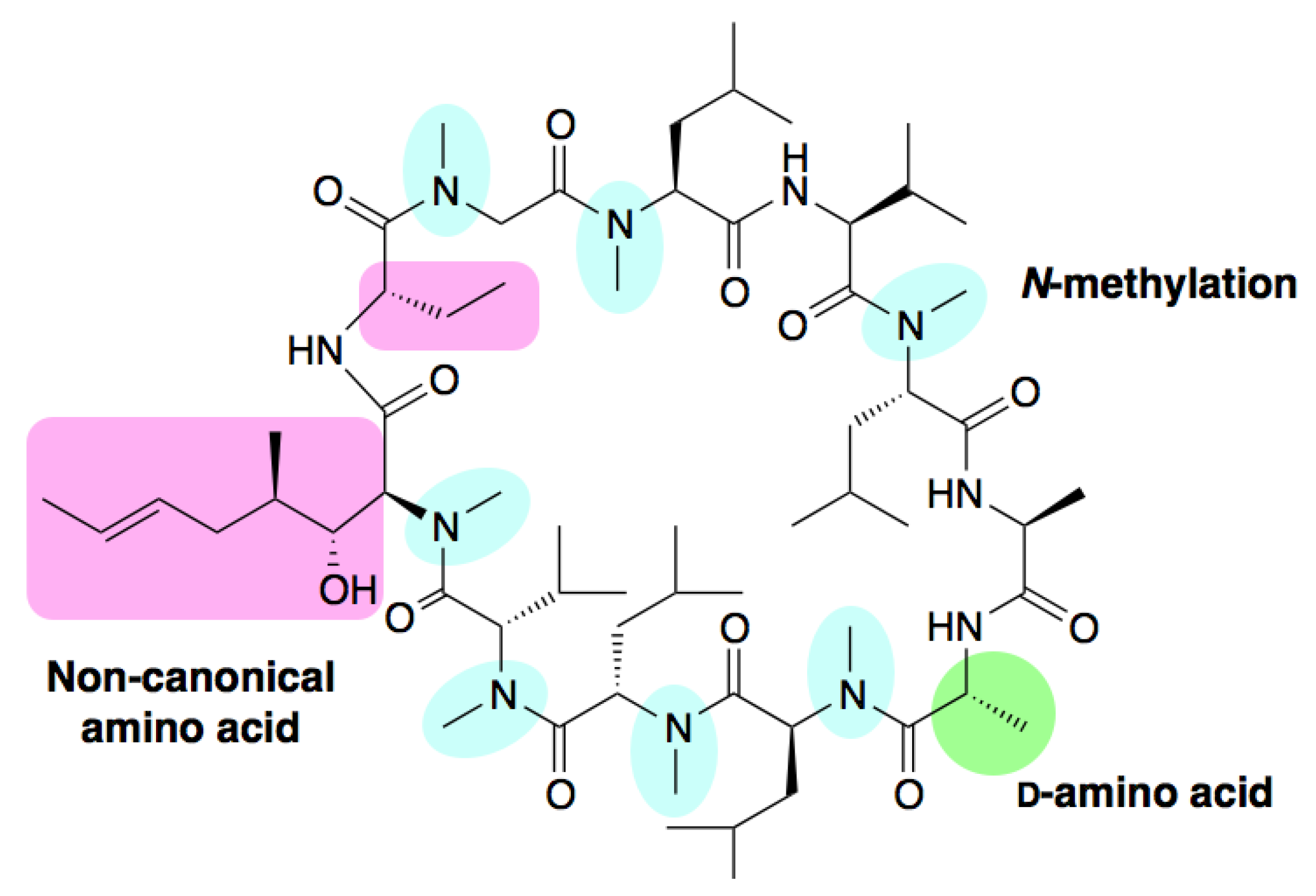
3. Flexizymes and the FIT System: Tools for Genetic Code Reprogramming and the Rapid Synthesis of Natural Product-inspired Non-standard Peptides
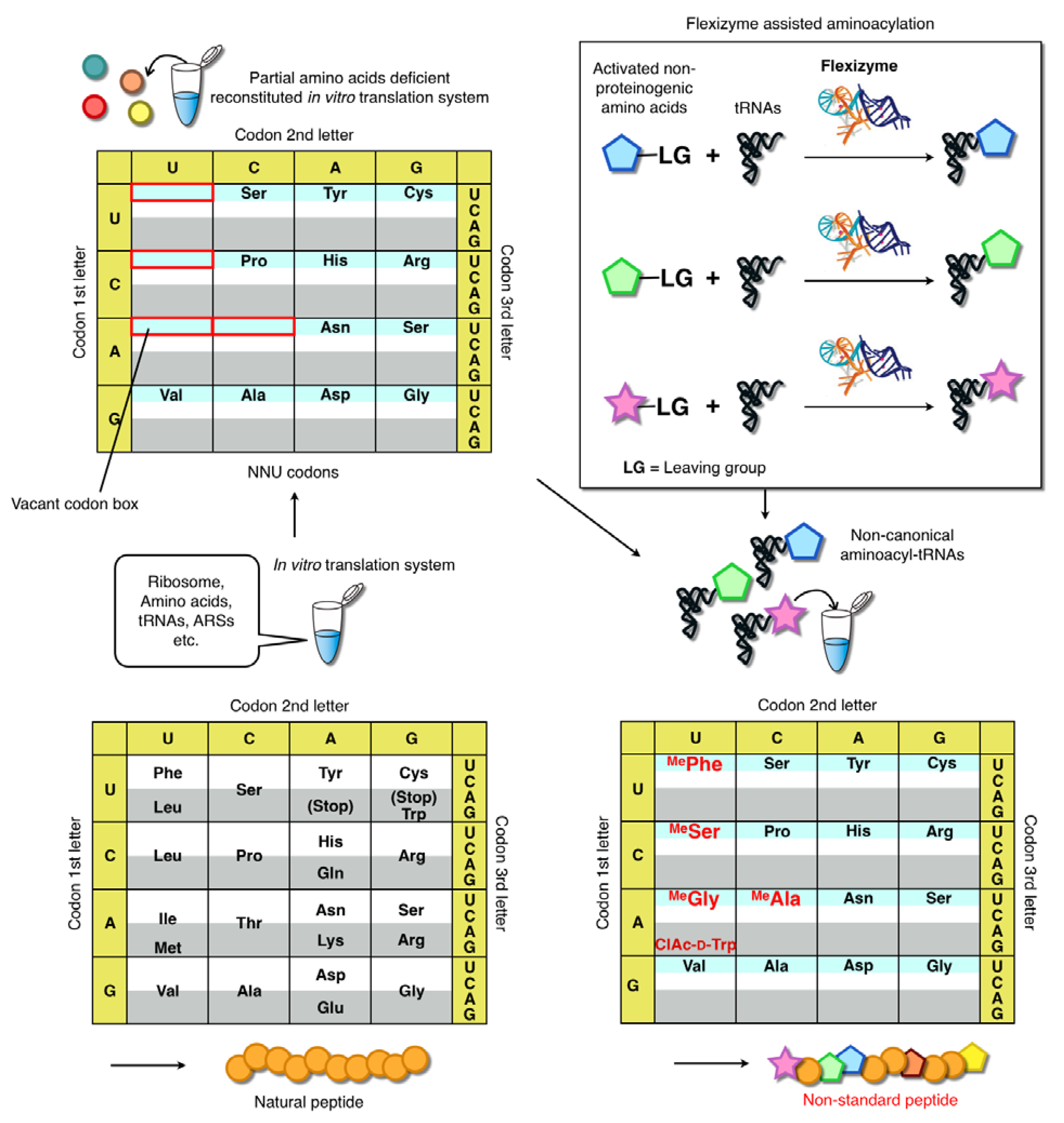
3.1. Limitations of the Natural Genetic Code and Use of Expanded Genetic Codes: Towards Genetic Reprograming
3.2. Flexizymes
3.3. Orthogonal tRNAs
3.4. Genetic Code Reprogramming in the FIT System
3.5. Ribosomal Synthesis of Biopolymers in the FIT System
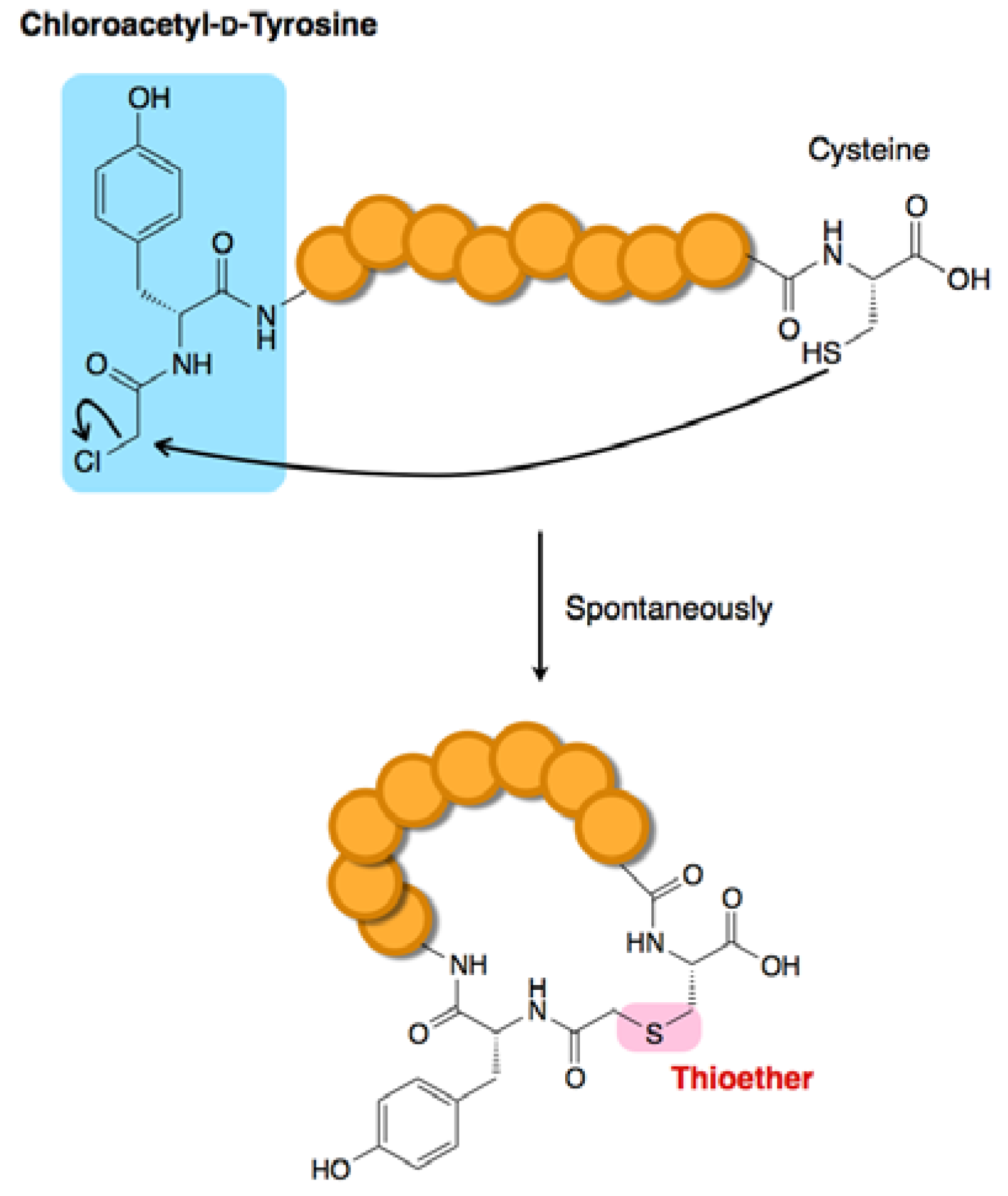
3.6. Macrocyclization Methodologies Compatible with the FIT System
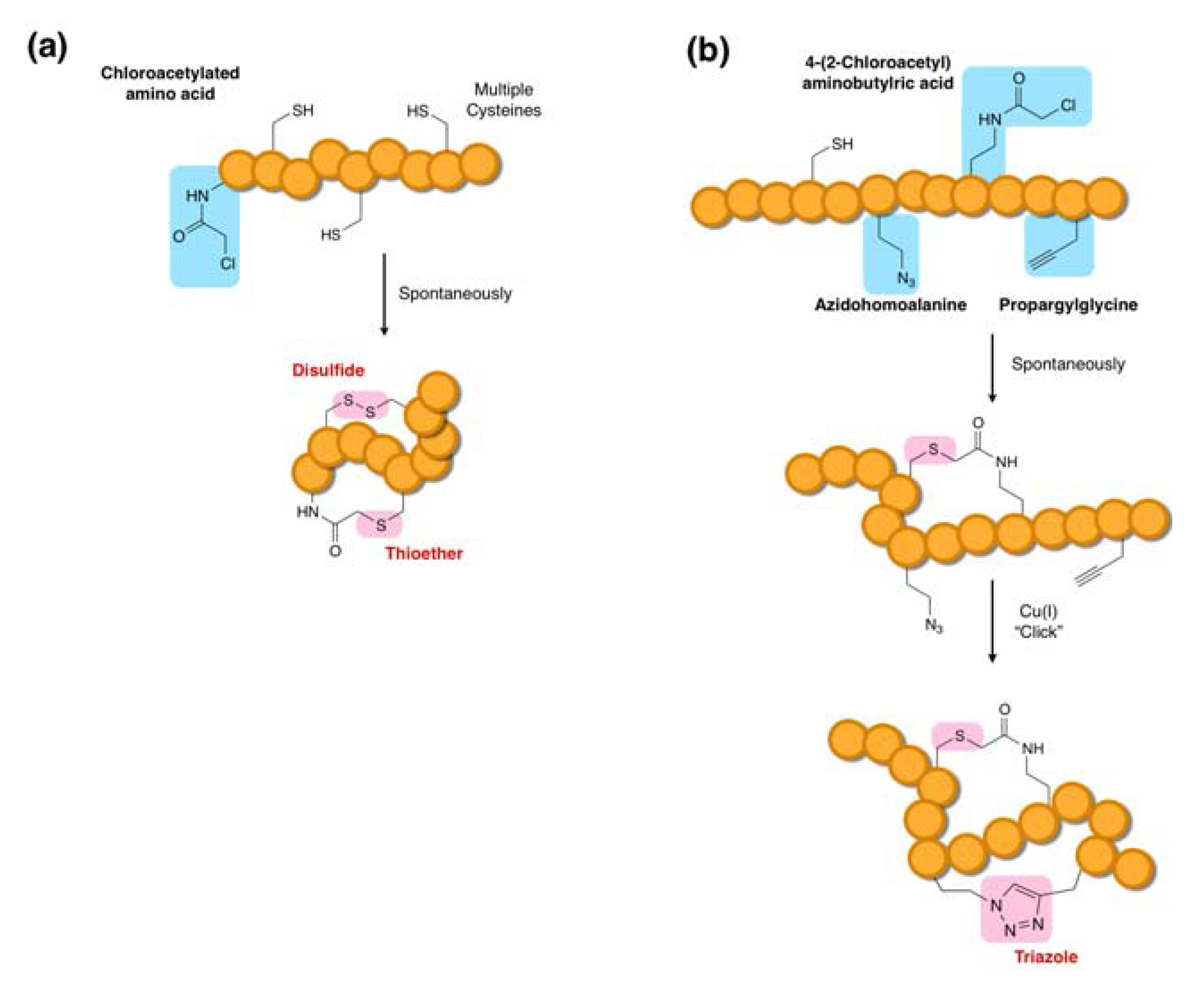
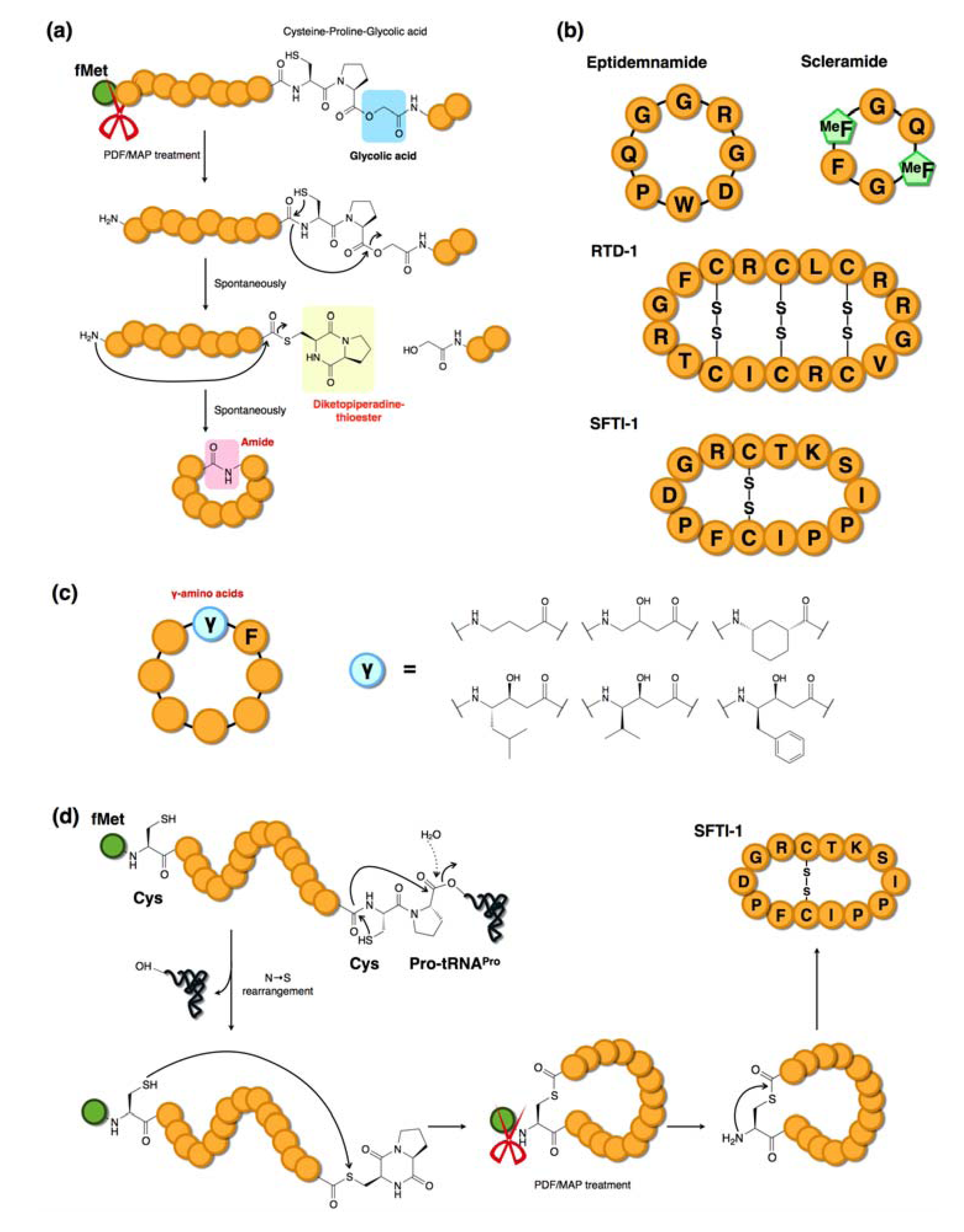
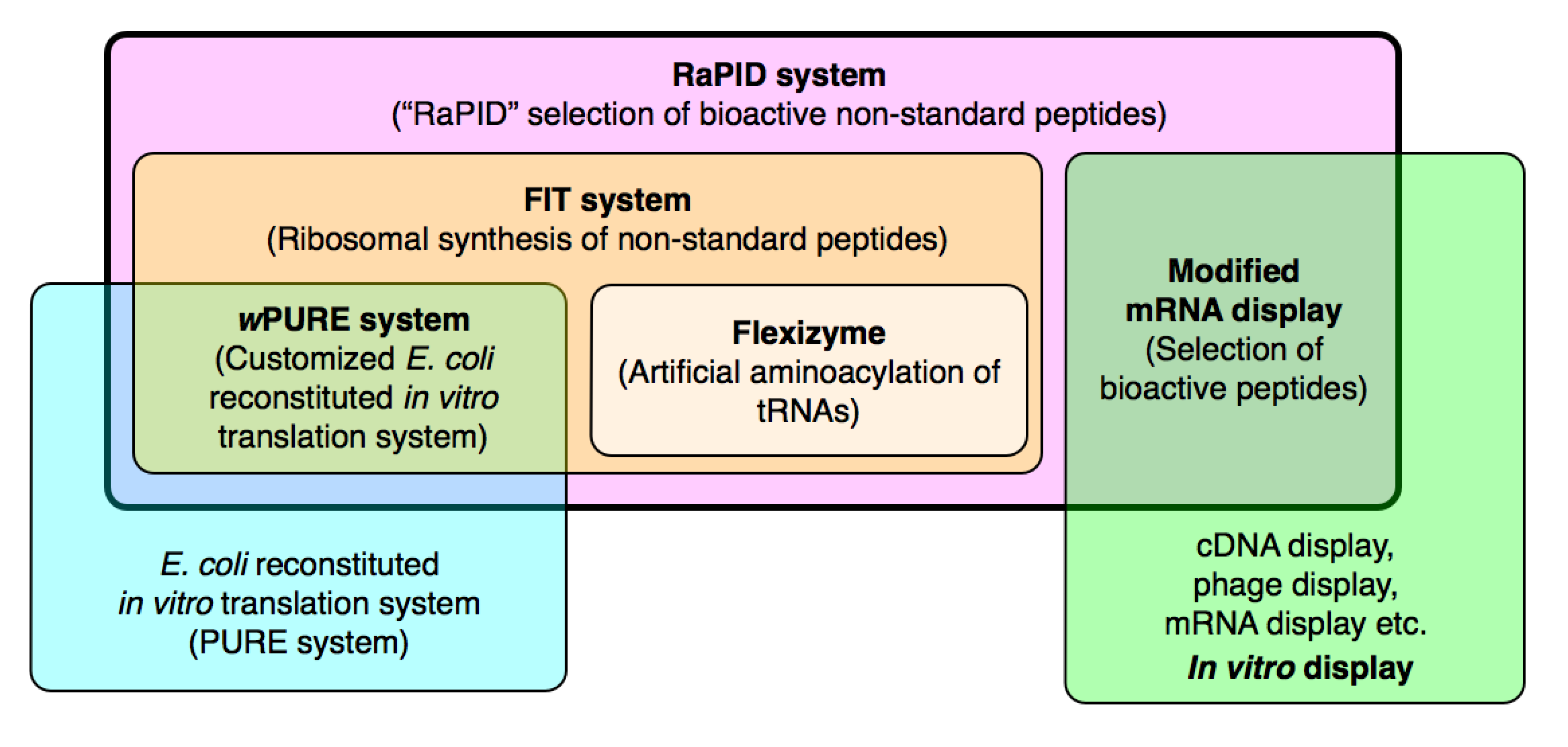
4. The RaPID System: An Enabling Technology for the Rapid Discovery of Natural Product-inspired Bioactive Peptides
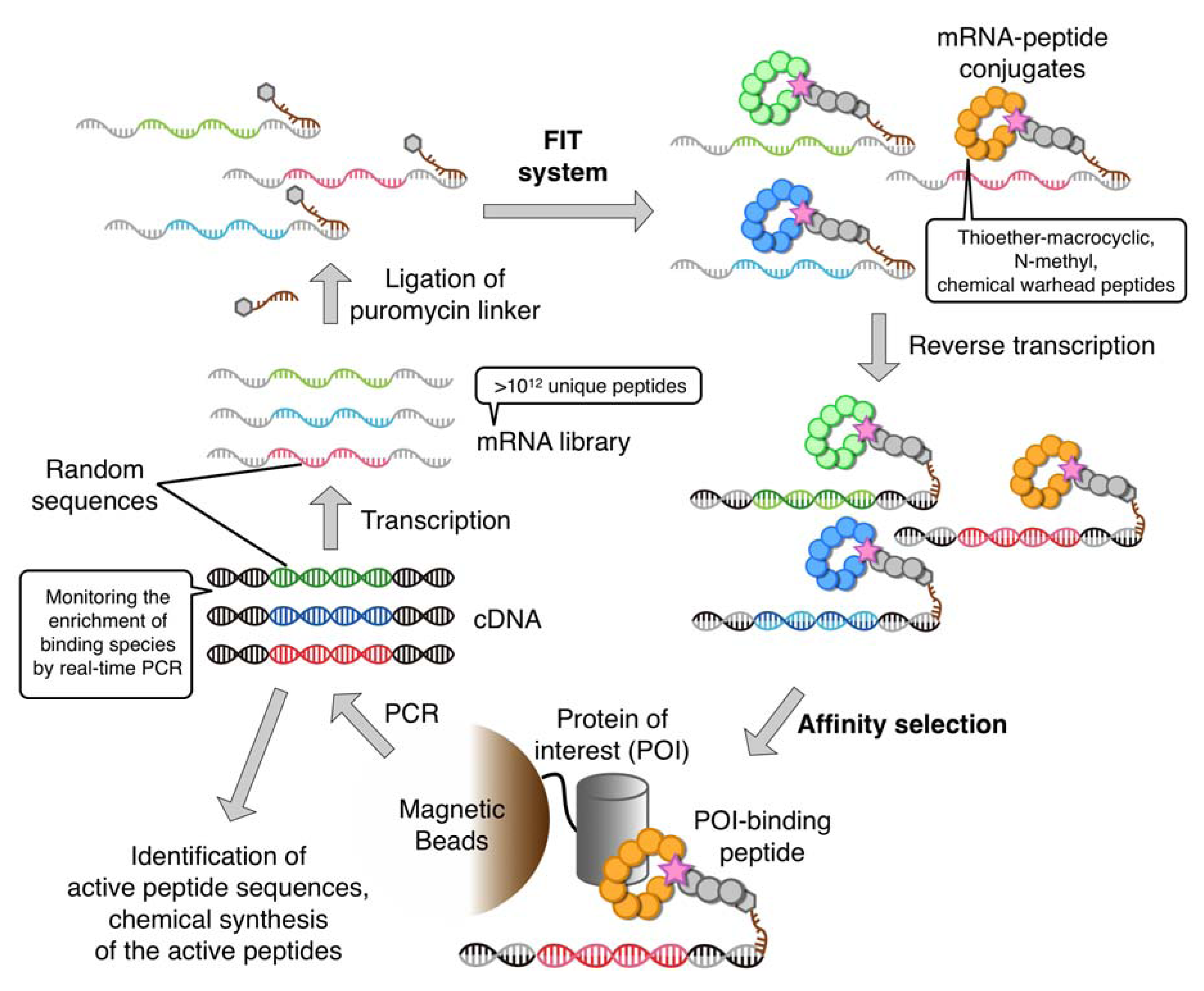
5. Bioactive Non-Standard Thioether-Macrocyclic Peptides Identified by the RaPID System
5.1. AKT2-Isoform Selective Inhibitors
5.2. SIRT2-Isoform Selective Inhibitors with a Mechanism-Based Warhead
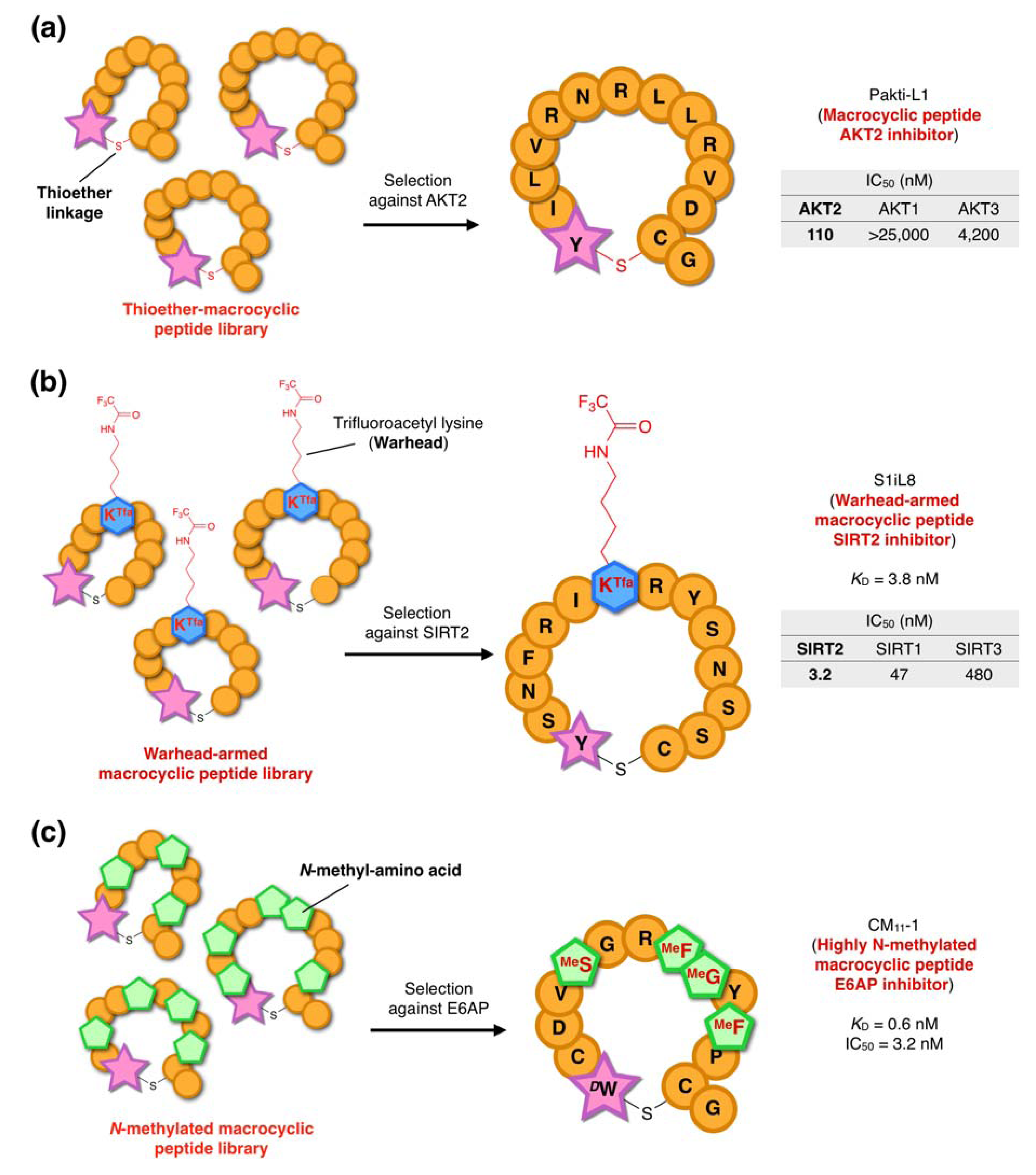
5.3. Thioether-macrocyclic N-methyl-peptide inhibitors against ubiquitin ligase E6AP
6. Other Methodologies for the Selection of Non-Standard Peptides
6.1. Expression of Non-Standard Peptides in a Reconstituted in Vitro Translation System via in Situ Mischarging of tRNAs by Wildtype ARSs
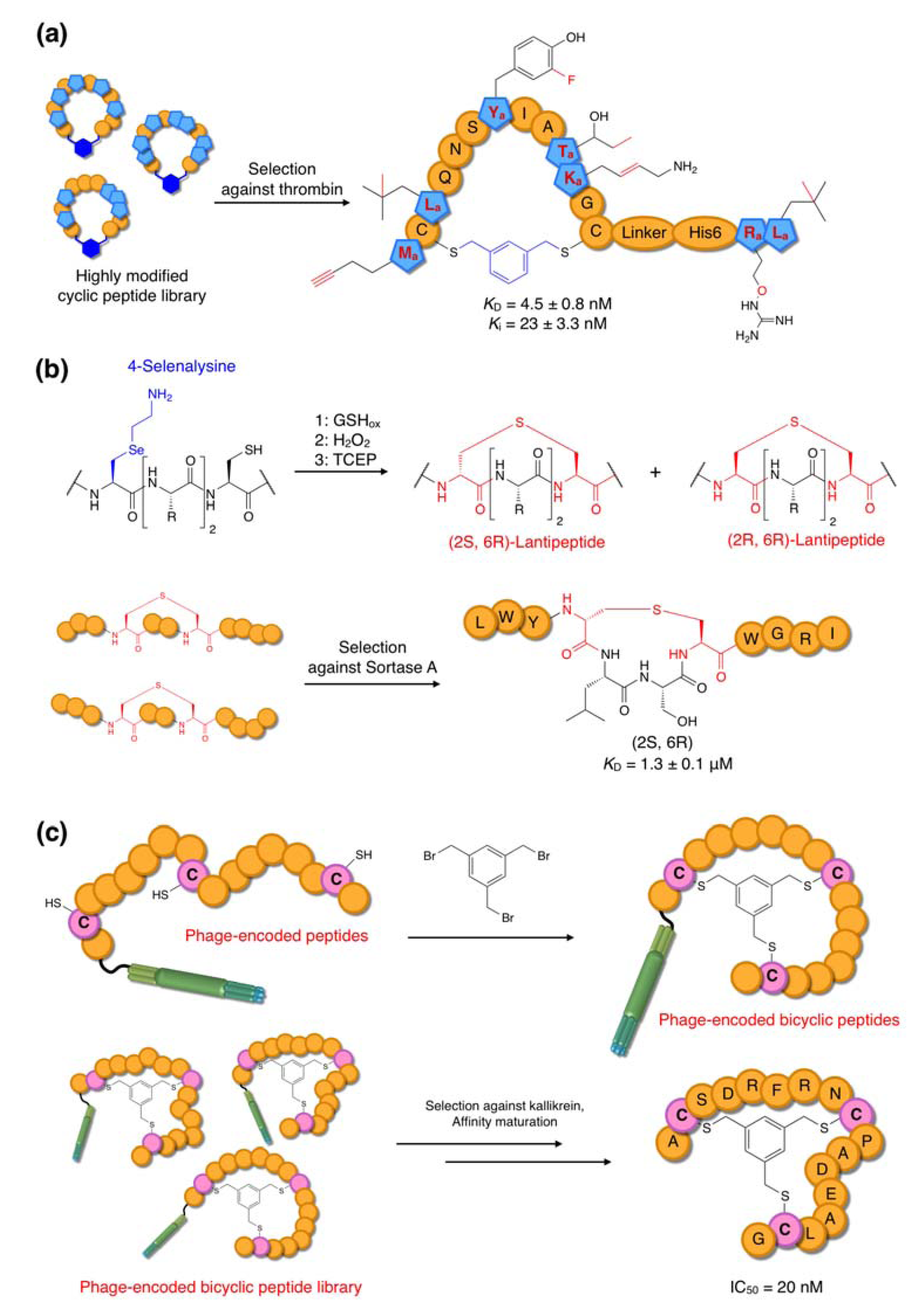
6.2. Selection of Bioactive Non-Standard Peptides and Lantipeptide-like Peptides
6.3. Selection of Macrocyclic Peptides Cross-Linked via Two Amide Bonds
6.4. Selection of Phage-Encoded Bicyclic Peptides
7. Conclusions
Acknowledgments
References
- Kling, J. Fresh from the biologic pipeline-2010. Nat. Biotechnol. 2011, 29, 197–200. [Google Scholar] [CrossRef]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Bock, J.E.; Gavenonis, J.; Kritzer, J.A. Getting in shape: Controlling peptide bioactivity and bioavailability using conformational constraints. ACS Chem. Biol. 2013. [Google Scholar] [CrossRef]
- Rezai, T.; Bock, J.E.; Zhou, M.V.; Kalyanaraman, C.; Lokey, R.S.; Jacobson, M.P. Conformational flexibility, internal hydrogen bonding, and passive membrane permeability: Successful in silico prediction of the relative permeabilities of cyclic peptides. J. Am. Chem. Soc. 2006, 128, 14073–14080. [Google Scholar] [CrossRef]
- White, T.R.; Renzelman, C.M.; Rand, A.C.; Rezai, T.; McEwen, C.M.; Gelev, V.M.; Turner, R.A.; Linington, R.G.; Leung, S.S.F.; Kalgutkar, A.S.; et al. On-resin N-methylation of cyclic peptides for discovery of orally bioavailable scaffolds. Nat. Chem. Biol. 2011, 7, 810–817. [Google Scholar] [CrossRef]
- Rand, A.C.; Leung, S.S.F.; Eng, H.; Rotter, C.J.; Sharma, R.; Kalgutkar, A.S.; Zhang, Y.Z.; Varma, M.V.; Farley, K.A.; Khunte, B.; et al. Optimizing PK properties of cyclic peptides: The effect of side chain substitutions on permeability and clearance. MedChemComm 2012, 3, 1282–1289. [Google Scholar] [CrossRef]
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery - an underexploited structural class. Nat. Rev. Drug Discovery 2008, 7, 608–624. [Google Scholar] [CrossRef]
- Frankel, A.; Millward, S.W.; Roberts, R.W. Encodamers: Unnatural peptide oligomers encoded in RNA. Chem. Biol. 2003, 10, 1043–1050. [Google Scholar] [CrossRef]
- Buchman, G.W.; Banerjee, S.; Hansen, J.N. Structure, expression, and evolution of a gene encoding the precursor of nisin, a small protein antibiotic. J. Biol. Chem. 1988, 263, 16260–16266. [Google Scholar]
- Nolan, E.M.; Walsh, C.T. How nature morphs peptide scaffolds into antibiotics. Chembiochem 2009, 10, 34–53. [Google Scholar] [CrossRef]
- Sieber, S.A.; Marahiel, M.A. Molecular mechanisms underlying nonribosomal peptide synthesis: Approaches to new antibiotics. Chem. Rev. 2005, 105, 715–738. [Google Scholar] [CrossRef]
- Fischbach, M.A.; Walsh, C.T. Assembly-line enzymology for polyketide and nonribosomal peptide antibiotics: Logic, machinery, and mechanisms. Chem. Rev. 2006, 106, 3468–3496. [Google Scholar] [CrossRef]
- Wu, M.C.; Law, B.; Wilkinson, B.; Micklefield, J. Bioengineering natural product biosynthetic pathways for therapeutic applications. Curr. Opin. Biotechnol. 2012, 23, 931–940. [Google Scholar] [CrossRef]
- O'connor, S.E.; Walsh, C.T.; Liu, F. Biosynthesis of epothilone intermediates with alternate starter units: Engineering polyketide-nonribosomal interfaces. Angew. Chem. Int. Ed. 2003, 42, 3917–3921. [Google Scholar] [CrossRef]
- Nemoto, N.; MiyamotoSato, E.; Husimi, Y.; Yanagawa, H. In vitro virus: Bonding of mRNA bearing puromycin at the 3'-terminal end to the C-terminal end of its encoded protein on the ribosome in vitro. FEBS Lett. 1997, 414, 405–408. [Google Scholar] [CrossRef]
- Roberts, R.W.; Szostak, J.W. RNA-peptide fusions for the in vitro selection of peptides and proteins. Proc. Natl. Acad. Sci. USA 1997, 94, 12297–12302. [Google Scholar] [CrossRef]
- Shimizu, Y.; Inoue, A.; Tomari, Y.; Suzuki, T.; Yokogawa, T.; Nishikawa, K.; Ueda, T. Cell-free translation reconstituted with purified components. Nat. Biotechnol. 2001, 19, 751–755. [Google Scholar] [CrossRef]
- Reid, P.C.; Goto, Y.; Katoh, T.; Suga, H. Charging of tRNAs using ribozymes and selection of cyclic peptides containing thioethers. Methods. Mol. Biol. 2012, 805, 335–348. [Google Scholar] [CrossRef]
- Suga, H.; Hayashi, G.; Terasaka, N. The RNA origin of transfer RNA aminoacylation and beyond. Philos. T. R. Soc. B. 2011, 366, 2959–2964. [Google Scholar] [CrossRef]
- Ohuchi, M.; Murakami, H.; Suga, H. The flexizyme system: A highly flexible tRNA aminoacylation tool for the translation apparatus. Curr. Opin. Chem. Biol. 2007, 11, 537–542. [Google Scholar] [CrossRef]
- Kourouklis, D.; Murakami, H.; Suga, H. Programmable ribozymes for mischarging tRNA with nonnatural amino acids and their applications to translation. Methods 2005, 36, 239–244. [Google Scholar] [CrossRef]
- Goto, Y.; Katoh, T.; Suga, H. Flexizymes for genetic code reprogramming. Nat. Protoc. 2011, 6, 779–790. [Google Scholar] [CrossRef]
- Morimoto, J.; Hayashi, Y.; Iwasaki, K.; Suga, H. Flexizymes: Their evolutionary history and the origin of catalytic function. Acc. Chem. Res. 2011, 44, 1359–1368. [Google Scholar] [CrossRef]
- Goto, Y.; Suga, H. Flexizymes as a tRNA acylation tool facilitating genetic code reprogramming. Methods. Mol. Biol. 2012, 848, 465–478. [Google Scholar] [CrossRef]
- Katoh, T.; Goto, Y.; Reza, M.S.; Suga, H. Ribosomal synthesis of backbone macrocyclic peptides. Chem. Commun. 2011, 47, 9946–9958. [Google Scholar] [CrossRef]
- Kang, T.J.; Suga, H. Ribosomal synthesis of nonstandard peptides. Biochem. Cell Biol. 2008, 86, 92–99. [Google Scholar] [CrossRef]
- Ohta, A.; Yamagishi, Y.; Suga, H. Synthesis of biopolymers using genetic code reprogramming. Curr. Opin. Chem. Biol. 2008, 12, 159–167. [Google Scholar] [CrossRef]
- Young, T.S.; Schultz, P.G. Beyond the Canonical 20 Amino Acids: Expanding the Genetic Lexicon. J. Biol. Chem. 2010, 285, 11039–11044. [Google Scholar] [CrossRef]
- Wang, K.H.; Schmied, W.H.; Chin, J.W. Reprogramming the Genetic Code: From Triplet to Quadruplet Codes. Angew. Chem. Int. Ed. 2012, 51, 2288–2297. [Google Scholar] [CrossRef]
- Robertson, S.A.; Ellman, J.A.; Schultz, P.G. A general and efficient route for chemical aminoacylation of transfer-RNAs. J. Am. Chem. Soc. 1991, 113, 2722–2729. [Google Scholar] [CrossRef]
- Xiao, H.; Murakami, H.; Suga, H.; Ferre-D'Amare, A.R. Structural basis of specific tRNA aminoacylation by a small in vitro selected ribozyme. Nature 2008, 454, 358–361. [Google Scholar] [CrossRef]
- Murakami, H.; Ohta, A.; Ashigai, H.; Suga, H. A highly flexible tRNA acylation method for non-natural polypeptide synthesis. Nat. Methods 2006, 3, 357–359. [Google Scholar] [CrossRef]
- Niwa, N.; Yamagishi, Y.; Murakami, H.; Suga, H. A flexizyme that selectively charges amino acids activated by a water-friendly leaving group. Bioorg. Med. Chem. Lett. 2009, 19, 3892–3894. [Google Scholar] [CrossRef]
- Kawakami, T.; Murakami, H.; Suga, H. Ribosomal synthesis of polypeptoids and peptoid-peptide hybrids. J. Am. Chem. Soc. 2008, 130, 16861–16863. [Google Scholar] [CrossRef]
- Kawakami, T.; Murakami, H.; Suga, H. Messenger RNA-programmed incorporation of multiple N-methyl-amino acids into linear and cyclic peptides. Chem. Biol. 2008, 15, 32–42. [Google Scholar] [CrossRef]
- Goto, Y.; Ohta, A.; Sako, Y.; Yamagishi, Y.; Murakami, H.; Suga, H. Reprogramming the translation initiation for the synthesis of physiologically stable cyclic peptides. ACS Chem. Biol. 2008, 3, 120–129. [Google Scholar] [CrossRef]
- Goto, Y.; Murakami, H.; Suga, H. Initiating translation with D-amino acids. RNA 2008, 14, 1390–1398. [Google Scholar] [CrossRef]
- Goto, Y.; Suga, H. Translation initiation with initiator tRNA charged with exotic peptides. J. Am. Chem. Soc. 2009, 131, 5040–5041. [Google Scholar] [CrossRef]
- Ohta, A.; Murakami, H.; Higashimura, E.; Suga, H. Synthesis of polyester by means of genetic code reprogramming. Chem. Biol. 2007, 14, 1315–1322. [Google Scholar] [CrossRef]
- Liu, D.R.; Magliery, T.J.; Pasternak, M.; Schultz, P.G. Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc. Natl. Acad. Sci. USA 1997, 94, 10092–10097. [Google Scholar] [CrossRef]
- Murakami, H.; Saito, H.; Suga, H. A versatile tRNA aminoacylation catalyst based on RNA. Chem. Biol. 2003, 10, 655–662. [Google Scholar] [CrossRef]
- Kawakami, T.; Ohta, A.; Ohuchi, M.; Ashigai, H.; Murakami, H.; Suga, H. Diverse backbone-cyclized peptides via codon reprogramming. Nat. Chem. Biol. 2009, 5, 888–890. [Google Scholar] [CrossRef]
- Forster, A.C.; Tan, Z.P.; Nalam, M.N.L.; Lin, H.N.; Qu, H.; Cornish, V.W.; Blacklow, S.C. Programming peptidomimetic syntheses by translating genetic codes designed de novo. Proc. Natl. Acad. Sci. USA 2003, 100, 6353–6357. [Google Scholar]
- Fahnestock, S.; Rich, A. Ribosome-catalyzed polyester formation. Science 1971, 173, 340–343. [Google Scholar]
- Iwasaki, K.; Goto, Y.; Katoh, T.; Suga, H. Selective thioether macrocyclization of peptides having the N-terminal 2-chloroacetyl group and competing two or three cysteine residues in translation. Org. Biomol. Chem. 2012, 10, 5783–5786. [Google Scholar] [CrossRef]
- Sako, Y.; Morimoto, J.; Murakami, H.; Suga, H. Ribosomal synthesis of bicyclic peptides via two orthogonal inter-side-chain reactions. J. Am. Chem. Soc. 2008, 130, 7232–7234. [Google Scholar] [CrossRef]
- Kawakami, T.; Aimoto, S. The use of a cysteinyl prolyl ester (CPE) autoactivating unit in peptide ligation reactions. Tetrahedron 2009, 65, 3871–3877. [Google Scholar] [CrossRef]
- Ohshiro, Y.; Nakajima, E.; Goto, Y.; Fuse, S.; Takahashi, T.; Doi, T.; Suga, H. Ribosomal synthesis of backbone-macrocyclic peptides containing gamma-amino acids. ChemBioChem 2011, 12, 1183–1187. [Google Scholar] [CrossRef]
- Kang, T.J.; Hayashi, Y.; Suga, H. Synthesis of the backbone cyclic peptide sunflower trypsin inhibitor-1 promoted by the induced peptidyl-tRNA drop-off. Angew. Chem. Int. Ed. 2011, 50, 2159–2161. [Google Scholar] [CrossRef]
- Zahnd, C.; Amstutz, P.; Pluckthun, A. Ribosome display: Selecting and evolving proteins in vitro that specifically bind to a target. Nat. Methods 2007, 4, 269–279. [Google Scholar] [CrossRef]
- Yonezawa, M.; Doi, N.; Kawahashi, Y.; Higashinakagawa, T.; Yanagawa, H. DNA display for in vitro selection of diverse peptide libraries. Nucleic Acids Res. 2003, 31, e118. [Google Scholar] [CrossRef]
- Hanes, J.; Pluckthun, A. In vitro selection and evolution of functional proteins by using ribosome display. Proc. Natl. Acad. Sci. USA 1997, 94, 4937–4942. [Google Scholar] [CrossRef]
- Kurz, M.; Gu, K.; Lohse, P.A. Psoralen photo-crosslinked mRNA-puromycin conjugates: A novel template for the rapid and facile preparation of mRNA-protein fusions. Nucleic Acids Res. 2000, 28, e83. [Google Scholar] [CrossRef]
- Tabuchi, I.; Soramoto, S.; Suzuki, M.; Nishigaki, K.; Nemoto, N.; Husimi, Y. An efficient ligation method in the making of an in vitro virus for in vitro protein evolution. Biol. Proced. Online 2002, 4, 49–54. [Google Scholar] [CrossRef]
- Liu, R.; Barrick, J.E.; Szostak, J.W.; Roberts, R.W. Optimized synthesis of RNA-protein fusions for in vitro protein selection. Methods Enzymol. 2000, 318, 268–293. [Google Scholar] [CrossRef]
- Cotten, S.W.; Zou, J.; Wang, R.; Huang, B.C.; Liu, R. mRNA display-based selections using synthetic peptide and natural protein libraries. Methods. Mol. Biol. 2012, 805, 287–297. [Google Scholar] [CrossRef]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Bleicher, K.H.; Bohm, H.J.; Muller, K.; Alanine, A.I. Hit and lead generation: Beyond high-throughput screening. Nat. Rev. Drug Discovery 2003, 2, 369–378. [Google Scholar] [CrossRef]
- Bellacosa, A.; Kumar, C.C.; Di Cristofano, A.; Testa, J.R. Activation of AKT kinases in cancer: Implications for therapeutic targeting. Adv. Cancer Res. 2005, 94, 29–86. [Google Scholar] [CrossRef]
- Hayashi, Y.; Morimoto, J.; Suga, H. In vitro selection of anti-Akt2 thioether-macrocyclic peptides leading to isoform-selective inhibitors. ACS Chem. Biol. 2012, 7, 607–613. [Google Scholar] [CrossRef]
- North, B.J.; Marshall, B.L.; Borra, M.T.; Denu, J.M.; Verdin, E. The human Sir2 ortholog, SIRT2, is an NAD(+)-dependent tubulin deacetylase. Mol. Cell 2003, 11, 437–444. [Google Scholar] [CrossRef]
- Vaquero, A.; Scher, M.B.; Lee, D.H.; Sutton, A.; Cheng, H.L.; Alt, F.W.; Serrano, L.; Sternglanz, R.; Reinberg, D. SirT2 is a histone deacetylase with preference for histone H4 Lys 16 during mitosis. Genes Dev. 2006, 20, 1256–1261. [Google Scholar] [CrossRef]
- Smith, B.C.; Denu, J.M. Mechanism-based inhibition of Sir2 deacetylases by thioacetyl-lysine peptide. Biochemistry 2007, 46, 14478–14486. [Google Scholar] [CrossRef]
- Morimoto, J.; Hayashi, Y.; Suga, H. Discovery of macrocyclic peptides armed with a mechanism-based warhead: Isoform-selective inhibition of human deacetylase SIRT2. Angew. Chem. Int. Ed. 2012, 51, 3423–3427. [Google Scholar] [CrossRef]
- Yamagishi, Y.; Shoji, I.; Miyagawa, S.; Kawakami, T.; Katoh, T.; Goto, Y.; Suga, H. Natural product-like macrocyclic N-methyl-peptide inhibitors against a ubiquitin ligase uncovered from a ribosome-expressed de novo library. Chem. Biol. 2011, 18, 1562–1570. [Google Scholar] [CrossRef]
- Biron, E.; Chatterjee, J.; Ovadia, O.; Langenegger, D.; Brueggen, J.; Hoyer, D.; Schmid, H.A.; Jelinek, R.; Gilon, C.; Hoffman, A.; Kessler, H. Improving oral bioavailability of peptides by multiple N-methylation: Somatostatin analogues. Angew. Chem. Int. Ed. 2008, 47, 2595–2599. [Google Scholar] [CrossRef]
- Kumar, S.; Talis, A.L.; Howley, P.M. Identification of HHR23A as a substrate for E6-associated protein-mediated ubiquitination. J. Biol. Chem. 1999, 274, 18785–18792. [Google Scholar] [CrossRef]
- Louria-Hayon, I.; Alsheich-Bartok, O.; Levav-Cohen, Y.; Silberman, I.; Berger, M.; Grossman, T.; Matentzoglu, K.; Jiang, Y.H.; Muller, S.; Scheffner, M.; Haupt, S.; Haupt, Y. E6AP promotes the degradation of the PML tumor suppressor. Cell Death Differ. 2009, 16, 1156–1166. [Google Scholar] [CrossRef]
- Hoeller, D.; Dikic, I. Targeting the ubiquitin system in cancer therapy. Nature 2009, 458, 438–444. [Google Scholar] [CrossRef]
- Schlippe, Y.V.G.; Hartman, M.C.T.; Josephson, K.; Szostak, J.W. In vitro selection of highly modified cyclic peptides that act as tight binding inhibitors. J. Am. Chem. Soc. 2012, 134, 10469–10477. [Google Scholar] [CrossRef]
- Hartman, M.C.T.; Josephson, K.; Szostak, J.W. Enzymatic aminoacylation of tRNA with unnatural amino acids. Proc. Natl. Acad. Sci. USA 2006, 103, 4356–4361. [Google Scholar] [CrossRef]
- Hartman, M.C.T.; Josephson, K.; Lin, C.W.; Szostak, J.W. An expanded set of amino acid analogs for the ribosomal translation of unnatural peptides. PLoS One 2007, 2, e972. [Google Scholar] [CrossRef]
- Subtelny, A.O.; Hartman, M.C.T.; Szostak, J.W. Ribosomal synthesis of N-methyl peptides. J. Am. Chem. Soc. 2008, 130, 6131–6136. [Google Scholar] [CrossRef]
- Goto, Y.; Iwasaki, K.; Torikai, K.; Murakami, H.; Suga, H. Ribosomal synthesis of dehydrobutyrine- and methyllanthionine-containing peptides. Chem. Commun. 2009, 3419–3421. [Google Scholar]
- Hofmann, F.T.; Szostak, J.W.; Seebeck, F.P. In vitro selection of functional lantipeptides. J. Am. Chem. Soc. 2012, 134, 8038–8041. [Google Scholar] [CrossRef]
- Heinis, C.; Rutherford, T.; Freund, S.; Winter, G. Phage-encoded combinatorial chemical libraries based on bicyclic peptides. Nat. Chem. Biol. 2009, 5, 502–507. [Google Scholar] [CrossRef]
- Knerr, P.J.; van der Donk, W.A. Discovery, biosynthesis, and engineering of lantipeptides. Annu. Rev. Biochem. 2012, 81, 479–505. [Google Scholar] [CrossRef]
- Knerr, P.J.; van der Donk, W.A. Chemical synthesis and biological activity of analogues of the lantibiotic epilancin 15X. J. Am. Chem. Soc. 2012, 134, 7648–7651. [Google Scholar] [CrossRef]
- Liu, W.; Chan, A.S.H.; Liu, H.Q.; Cochrane, S.A.; Vederas, J.C. Solid supported chemical syntheses of both components of the lantibiotic lacticin 3147. J. Am. Chem. Soc. 2011, 133, 14216–14219. [Google Scholar] [CrossRef]
- Ross, A.C.; Liu, H.Q.; Pattabiraman, V.R.; Vederas, J.C. Synthesis of the lantibiotic lactocin S using peptide cyclizations on solid phase. J. Am. Chem. Soc. 2010, 132, 462–463. [Google Scholar] [CrossRef]
- Seebeck, F.P.; Ricardo, A.; Szostak, J.W. Artificial lantipeptides from in vitro translations. Chem. Commun. 2011, 47, 6141–6143. [Google Scholar] [CrossRef]
- Mazmanian, S.K.; Liu, G.; Hung, T.T.; Schneewind, O. Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall. Science 1999, 285, 760–763. [Google Scholar] [CrossRef]
- Millward, S.W.; Takahashi, T.T.; Roberts, R.W. A general route for post-translational cyclization of mRNA display libraries. J. Am. Chem. Soc. 2005, 127, 14142–14143. [Google Scholar] [CrossRef]
- Millward, S.W.; Fiacco, S.; Austin, R.J.; Roberts, R.W. Design of cyclic peptides that bind protein surfaces with antibody-like affinity. ACS Chem. Biol. 2007, 2, 625–634. [Google Scholar] [CrossRef]
- Weinstein, L.S.; Chen, M.; Xie, T.; Liu, J. Genetic diseases associated with heterotrimeric G proteins. Trends Pharmacol. Sci. 2006, 27, 260–266. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ito, K.; Passioura, T.; Suga, H. Technologies for the Synthesis of mRNA-Encoding Libraries and Discovery of Bioactive Natural Product-Inspired Non-Traditional Macrocyclic Peptides. Molecules 2013, 18, 3502-3528. https://doi.org/10.3390/molecules18033502
Ito K, Passioura T, Suga H. Technologies for the Synthesis of mRNA-Encoding Libraries and Discovery of Bioactive Natural Product-Inspired Non-Traditional Macrocyclic Peptides. Molecules. 2013; 18(3):3502-3528. https://doi.org/10.3390/molecules18033502
Chicago/Turabian StyleIto, Kenichiro, Toby Passioura, and Hiroaki Suga. 2013. "Technologies for the Synthesis of mRNA-Encoding Libraries and Discovery of Bioactive Natural Product-Inspired Non-Traditional Macrocyclic Peptides" Molecules 18, no. 3: 3502-3528. https://doi.org/10.3390/molecules18033502
APA StyleIto, K., Passioura, T., & Suga, H. (2013). Technologies for the Synthesis of mRNA-Encoding Libraries and Discovery of Bioactive Natural Product-Inspired Non-Traditional Macrocyclic Peptides. Molecules, 18(3), 3502-3528. https://doi.org/10.3390/molecules18033502



