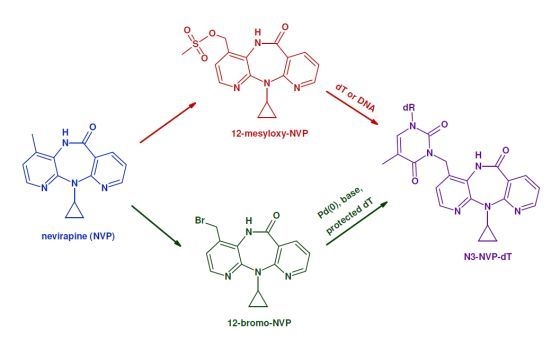
2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine
Abstract
Share and Cite
Antunes, A.M.M.; Wolf, B.; Oliveira, M.C.; Beland, F.A.; Marques, M.M. 2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine. Molecules 2013, 18, 4955-4971. https://doi.org/10.3390/molecules18054955
Antunes AMM, Wolf B, Oliveira MC, Beland FA, Marques MM. 2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine. Molecules. 2013; 18(5):4955-4971. https://doi.org/10.3390/molecules18054955
Chicago/Turabian StyleAntunes, Alexandra M. M., Benjamin Wolf, M. Conceição Oliveira, Frederick A. Beland, and M. Matilde Marques. 2013. "2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine" Molecules 18, no. 5: 4955-4971. https://doi.org/10.3390/molecules18054955
APA StyleAntunes, A. M. M., Wolf, B., Oliveira, M. C., Beland, F. A., & Marques, M. M. (2013). 2'-Deoxythymidine Adducts from the Anti-HIV Drug Nevirapine. Molecules, 18(5), 4955-4971. https://doi.org/10.3390/molecules18054955