Carvacrol: From Ancient Flavoring to Neuromodulatory Agent
Abstract
:1. Introduction
2. Results and Discussion



3. Experimental Section
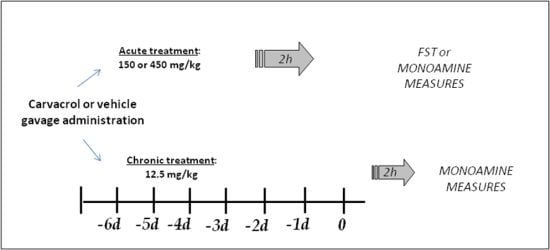
3.1. Animals and Treatment
3.2. Measurement of Monoamine Neurotransmitter Levels
3.3. Forced Swimming Test
3.4. Statistical Analysis
4. Conclusions
Acknowledgments
Conflicts of Interest
References and Notes
- Kintzios, S.E. Oregano: The Genera Origanum and Lippia; Taylor & Francis: New York, NY, USA, 2002. [Google Scholar]
- Regulation (EC) No 2232/96 of the European Parliament and of the Council on 28 October 1996, Commission decision of 23 February 1999 adopting a register of flavouring substances used in or on foodstuffs. In Official Journal L 84: 1999/217/EC; pp. 1–37.
- EAFUS: A Food Additive Database; Centre for Food Safety and Applied Nutrition, U.S. Food and Drug Administration: Washington, DC, USA, 2006.
- Burt, S. Essential oils: their antibacterial properties and potential applications in foods-a review. Int. J. Food Microbiol 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Edris, A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother. Res 2007, 21, 308–323. [Google Scholar] [CrossRef]
- Bergström, L.S.; Lynöe, N. Enhancing concentration, mood and memory in healthy individuals: an empirical study of attitudes among general practitioners and the general population. Scand J. Public. Health 2008, 36, 532–537. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Fungicidal activity of thymol and carvacrol by disrupting ergosterol biosynthesis and membrane integrity against Candida. Eur. J. Clin. Microbiol. Infect Dis 2011, 30, 41–50. [Google Scholar] [CrossRef]
- Dambolena, J.S.; Zygadlo, J.A.; Rubinstein, H.R. Antifumonisin activity of natural phenolic compounds. A structure-property-activity relationship study. Int. J. Food Microbiol 2011, 145, 140–146. [Google Scholar] [CrossRef]
- Tang, X.; Chen, S.; Wang, L. Purification and identification of carvacrol from the root of Stellera chamaejasme and research on its insecticidal activity. Nat. Prod. Res 2011, 25, 320–325. [Google Scholar] [CrossRef]
- Nostro, A.; Papalia, T. Antimicrobial activity of carvacrol: current progress and future prospectives. Recent. Pat. Antiinfect. Drug Discov 2012, 7, 28–35. [Google Scholar] [CrossRef]
- Koparal, A.T.; Zeytinoglu, M. Effects of Carvacrol on a Human Non-Small Cell Lung Cancer (NSCLC) Cell Line, A549. Cytotechnology 2003, 43, 149–154. [Google Scholar] [CrossRef]
- Karkabounas, S.; Kostoula, O.K.; Daskalou, T.; Veltsistas, P.; Karamouzis, M.; Zelovitis, I.; Metsios, A.; Lekkas, P.; Evangelou, A.M.; Kotsis, N.; et al. Anticarcinogenic and antiplatelet effects of carvacrol. Exp. Oncol 2006, 28, 121–125. [Google Scholar]
- Mezzoug, N.; Elhadri, A.; Dallouh, A.; Amkiss, S.; Skali, N.S.; Abrini, J.; Zhiri, A.; Baudoux, D.; Diallo, B.; El Jaziri, M.; et al. Investigation of the mutagenic and antimutagenic effects of Origanum compactum essential oil and some of its constituents. Mutat. Res 2007, 629, 100–110. [Google Scholar] [CrossRef]
- Sokmen, A.; Sokmen, M.; Daferera, D.; Polissiou, M.; Candan, F.; Unlu, M.; Akpulat, H.A. The in vitro antioxidant and antimicrobial activities of the essential oil and methanol extracts of Achillea biebersteini Afan. (Asteraceae). Phytother. Res 2004, 18, 451–456. [Google Scholar] [CrossRef]
- Canbek, M.; Uyanoglu, M.; Bayramoglu, G.; Senturk, H.; Erkasap, N.; Koken, T.; Uslu, S.; Demirustu, C.; Aral, E.; Can Baser, K.H. Effects of carvacrol on defects of ischemia-reperfusion in the rat liver. Phytomedicine 2008, 15, 447–452. [Google Scholar] [CrossRef]
- Savelev, S.U.; Okello, E.J.; Perry, E.K. Butyryl- and acetyl-cholinesterase inhibitory activities in essential oils of Salvia species and their constituents. Phytother. Res 2004, 18, 315–324. [Google Scholar] [CrossRef]
- Hotta, M.; Nakata, R.; Katsukawa, M.; Hori, K.; Takahashi, S.; Inoue, H. Carvacrol, a component of thyme oil, activates PPARalpha and gamma and suppresses COX-2 expression. J. Lipid. Res 2010, 51, 132–139. [Google Scholar] [CrossRef]
- Trabace, L.Z.M.; Morgese, M.G.; Tucci, P.; Colaianna, M.; Schiavone, S.; Avato, P.; Cuomo, V. Estrous cycle affects the neurochemical and neurobehavioral profile of carvacrol-treated female rats. Toxicol. Appl. Pharmacol 2011, 255, 169–175. [Google Scholar] [CrossRef]
- Parnas, M.; Peters, M.; Dadon, D.; Lev, S.; Vertkin, I.; Slutsky, I.; Minke, B. Carvacrol is a novel inhibitor of Drosophila TRPL and mammalian TRPM7 channels. Cell Calcium 2009, 45, 300–339. [Google Scholar] [CrossRef]
- Yu, H.; Zhang, Z.L.; Chen, J.; Pei, A.; Hua, F.; Qian, X.; He, J.; Liu, C.F.; Xu, X. Carvacrol, a food-additive, provides neuroprotection on focal cerebral ischemia/reperfusion injury in mice. PLoS One 2012, e33584. [Google Scholar]
- Jukic, M.; Politeo, O.; Maksimovic, M.; Milos, M. In vitro acetylcholinesterase inhibitory properties of thymol, carvacrol and their derivatives thymoquinone and thymohydroquinone. Phytother. Res 2007, 21, 259–261. [Google Scholar] [CrossRef]
- Melo, F.H.; Venancio, E.T.; de Sousa, D.P.; de Franca Fonteles, M.M.; de Vasconcelos, S.M.; Viana, G.S.; de Sousa, F.C. Anxiolytic-like effect of Carvacrol (5-isopropyl-2-methylphenol) in mice: Involvement with GABAergic transmission. Fundam. Clin. Pharmacol 2010, 24, 437–443. [Google Scholar]
- Melo, F.H.; Moura, B.A.; de Sousa, D.P.; de Vasconcelos, S.M.; Macedo, D.S.; Fonteles, M.M.; Viana, G.S.; de Sousa, F.C. Antidepressant-like effect of carvacrol (5-Isopropyl-2-methylphenol) in mice: involvement of dopaminergic system. Fundam. Clin. Pharmacol 2011, 25, 362–367. [Google Scholar] [CrossRef]
- Mechan, A.O.; Fowler, A.; Seifert, N.; Rieger, H.; Wohrle, T.; Etheve, S.; Wyss, A.; Schuler, G.; Colletto, B.; Kilpert, C.; et al. Monoamine reuptake inhibition and mood-enhancing potential of a specified oregano extract. Br. J. Nutr 2011, 105, 1150–1163. [Google Scholar] [CrossRef]
- Efterpi, C.; Eleftherios, B.; Ilias, G.; Panagiota, F.P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Furia, T.E.; Bellanca, N. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Cleveland, OH, USA, 1975. [Google Scholar]
- Kirsti Kaarina, P.K.N.; Seija Marjatta, M.K. Processing, effects and use of Oregano and marjoram in foodstuffs and in food preparation. In The Design Life of Structures; Spon Press: London, UK, 1998. [Google Scholar]
- Bhattaram, V.A.; Graefe, U.; Kohlert, C.; Veit, M.; Derendorf, H. Pharmacokinetics and bioavailability of herbal medicinal products. Phytomedicine 2002, 9, 1–33. [Google Scholar]
- De Vincenzi, M.; Stammati, A.; De Vincenzi, A.; Silano, M. Constituents of aromatic plants: Carvacrol. Fitoterapia 2004, 75, 801–804. [Google Scholar] [CrossRef]
- Stammati, A.; Bonsi, P.; Zucco, F.; Moezelaar, R.; Alakomi, H.L.; von Wright, A. Toxicity of selected plant volatiles in microbial and mammalian short-term assays. Food Chem. Toxicol 1999, 37, 813–823. [Google Scholar] [CrossRef]
- Baser, K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Des 2008, 14, 3106–3119. [Google Scholar] [CrossRef]
- Lucki, I. The spectrum of behaviors influenced by serotonin. Biol. Psychiatry 1998, 44, 151–162. [Google Scholar] [CrossRef]
- Missale, C.; Nash, S.R.; Robinson, S.W.; Jaber, M.; Caron, M.G. Dopamine receptors: from structure to function. Physiol. Rev 1998, 78, 189–225. [Google Scholar]
- Golden, R.N.; Gilmore, J.H. Serotonin and mood disorders. Psychiatr. Ann 1990, 20, 580–586. [Google Scholar]
- Golden, R.N.; Gilmore, J.H.; Corrigan, M.H.; Ekstrom, R.D.; Knight, B.T.; Garbutt, J.C. Serotonin, suicide, and aggression: clinical studies. J. Clin. Psychiatr 1991, 52, 61–69. [Google Scholar]
- Fernstrom, J.D.; Wurtman, R.J. Brain serotonin content: Increase following ingestion of carbohydrate diet. Science 1971, 174, 1023–1025. [Google Scholar]
- Fremeau, R.T., Jr.; Duncan, G.E.; Fornaretto, M.G.; Dearry, A.; Gingrich, J.A.; Breese, G.R.; Caron, M.G. Localization of D1 dopamine receptor mRNA in brain supports a role in cognitive, affective, and neuroendocrine aspects of dopaminergic neurotransmission. Proc. Natl. Acad. Sci. USA 1991, 88, 3772–3776. [Google Scholar] [CrossRef]
- Beninger, R.J. The role of dopamine in locomotor activity and learning. Brain Res 1983, 287, 173–196. [Google Scholar]
- Wise, R.A. The role of reward pathways in the development of drug dependence. Pharmacol. Ther 1987, 35, 227–263. [Google Scholar] [CrossRef]
- Manji, H.K.; Drevets, W.C.; Charney, D.S. The cellular neurobiology of depression. Nat. Med 2001, 7, 541–547. [Google Scholar] [CrossRef]
- Morilak, D.A.; Frazer, A. Antidepressants and brain monoaminergic systems: a dimensional approach to understanding their behavioural effects in depression and anxiety disorders. Int. J. Neuropsychopharmacol 2004, 7, 193–218. [Google Scholar] [CrossRef]
- Invernizzi, R.W.; Sacchetti, G.; Parini, S.; Acconcia, S.; Samanin, R. Flibanserin, a potential antidepressant drug, lowers 5-HT and raises dopamine and noradrenaline in the rat prefrontal cortex dialysate: role of 5-HT(1A) receptors. Br. J. pharmacol 2003, 139, 1281–1288. [Google Scholar] [CrossRef]
- Kennedy, D.O.; Wake, G.; Savelev, S.; Tildesley, N.T.; Perry, E.K.; Wesnes, K.A.; Scholey, A.B. Modulation of mood and cognitive performance following acute administration of single doses of Melissa officinalis (Lemon balm) with human CNS nicotinic and muscarinic receptor-binding properties. Neuropsychopharmacology 2003, 28, 1871–1881. [Google Scholar] [CrossRef]
- Kumar, G.P.; Khanum, F. Neuroprotective potential of phytochemicals. Pharmacogn. Rev 2012, 6, 81–90. [Google Scholar]
- Linde, K.; Berner, M.M.; Kriston, L. St John’s wort for major depression. Cochrane Database Syst. Rev 2008, 4, CD000448. [Google Scholar]
- Yoshitake, T.; Yoshitake, S.; Kehr, J. The Ginkgo biloba extract EGb 761(R) and its main constituent flavonoids and ginkgolides increase extracellular dopamine levels in the rat prefrontal cortex. Br. J. Pharmacol 2010, 1, 659–668. [Google Scholar] [CrossRef]
- Pierce, R.C.; Kumaresan, V. The mesolimbic dopamine system: The final common pathway for the reinforcing effect of drugs of abuse? Neurosci. Biobehav. Rev 2006, 30, 215–238. [Google Scholar] [CrossRef]
- Cook, J.D.; Schanberg, S.M. Effect of methamphetamine on norepinephrine metabolism in various regions of brain. J. Pharmacol. Exp. Ther 1975, 195, 87–93. [Google Scholar]
- Breese, G.R.; Cooper, B.R.; Mueller, R.A. Evidence for involvement of 5-hydroxytryptamine in the actions of amphetamine. Br. J. Pharmacol 1974, 52, 307–314. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C.R.R. The Rat Brain in Stereotaxic Coordinates; Elsevier Academic Press: New York, NY, USA, 1998. [Google Scholar]
- Cryan, J.F.; Valentino, R.J.; Lucki, I. Assessing substrates underlying the behavioral effects of antidepressants using the modified rat forced swimming test. Neurosci. Biobehav. Rev 2005, 29, 547–569. [Google Scholar] [CrossRef]
- Sample Availability: Not aviable.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zotti, M.; Colaianna, M.; Morgese, M.G.; Tucci, P.; Schiavone, S.; Avato, P.; Trabace, L. Carvacrol: From Ancient Flavoring to Neuromodulatory Agent. Molecules 2013, 18, 6161-6172. https://doi.org/10.3390/molecules18066161
Zotti M, Colaianna M, Morgese MG, Tucci P, Schiavone S, Avato P, Trabace L. Carvacrol: From Ancient Flavoring to Neuromodulatory Agent. Molecules. 2013; 18(6):6161-6172. https://doi.org/10.3390/molecules18066161
Chicago/Turabian StyleZotti, Margherita, Marilena Colaianna, Maria Grazia Morgese, Paolo Tucci, Stefania Schiavone, Pinarosa Avato, and Luigia Trabace. 2013. "Carvacrol: From Ancient Flavoring to Neuromodulatory Agent" Molecules 18, no. 6: 6161-6172. https://doi.org/10.3390/molecules18066161
APA StyleZotti, M., Colaianna, M., Morgese, M. G., Tucci, P., Schiavone, S., Avato, P., & Trabace, L. (2013). Carvacrol: From Ancient Flavoring to Neuromodulatory Agent. Molecules, 18(6), 6161-6172. https://doi.org/10.3390/molecules18066161