89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges
Abstract
:1. Introduction
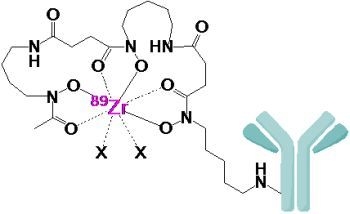
2. Radiolabeling of Bioactive Molecules with 89Zr

3. Introduction of Desferrioxamine B into Bioactive Molecules
3.1. Introduction of DFO via Thiol Conjugation


3.2. Introduction of DFO via Click Chemistry

3.3. Introduction of DFO via Acid-Amide or Thiourea Formation


4. Stability of the 89Zr-Desferrioxamine B Complex
5. 89Zr-labeled Bioactive Compounds
5.1. Peptides, Antibody Fragments and Serum Proteins
5.2. Nanoparticles and Microspheres
5.3. Antibodies
| Antibody used | Epitope | Target tissue | Preclinical | Clinical |
|---|---|---|---|---|
| 323/A3 | 17.1 A | squamous-cell carcinoma and others | [54] | |
| 5A10 | “free” prostate-specific antigen (fPSA) | osseous prostate cancer lesions | [87] | |
| 7E11 | prostate-specific membrane antigen (PSMA) | prostate cancer | [51] | |
| bevacizumab | vascular endothelial growth factor (VEGF) | tumor angiogenic vessels | [24,93,86,92,97] | |
| cetuximab | epidermal growth factor receptor | various cancers | [29,47,72,97] | |
| CD45R | B220 | B cells | [48] | |
| cG250 | G250 | renal cell carcinoma (RCC) | [94] | |
| cU36 | CD44v6 | head and neck squamous cell carcinoma (HNSCC) human studies | [8,19,47,58] | [16,28] |
| DN30 | c-Met receptor | gastric cancer, Met/head and neck cancer | [57] | |
| E48 | 22 kDa antigen | squamous-cell carcinoma and others | [54] | |
| E4G10 | vascular endothelial cadherin (VE-cad) | tumor angiogenic vessels | [33] | |
| fresolimumab | Transforming growth factor-β (TGF-β) | highly invasive or metastatic tumors as glioblastomas and human breast cancer | [52] | |
| hRS7 | epithelial glycoprotein-1 (EGP-1) | epithelial carcinomas | [59] | |
| ibritumomab tiuxetan (Zevalin) | CD20 | NHL (non-Hodgkin’s lymphoma) human studies | [53] | [53,95] |
| J591 | prostate-specific membrane antigen (PSMA) | prostate cancer | [2246] | |
| Onartuzumab | hepatocyte growth factor receptor (Met) | gastric carcinoma and glioblastoma | [89] | |
| panitumumab | (EGFR/HER1) | colorectal cancer, head and neck tumors | [61,90,98] | |
| PGN635 | phosphatidylserine | apoptosis | [91] | |
| R1507 | insulin-like growth factor receptor 1 | breast cancer | [71] | |
| ranibizumab | VEGF | tumor induced angiogenesis | [88] | |
| rituximab | CD20 | malign lymphoma cells | [47,62] | |
| trastuzumab | human epidermal growth factor receptor 2 (HER2) | breast cancer, ovarian, colorectal carcinoma human studies | [23,25,26,27,50,55,56,96] | [93,99,100] |
| TRC105 | CD105 | angiogenic endothelial cells | [60,101] |
6. Conclusions and Outlook
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Hong, H.; Cai, W. PET tracers based on Zirconium-89. Curr. Radiopharm. 2011, 4, 131–139. [Google Scholar] [CrossRef]
- Severin, G.W.; Engle, J.W.; Barnhart, T.E.; Nickles, R.J. Zr-89 Radiochemistry for positron emission tomography. Med. Chem. 2011, 7, 389–394. [Google Scholar]
- Nayak, T.K.; Brechbiel, M.W. Radioimmunoimaging with longer-lived positron-emitting radionuclides: Potentials and challenges. Bioconjug. Chem. 2009, 20, 825–841. [Google Scholar] [CrossRef]
- Deri, M.A.; Zeglis, B.M.; Francesconi, L.C.; Lewis, J.S. PET imaging with Zr-89: From radiochemistry to the clinic. Nucl. Med. Biol. 2013, 40, 3–14. [Google Scholar] [CrossRef]
- Holland, J.P.; Sheh, Y.C.; Lewis, J.S. Standardized methods for the production of high specific-activity zirconium-89. Nucl. Med. Biol. 2009, 36, 729–739. [Google Scholar]
- Baillie, A.D.; Ledingham, K.W.D.; Lynch, J.G.; Campbell, M. Electron capture to positron emission ratios in the allowed decay of 89Zr. J. Phys. G Nucl. Phys. 1979, 5, 1433–1444. [Google Scholar] [CrossRef]
- Lubberink, M.; Herzog, H. Quantitative imaging of I-124 and Y-86 with PET. Eur. J. Nucl. Med. Mol. I 2011, 38, 10–18. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boellaard, R.; Stigter-van Walsum, M.; Snow, G.; van Dongen, G.A. 89Zr immuno-PET: Comprehensive procedures for the production of 89Zr-labeled monoclonal antibodies. J. Nucl. Med. 2003, 44, 1271–1281. [Google Scholar]
- Meijs, W.E.; Herscheid, J.D.M.; Haisma, H.J.; Wijbrandts, R.; Vanlangevelde, F.; Vanleuffen, P.J.; Mooy, R.; Pinedo, H.M. Production of highly pure no-carrier added Zr-89 for the labeling of antibodies with a positron emitter. Appl. Radiat. Isotopes 1994, 45, 1143–1147. [Google Scholar] [CrossRef]
- Taghilo, M.; Kakavand, T.; Rajabifar, S.; Sarabadani, P. Cyclotron production of 89Zr: A potent radionuclide for positron emission tomography. Int. J. Phys. Sci. 2012, 7, 1321–1325. [Google Scholar]
- Dejesus, O.T.; Nickles, R.J. Production and Purification of Zr-89, a Potential Pet Antibody Label. Appl. Radiat. Isotopes 1990, 41, 789–790. [Google Scholar] [CrossRef]
- Kasbollah, A.; Eu, P.; Cowell, S.; Deb, P. Review on Production of 89Zr in a Medical Cyclotron for PET Radiopharmaceuticals. J. Nucl. Med. Technol. 2013, 41, 35–41. [Google Scholar] [CrossRef]
- Sadeghi, M.; Enferadi, M.; Bakhtiari, M. Accelerator production of the positron emitter zirconium-89. Ann. Nucl. Energy 2012, 41, 97–103. [Google Scholar] [CrossRef]
- Lobo, E.D.; Hansen, R.J.; Balthasar, J.P. Antibody pharmacokinetics and pharmacodynamics. J. Pharm. Sci. 2004, 93, 2645–2668. [Google Scholar] [CrossRef]
- Imai, K.; Takaoka, A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer 2006, 6, 714–727. [Google Scholar] [CrossRef]
- Borjesson, P.K.; Jauw, Y.W.; de Bree, R.; Roos, J.C.; Castelijns, J.A.; Leemans, C.R.; van Dongen, G.A.; Boellaard, R. Radiation dosimetry of 89Zr-labeled chimeric monoclonal antibody U36 as used for immuno-PET in head and neck cancer patients. J. Nucl. Med. 2009, 50, 1828–1836. [Google Scholar] [CrossRef]
- Brix, G.; Lechel, U.; Glatting, G.; Ziegler, S.I.; Munzing, W.; Muller, S.P.; Beyer, T. Radiation exposure of patients undergoing whole-body dual-modality 18F-FDG PET/CT examinations. J. Nucl. Med. 2005, 46, 608–613. [Google Scholar]
- van Dongen, G.A.M.S.; Visser, G.W.M.; Hooge, M.N.L.D.; De Vries, E.G.; Perk, L.R. Immuno-PET: A navigator in monoclonal antibody development and applications. Oncologist 2007, 12, 1379–1389. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.; Boellaard, R.; Boerman, O.C.; van Eerd, J.; Snow, G.B.; Lammertsma, A.A.; van Dongen, G.A. Quantitative 89Zr immuno-PET for in vivo scouting of 90Y-labeled monoclonal antibodies in xenograft-bearing nude mice. J. Nucl. Med. 2003, 44, 1663–1670. [Google Scholar]
- Herzog, H.; Tellmann, L.; Qaim, S.M.; Spellerberg, S.; Schmid, A.; Coenen, H.H. PET quantitation and imaging of the non-pure positronemitting iodine isotope I-124. Appl. Radiat. Isotopes 2002, 56, 673–679. [Google Scholar] [CrossRef]
- Thorek, D.L.J.; Robertson, R.; Bacchus, W.A.; Hahn, J.; Rothberg, J.; Beattie, B.J.; Grimm, J. Cerenkov imaging-a new modality for molecular imaging. Am. J. Nucl. Med. Mol. Imaging 2012, 2, 163–173. [Google Scholar]
- Ruggiero, A.; Holland, J.P.; Lewis, J.S.; Grimm, J. Cerenkov Luminescence Imaging of Medical Isotopes. J. Nucl. Med. 2010, 51, 1123–1130. [Google Scholar] [CrossRef]
- Holland, J.P.; Normand, G.; Ruggiero, A.; Lewis, J.S.; Grimm, J. Intraoperative Imaging of Positron Emission Tomographic Radiotracers Using Cerenkov Luminescence Emissions. Mol. Imaging 2011, 10, 177–186. [Google Scholar]
- Nagengast, W.B.; de Vries, E.G.; Hospers, G.A.; Mulder, N.H.; de Jong, J.R.; Hollema, H.; Brouwers, A.H.; van Dongen, G.A.; Perk, L.R.; Lub-de Hooge, M.N. In vivo VEGF imaging with radiolabeled bevacizumab in a human ovarian tumor xenograft. J. Nucl. Med. 2007, 48, 1313–1319. [Google Scholar] [CrossRef]
- Munnink, T.H.O.; de Korte, M.A.; Nagengast, W.B.; Timmer-Bosscha, H.; Schroder, C.P.; de Jong, J.R.; van Dongen, G.A.M.S.; Jensen, M.R.; Quadt, C.; Lub-de Hooge, M.N.; et al. Zr-89-trastuzumab PET visualises HER2 downregulation by the HSP90 inhibitor NVP-AUY922 in a human tumour xenograft. Eur. J. Cancer 2010, 46, 678–684. [Google Scholar] [CrossRef]
- Dijkers, E.C.F.; Kosterink, J.G.W.; Rademaker, A.P.; Perk, L.R.; van Dongen, G.A.M.S.; Bart, J.; de Jong, J.R.; de Vries, E.G.E.; Lub-de Hooge, M.N. Development and Characterization of Clinical-Grade Zr-89-Trastuzumab for HER2/neu ImmunoPET Imaging. J. Nucl. Med. 2009, 50, 974–981. [Google Scholar] [CrossRef]
- Chang, A.J.; DeSilva, R.; Jain, S.; Lears, K.; Rogers, B.; Lapi, S. 89Zr-radiolabeled trastuzumab imaging in orthotopic and metastatic breast tumors. Pharmaceuticals 2012, 5, 79–93. [Google Scholar] [CrossRef]
- Borjesson, P.K.; Jauw, Y.W.; Boellaard, R.; de Bree, R.; Comans, E.F.; Roos, J.C.; Castelijns, J.A.; Vosjan, M.J.; Kummer, J.A.; Leemans, C.R.; et al. Performance of immuno-positron emission tomography with zirconium-89-labeled chimeric monoclonal antibody U36 in the detection of lymph node metastases in head and neck cancer patients. Clin. Cancer Res. 2006, 12, 2133–2140. [Google Scholar] [CrossRef]
- Aerts, H.J.W.L.; Dubois, L.; Perk, L.; Vermaelen, P.; van Dongen, G.A.M.S.; Wouters, B.G.; Lambin, P. Disparity Between In Vivo EGFR Expression and (89)Zr-Labeled Cetuximab Uptake Assessed with PET. J. Nucl. Med. 2009, 50, 123–131. [Google Scholar]
- Jacobson, O.; Zhu, L.; Niu, G.; Weiss, I.D.; Szajek, L.P.; Ma, Y.; Sun, X.; Yan, Y.; Kiesewetter, D.O.; Liu, S.; et al. MicroPET imaging of integrin alphavbeta3 expressing tumors using 89Zr-RGD peptides. Mol. Imaging Biol. 2011, 13, 1224–1233. [Google Scholar] [CrossRef]
- Keliher, E.J.; Yoo, J.; Nahrendorf, M.; Lewis, J.S.; Marinelli, B.; Newton, A.; Pittet, M.J.; Weissleder, R. Zr-89-Labeled Dextran Nanoparticles Allow in vivo Macrophage Imaging. Bioconjug. Chem. 2011, 22, 2383–2389. [Google Scholar] [CrossRef]
- Avila-Rodriguez, M.A.; Selwyn, R.G.; Hampel, J.A.; Thornadsen, B.R.; DeJesus, O.T.; Converse, A.K.; Nickles, R.J. Positron-emitting resin microspheres as surrogates of Y-90 SIR-Spheres: A radiolabeling and stability study. Nucl. Med. Biol. 2007, 34, 585–590. [Google Scholar] [CrossRef]
- Ruggiero, A.; Villa, C.H.; Holland, J.; Sprinkle, S.R.; May, C.; Lewis, J.S.; Scheinberg, D.A.; McDevitt, M.R. Imaging and treating tumor vasculature with targeted radiolabeled carbon nanotubes. Int. J. Nanomed. 2010, 5, 783–802. [Google Scholar]
- Abou, D.S.; Thorek, D.L.; Ramos, N.N.; Pinkse, M.W.; Wolterbeek, H.T.; Carlin, S.D.; Beattie, B.J.; Lewis, J.S. (89)Zr-Labeled Paramagnetic Octreotide-Liposomes for PET-MR Imaging of Cancer. Pharm. Res. 2013, 30, 878–888. [Google Scholar] [CrossRef]
- Evans, M.J.; Holland, J.P.; Rice, S.L.; Doran, M.G.; Cheal, S.M.; Campos, C.; Carlin, S.D.; Mellinghoff, I.K.; Sawyers, C.L.; Lewis, J.S. Imaging Tumor Burden in the Brain with Zr-89-Transferrin. J. Nucl. Med. 2013, 54, 90–95. [Google Scholar] [CrossRef]
- Holland, J.P.; Evans, M.J.; Rice, S.L.; Wongvipat, J.; Sawyers, C.L.; Lewis, J.S. Annotating MYC status with Zr-89-transferrin imaging. Nat. Med. 2012, 18, 1586–1597. [Google Scholar] [CrossRef]
- Meijs, W.E.; Haisma, H.J.; VanderSchors, R.; Wijbrandts, R.; VandenOever, K.; Klok, R.P.; Pinedo, H.M.; Herscheid, J.D.M. A facile method for the labeling of proteins with zirconium isotopes. Nucl. Med. Biol. 1996, 23, 439–448. [Google Scholar] [CrossRef]
- Sotogaku, N.; Endo, K.; Hirunuma, R.; Enomoto, S.; Ambe, S.; Ambe, F. Biochemical reactions of various trace elements with blood components and transport proteins. J. Radioanal. Nucl. Ch. 1999, 239, 429–432. [Google Scholar] [CrossRef]
- Abou, D.S.; Ku, T.; Smith-Jones, P.M. In vivo biodistribution and accumulation of Zr-89 in mice. Nucl. Med. Biol. 2011, 38, 675–681. [Google Scholar] [CrossRef]
- Amano, R.; Enomoto, S.; Nobuta, M.; Sakamoto, M.; Tsujioka, R.; Ambe, F. Bone uptake of vanadium in mice: Simultaneous tracing of V, Se, Sr, Y, Zr, Ru and Rh using a radioactive multitracer. J. Trace Elem. Med. Biol. 1996, 10, 145–148. [Google Scholar] [CrossRef]
- Meijs, W.E.; Herscheid, J.D.M.; Haisma, H.J.; Pinedo, H.M. Evaluation of Desferal as a Bifunctional Chelating Agent for Labeling Antibodies with Zr-89. Appl. Radiat. Isotopes 1992, 43, 1443–1447. [Google Scholar] [CrossRef]
- Wadas, T.J.; Wong, E.H.; Weisman, G.R.; Anderson, C.J. Coordinating radiometals of copper, Gallium, Indium, Yttrium, and zirconium for PET and SPECT imaging of disease. Chem. Rev. 2010, 110, 2858–2902. [Google Scholar] [CrossRef]
- Kiss, T.; Farkas, E. Metal-binding ability of desferrioxamine B. J. Inclus. Phenom. Mol. 1998, 32, 385–403. [Google Scholar] [CrossRef]
- Hernlem, B.J.; Vane, L.M.; Sayles, G.D. Stability constants for complexes of the siderophore desferrioxamine B with selected heavy metal cations. Inorg. Chim. Acta 1996, 244, 179–184. [Google Scholar] [CrossRef]
- Baroncelli, F.; Grossi, G. The complexing power of hydroxamic acids and its effect on the behaviour of organic extractants in the reprocessing of irradiated fuels—I the complexes between benzohydroxamic acid and zirconium, iron (III) and uranium (VI). J. Inorg. Nucl. Chem. 1965, 27, 1085–1092. [Google Scholar] [CrossRef]
- Holland, J.P.; Divilov, V.; Bander, N.H.; Smith-Jones, P.M.; Larson, S.M.; Lewis, J.S. Zr-89-DFO-J591 for ImmunoPET of Prostate-Specific Membrane Antigen Expression In Vivo. J. Nucl. Med. 2010, 51, 1293–1300. [Google Scholar] [CrossRef]
- Perk, L.R.; Vosjan, M.J.W.D.; Visser, G.W.M.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A.M.S. p-Isothiocyanatobenzyl-desferrioxamine: A new bifunctional chelate for facile radiolabeling of monoclonal antibodies with zirconium-89 for immuno-PET imaging. Eur. J. Nucl. Med. Mol. I 2010, 37, 250–259. [Google Scholar] [CrossRef]
- Walther, M.; Gebhardt, P.; Grosse-Gehling, P.; Wurbach, L.; Irmler, I.; Preusche, S.; Khalid, M.; Opfermann, T.; Kamradt, T.; Steinbach, J.; et al. Implementation of Zr-89 production and in vivo imaging of B-cells in mice with Zr-89-labeled anti-B-cell antibodies by small animal PET/CT. Appl. Radiat. Isotopes 2011, 69, 852–857. [Google Scholar] [CrossRef]
- Vosjan, M.J.; Perk, L.R.; Visser, G.W.; Budde, M.; Jurek, P.; Kiefer, G.E.; van Dongen, G.A. Conjugation and radiolabeling of monoclonal antibodies with zirconium-89 for PET imaging using the bifunctional chelate p-isothiocyanatobenzyl-desferrioxamine. Nat. Protoc. 2010, 5, 739–743. [Google Scholar] [CrossRef]
- Holland, J.P.; Caldas-Lopes, E.; Divilov, V.; Longo, V.A.; Taldone, T.; Zatorska, D.; Chiosis, G.; Lewis, J.S. Measuring the Pharmacodynamic Effects of a Novel Hsp90 Inhibitor on HER2/neu Expression in Mice Using Zr-89-DFO-Trastuzumab. PLoS One 2010, 5, e8859. [Google Scholar] [CrossRef]
- Ruggiero, A.; Holland, J.P.; Hudolin, T.; Shenker, L.; Koulova, A.; Bander, N.H.; Lewis, J.S.; Grimm, J. Targeting the Internal Epitope of Prostate-Specific Membrane Antigen with Zr-89-7E11 Immuno-PET. J. Nucl. Med. 2011, 52, 1608–1615. [Google Scholar] [CrossRef]
- Munnink, T.H.O.; Arjaans, M.E.A.; Timmer-Bosscha, H.; Schroder, C.P.; Hesselink, J.W.; Vedelaar, S.R.; Walenkamp, A.M.E.; Reiss, M.; Gregory, R.C.; Lub-de Hooge, M.N.; et al. PET with the Zr-89-Labeled Transforming Growth Factor-beta Antibody Fresolimumab in Tumor Models. J. Nucl. Med. 2011, 52, 2001–2008. [Google Scholar] [CrossRef]
- Perk, L.R.; Visser, O.J.; Walsum, M.S.V.; Vosjan, M.J.W.D.; Visser, G.W.M.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A.M.S. Preparation and evaluation of Zr-89-Zevalin for monitoring of Y-90-Zevalin biodistribution with positron emission tomography. Eur. J. Nucl. Med. Mol. I 2006, 33, 1337–1345. [Google Scholar] [CrossRef]
- Meijs, W.E.; Haisma, H.J.; Klok, R.P.; vanGog, F.B.; Kievit, E.; Pinedo, H.M.; Herscheid, J.D.M. Zirconium-labeled monoclonal antibodies and their distribution in tumor-bearing nude mice. J. Nucl. Med. 1997, 38, 112–118. [Google Scholar]
- Tinianow, J.N.; Gill, H.S.; Ogasawara, A.; Flores, J.E.; Vanderbilt, A.N.; Luis, E.; Vandlen, R.; Darwish, M.; Junutula, J.R.; Williams, S.P.; et al. Site-specifically 89Zr-labeled monoclonal antibodies for ImmunoPET. Nucl. Med. Biol. 2010, 37, 289–297. [Google Scholar] [CrossRef]
- Zeglis, B.M.; Mohindra, P.; Weissmann, G.I.; Divilov, V.; Hilderbrand, S.A.; Weissleder, R.; Lewis, J.S. Modular strategy for the construction of radiometalated antibodies for positron emission tomography based on inverse electron demand Diels-Alder click chemistry. Bioconjug. Chem. 2011, 22, 2048–2059. [Google Scholar] [CrossRef]
- Perk, L.R.; Walsum, M.S.V.; Visser, G.W.M.; Kloet, R.W.; Vosjan, M.J.W.D.; Leemans, C.R.; Giaccone, G.; Albano, R.; Comoglio, P.M.; van Dongen, G.A.M.S. Quantitative PET imaging of Met-expressing human cancer xenografts with Zr-89-labelled monoclonal antibody DN30. Eur. J. Nucl. Med. Mol. I 2008, 35, 1857–1867. [Google Scholar] [CrossRef]
- Verel, I.; Visser, G.W.M.; Boerman, O.C.; van Eerd, J.E.M.; Finn, R.; Boellaard, R.; Vosjan, M.J.W.D.; Walsum, M.S.V.; Snow, G.B.; van Dongen, G.A.M.S. Long-lived positron emitters zirconium-89 and iodine-124 for scouting of therapeutic radioimmunoconjugates with PET. Cancer Biother. Radiol. 2003, 18, 655–661. [Google Scholar] [CrossRef]
- van Rij, C.M.; Sharkey, R.M.; Goldenberg, D.M.; Frielink, C.; Molkenboer, J.D.M.; Franssen, G.M.; van Weerden, W.M.; Oyen, W.J.G.; Boerman, O.C. Imaging of prostate cancer with Immuno-PET and Immuno-SPECT using a radiolabeled anti-EGP-1 monoclonal antibody. J. Nucl. Med. 2011, 52, 1601–1607. [Google Scholar] [CrossRef]
- Hong, H.; Severin, G.W.; Yang, Y.A.; Engle, J.W.; Zhang, Y.; Barnhart, T.E.; Liu, G.; Leigh, B.R.; Nickles, R.J.; Cai, W.B. Positron emission tomography imaging of CD105 expression with Zr-89-Df-TRC105. Eur. J. Nucl. Med. Mol. I 2012, 39, 138–148. [Google Scholar] [CrossRef]
- Nayak, T.K.; Garmestani, K.; Milenic, D.E.; Brechbiel, M.W. PET and MRI of metastatic peritoneal and pulmonary colorectal cancer in mice with human epidermal growth factor receptor 1-targeted Zr-89-labeled panitumumab. J. Nucl. Med. 2012, 53, 113–120. [Google Scholar] [CrossRef]
- Natarajan, A.; Habte, F.; Gambhir, S.S. Development of a novel long-lived immunopet tracer for monitoring lymphoma therapy in a humanized transgenic mouse model. Bioconjug. Chem. 2012, 23, 1221–1229. [Google Scholar] [CrossRef]
- Vosjan, M.J.W.D.; Vercammen, J.; Kolkman, J.A.; Stigter-van Walsum, M.; Revets, H.; van Dongen, G.A.M.S. Nanobodies targeting the hepatocyte growth factor: Potential new drugs for molecular cancer therapy. Mol. Cancer Ther. 2012, 11, 1017–1025. [Google Scholar] [CrossRef]
- O'Mara, R.E.; McAfee, J.G.; Subramanian, G. Rare earth nuclides as potential agents for skeletal imaging. J. Nucl. Med. 1969, 10, 49–51. [Google Scholar]
- Wright, E.G. Radiation-induced genomic instability in haemopoietic cells. Int. J. Radiat. Biol. 1998, 74, 681–687. [Google Scholar] [CrossRef]
- Banfi, A.; Bianchi, G.; Galotto, M.; Cancedda, R.; Quarto, R. Bone marrow stromal damage after chemo/radiotherapy: Occurrence, Consequences and possibilities of treatment. Leuk. Lymph. 2001, 42, 863–870. [Google Scholar] [CrossRef]
- Otsuka, F.L.; Fleischman, J.B.; Welch, M.J. Comparative studies using 125I- and 111In-labeled monoclonal antibodies. Int. J. Radiat. Appl. Instr. 1986, 13, 325–334. [Google Scholar] [CrossRef]
- Koizumi, M.; Endo, K.; Kunimatsu, M.; Sakahara, H.; Nakashima, T.; Kawamura, Y.; Watanabe, Y.; Saga, T.; Konishi, J.; Yamamuro, T.; et al. Ga-67-labeled antibodies for immunoscintigraphy and evaluation of tumor targeting of drug-antibody conjugates in mice. Cancer Res. 1988, 48, 1189–1194. [Google Scholar]
- Smithjones, P.M.; Stolz, B.; Bruns, C.; Albert, R.; Reist, H.W.; Fridrich, R.; Macke, H.R. Gallium-67/Gallium-68-[Dfo]-Octreotide—a Potential Radiopharmaceutical for Pet Imaging of Somatostatin Receptor-Positive Tumors-Synthesis and Radiolabeling in-Vitro and Preliminary in-Vivo Studies. J. Nucl. Med. 1994, 35, 317–325. [Google Scholar]
- OgiharaUmeda, I.; Sasaki, T.; Kojima, S.; Nishigori, H. Optimal radiolabeled liposomes for tumor imaging. J. Nucl. Med. 1996, 37, 326–332. [Google Scholar]
- Heskamp, S.; van Laarhoven, H.W.M.; Molkenboer-Kuenen, J.D.M.; Franssen, G.M.; Versleijen-Jonkers, Y.M.H.; Oyen, W.J.G.; van der Graaf, W.T.A.; Boerman, O.C. ImmunoSPECT and immunopet of IGF-1R expression with the radiolabeled antibody R1507 in a triple-negative breast cancer model. J. Nucl. Med. 2010, 51, 1565–1572. [Google Scholar] [CrossRef]
- Perk, L.R.; Visser, G.W.M.; Vosjan, M.J.W.D.; Stigter-van Walsum, M.; Tijink, B.M.; Leemans, C.R.; van Dongen, G.A.M.S. Zr-89 as a PET surrogate radioisotope for scouting biodistribution of the therapeutic radiometals Y-90 and Lu-117 in tumor-bearing nude mice after coupling to the internalizing antibody cetuximab. J. Nucl. Med. 2005, 46, 1898–1906. [Google Scholar]
- Woodin, K.S.; Heroux, K.J.; Boswell, C.A.; Wong, E.H.; Weisman, G.R.; Niu, W.J.; Tomellini, S.A.; Anderson, C.J.; Zakharov, L.N.; Rheingold, A.L. Kinetic inertness and electrochemical behavior of copper(II) tetraazamacrocyclic complexes: Possible implications for in vivo stability. Eur. J. Inorg. Chem. 2005, 23, 4829–4833. [Google Scholar]
- Heroux, K.J.; Woodin, K.S.; Tranchemontagne, D.J.; Widger, P.C.B.; Southwick, E.; Wong, E.H.; Weisman, G.R.; Tomellini, S.A.; Wadas, T.J.; Anderson, C.J.; et al. The long and short of it: The influence of N-carboxyethyl versus N-carboxymethyl pendant arms on in vitro and in vivo behavior of copper complexes of cross-bridged tetraamine macrocycles. Dalton Trans. 2007, 21, 2150–2162. [Google Scholar]
- Wangler, B.; Schirrmacher, R.; Bartenstein, P.; Wangler, C. Chelating agents and their use in radiopharmaceutical sciences. Mini Rev. Med.Chem. 2011, 11, 968–983. [Google Scholar] [CrossRef]
- Guerard, F.; Lee, Y.S.; Tripier, R.; Szajek, L.P.; Deschamps, J.R.; Brechbiel, M.W. Investigation of Zr(IV) and Zr-89(IV) complexation with hydroxamates: Progress towards designing a better chelator than desferrioxamine B for immuno-PET imaging. Chem. Commun. 2013, 49, 1002–1004. [Google Scholar] [CrossRef]
- Liu, S. Radiolabeled multimeric cyclic RGD peptides as integrin alpha(v)beta(3) targeted radiotracers for tumor imaging. Mol. Pharm. 2006, 3, 472–487. [Google Scholar] [CrossRef]
- Li, Z.B.; Cai, W.B.; Cao, Q.Z.; Chen, K.; Wu, Z.H.; He, L.N.; Chen, X.Y. 64Cu-Labeled Tetrameric and octameric RGD peptides for small-animal PET of Tumor alpha(v)beta(3) integrin expression. J. Nucl. Med. 2007, 48, 1162–1171. [Google Scholar] [CrossRef]
- Hoeben, B.A.W.; Kaanders, J.H.A.M.; Franssen, G.M.; Troost, E.G.C.; Rijken, P.F.J.W.; Oosterwijk, E.; van Dongen, G.A.M.S.; Oyen, W.J.G.; Boerman, O.C.; Bussink, J. PET of hypoxia with Zr-89-labeled cG250-F(ab ')(2) in head and neck tumors. J. Nucl. Med. 2010, 51, 1076–1083. [Google Scholar]
- Munnink, T.H.O.; de Vries, E.G.E.; Vedelaar, S.R.; Timmer-Bosscha, H.; Schroder, C.P.; Brouwers, A.H.; Lub-de Hooge, M.N. Lapatinib and 17AAG reduce Zr-89-trastuzumab-F(ab')(2) uptake in SKBR3 tumor xenografts. Mol. Pharm. 2012, 9, 2995–3002. [Google Scholar]
- Heneweer, C.; Holland, J.P.; Divilov, V.; Carlin, S.; Lewis, J.S. Magnitude of enhanced permeability and retention effect in tumors with different phenotypes: Zr-89-albumin as a model system. J. Nucl. Med. 2011, 52, 625–633. [Google Scholar] [CrossRef]
- Heuveling, D.A.; Visser, G.W.M.; Baclayon, M.; Roos, W.H.; Wuite, G.J.L.; Hoekstra, O.S.; Leemans, C.R.; de Bree, R.; van Dongen, G.A.M.S. Zr-89-Nanocolloidal albumin-based PET/CT lymphoscintigraphy for sentinel node detection in head and neck cancer: preclinical results. J. Nucl. Med. 2011, 52, 1580–1584. [Google Scholar] [CrossRef]
- Heuveling, D.A.; van Schie, A.; Vugts, D.J.; Hendrikse, N.H.; Yaqub, M.; Hoekstra, O.S.; Karagozoglu, K.H.; Leemans, C.R.; van Dongen, G.A.; de Bree, R. Pilot study on the feasibility of PET/CT lymphoscintigraphy with 89Zr-nanocolloidal albumin for sentinel node identification in oral cancer patients. J. Nucl. Med. 2013, 54, 585–589. [Google Scholar]
- Vugts, D.J.; Visser, G.W.; van Dongen, G.A. 89)Zr-PET radiochemistry in the development and application of therapeutic monoclonal antibodies and other biologicals. Curr. Top. Med. Chem. 2013, 13, 446–457. [Google Scholar]
- van Dongen, G.A.M.S.; Vosjan, M.J.W.D. Immuno-positron emission tomography: Shedding light on clinical antibody therapy. Cancer Biother. Radiol. 2010, 25, 375–385. [Google Scholar]
- Nagengast, W.B.; de Korte, M.A.; Munnink, T.H.O.; Timmer-Bosscha, H.; den Dunnen, W.F.; Hollema, H.; de Jong, J.R.; Jensen, M.R.; Quadt, C.; Garcia-Echeverria, C.; et al. (89)Zr-bevacizumab PET of early antiangiogenic tumor response to treatment with HSP90 inhibitor NVP-AUY922. J. Nucl. Med. 2010, 51, 761–767. [Google Scholar] [CrossRef]
- Ulmert, D.; Evans, M.J.; Holland, J.P.; Rice, S.L.; Wongvipat, J.; Pettersson, K.; Abrahamsson, P.A.; Scardino, P.T.; Larson, S.M.; Lilja, H.; et al. Imaging androgen receptor signaling with a radiotracer targeting free prostate-specific antigen. Cancer Discov. 2012, 2, 320–327. [Google Scholar] [CrossRef]
- Nagengast, W.B.; Lub-de Hooge, M.N.; Oosting, S.F.; den Dunnen, W.F.; Warnders, F.J.; Brouwers, A.H.; de Jong, J.R.; Price, P.M.; Hollema, H.; Hospers, G.A.; et al. VEGF-PET imaging is a noninvasive biomarker showing differential changes in the tumor during sunitinib treatment. Cancer Res. 2011, 71, 143–153. [Google Scholar] [CrossRef]
- Jagoda, E.M.; Lang, L.X.; Bhadrasetty, V.; Histed, S.; Williams, M.; Kramer-Marek, G.; Mena, E.; Rosenblum, L.; Marik, J.; Tinianow, J.N.; et al. Immuno-PET of the hepatocyte growth factor receptor Met using the 1-Armed antibody onartuzumab. J. Nucl. Med. 2012, 53, 1592–1600. [Google Scholar] [CrossRef]
- Chang, A.J.; De Silva, R.A.; Lapi, S.E. Development and characterization of 89Zr-labeled panitumumab for immuno-positron emission tomographic imaging of the epidermal growth factor receptor. Mol. Imaging 2013, 12, 17–27. [Google Scholar]
- Ogasawara, A.; Tinianow, J.N.; Vanderbilt, A.N.; Gill, H.S.; Yee, S.; Flores, J.E.; Williams, S.P.; Ashkenazi, A.; Marik, J. ImmunoPET imaging of phosphatidylserine in pro-apoptotic therapy treated tumor models. Nucl. Med. Biol. 2013, 40, 15–22. [Google Scholar]
- van der Bilt, A.R.; Terwisscha van Scheltinga, A.G.; Timmer-Bosscha, H.; Schroder, C.P.; Pot, L.; Kosterink, J.G.; van der Zee, A.G.; Lub-de Hooge, M.N.; de Jong, S.; de Vries, E.G.; et al. Measurement of tumor VEGF-A levels with 89Zr-bevacizumab PET as an early biomarker for the antiangiogenic effect of everolimus treatment in an ovarian cancer xenograft model. Clin. Cancer Res. 2012, 18, 6306–6314. [Google Scholar] [CrossRef]
- Dijkers, E.C.; Oude Munnink, T.H.; Kosterink, J.G.; Brouwers, A.H.; Jager, P.L.; de Jong, J.R.; van Dongen, G.A.; Schroder, C.P.; Lub-de Hooge, M.N.; de Vries, E.G. Biodistribution of 89Zr-trastuzumab and PET imaging of HER2-positive lesions in patients with metastatic breast cancer. Clin. Pharm. Ther. 2010, 87, 586–592. [Google Scholar]
- Brouwers, A.; Verel, I.; Van Eerd, J.; Visser, G.; Steffens, M.; Oosterwijk, E.; Corstens, F.; Oyen, W.; Van Dongen, G.; Boerman, O. PET radioimmunoscintigraphy of renal cell cancer using 89Zr-labeled cG250 monoclonal antibody in nude rats. Cancer Biother. Radiopharm. 2004, 19, 155–163. [Google Scholar]
- Rizvi, S.N.F.; Visser, O.J.; Vosjan, M.J.W.D.; van Lingen, A.; Hoekstra, O.S.; Zijlstra, J.M.; Huijgens, P.C.; van Dongen, G.A.M.S.; Lubberink, M. Biodistribution, radiation dosimetry and scouting of Y-90-ibritumomab tiuxetan therapy in patients with relapsed B-cell non-Hodgkin's lymphoma using Zr-89-ibritumomab tiuxetan and PET. Eur. J. Nucl. Med. Mol. I 2012, 39, 512–520. [Google Scholar] [CrossRef]
- van Scheltinga, A.G.T.T.; van Dam, G.M.; Nagengast, W.B.; Ntziachristos, V.; Hollema, H.; Herek, J.L.; Schroder, C.P.; Kosterink, J.G.W.; Lub-de Hoog, M.N.; de Vries, E.G.E. Intraoperative near-infrared fluorescence tumor imaging with vascular endothelial growth factor and human epidermal growth factor receptor 2 targeting antibodies. J. Nucl. Med. 2011, 52, 1778–1785. [Google Scholar] [CrossRef]
- Cohen, R.; Stammes, M.A.; de Roos, I.H.; Stigter-van Walsum, M.; Visser, G.W.; van Dongen, G.A. Inert coupling of IRDye800CW to monoclonal antibodies for clinical optical imaging of tumor targets. EJNMMI Res. 2011, 1, 31. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kurdziel, K.; Wei, L.; Riffle, L.; Kaur, G.; Hill, G.C.; Jacobs, P.M.; Tatum, J.L.; Doroshow, J.H.; Kalen, J.D. Zirconium-89 labeled panitumumab: A potential immuno-PET probe for HER1-expressing carcinomas. Nucl. Med. Biol. 2013, 40, 451–457. [Google Scholar]
- Munnink, T.H.O.; Dijkers, E.C.; Netters, S.J.; Lub-de Hooge, M.N.; Brouwers, A.H.; Haasjes, J.G.; Schroder, C.P.; de Vries, E.G. Trastuzumab pharmacokinetics influenced by extent human epidermal growth factor receptor 2-positive tumor load. J. Clin. Oncol. 2010, 28, E355–E356. [Google Scholar] [CrossRef]
- Gaykema, S.B.M.; Brouwers, A.H.; Hovenga, S.; Lub-de Hooge, M.N.; de Vries, E.G.E.; Schroder, C.P. Zirconium-89-trastuzumab positron emission tomography as a tool to solve a clinical dilemma in a patient with breast cancer. J. Clin. Oncol. 2012, 30, E74–E75. [Google Scholar] [CrossRef]
- Hong, H.; Zhang, Y.; Severin, G.W.; Yang, Y.N.; Engle, J.W.; Niu, G.; Nickles, R.J.; Chen, X.Y.; Leigh, B.R.; Barnhart, T.E.; et al. Multimodality Imaging of Breast Cancer Experimental Lung Metastasis with Bioluminescence and a Monoclonal Antibody Dual-Labeled with Zr-89 and IRDye 800CW. Mol. Pharm. 2012, 9, 2339–2349. [Google Scholar]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Fischer, G.; Seibold, U.; Schirrmacher, R.; Wängler, B.; Wängler, C. 89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules 2013, 18, 6469-6490. https://doi.org/10.3390/molecules18066469
Fischer G, Seibold U, Schirrmacher R, Wängler B, Wängler C. 89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules. 2013; 18(6):6469-6490. https://doi.org/10.3390/molecules18066469
Chicago/Turabian StyleFischer, Gabriel, Uwe Seibold, Ralf Schirrmacher, Björn Wängler, and Carmen Wängler. 2013. "89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges" Molecules 18, no. 6: 6469-6490. https://doi.org/10.3390/molecules18066469
APA StyleFischer, G., Seibold, U., Schirrmacher, R., Wängler, B., & Wängler, C. (2013). 89Zr, a Radiometal Nuclide with High Potential for Molecular Imaging with PET: Chemistry, Applications and Remaining Challenges. Molecules, 18(6), 6469-6490. https://doi.org/10.3390/molecules18066469