Understanding the Nucleophilic Character and Stability of the Carbanions and Alkoxides of 1-(9-Anthryl)ethanol and Derivatives
Abstract
:1. Introduction


2. Results and Discussion
| Alcohol | ΔacidG°(CH) | ΔacidG°(OH) | δΔacidG° |
|---|---|---|---|
| 1b (2b) | 349.8 (352.3) | 342.1 (356.2) | −7.7 (3.9) |
| 1c (2c) | 346.3 (347.8) | 339.3 (353.4) | −7.0 (5.6) |
| 1d (2d) | 345.1 (348.0) | 339.9 (353.3) | −5.2 (5.3) |
| 1e (2e) | 327.2 (328.5) | 330.3 (344.1) | 3.1 (15.6) |
| 1f(2f) | 319.9 (318.6) | 330.5 (343.7) | 10.6 (25.1) |
| 1g (2g) | 313.1 (311.9) | 325.6 (338.0) | 12.5 (26.1) |
 for 1b–1g. The linear correlation equation for 2b–2g is given in parenthesis. ΔacidG° = b + mY(values in kcal mol−1).
for 1b–1g. The linear correlation equation for 2b–2g is given in parenthesis. ΔacidG° = b + mY(values in kcal mol−1).
| Y | ΔacidG° | b | M | R2 |
|---|---|---|---|---|
 | CH | 344.9 | −23.0 | 0.99 |
| (347.1) | (−25.2) | (0.98) | ||
| OH | 339.2 | −9.7 | 0.96 | |
| (353.3) | (−1.0) | (0.97) | ||
 | CH | 345.6 | −20.1 | 0.97 |
| (343.6) | (−18.2) | (0.98) | ||
| OH | 338.9 | −7.7 | 0.95 | |
| (352.7) | (−8.3) | (0.95) | ||
 | CH | 472.5 | −24.7 | 0.98 |
| (488.0) | (−27.3) | (0.97) | ||
| OH | 393.3 | −10.4 | 0.98 | |
| (409.2) | (−10.8) | (0.99) |

 ), and the stability of the ion can be achieved from the link between f(r) and the energy E of the system f(r) =
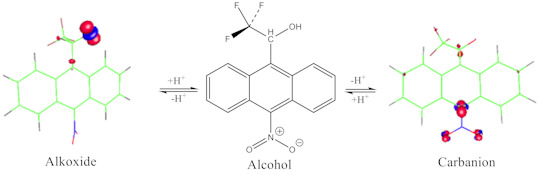
), and the stability of the ion can be achieved from the link between f(r) and the energy E of the system f(r) =  [11,12], where v(r) is the external potential associated to the nucleus. The amount of charge in the carbanions and alkoxides was examined specifically from the Fukui function for electrophilic attack f−(r) (Figure 2, Figure 3 and Figure 4) and the local HSAB principle [13,14]. Figure 2 shows that the largest values of f−(r) for 1a and 2a are located on the O and the C-11/C-10 atoms for the alkoxides and carbanions respectively. They are associated with softer nucleophilic regions (s−(r) = Sf−(r)), by giving up electronic charge and are especially reactive toward soft electrophiles [13]. Therefore, charge localization occurs on the strongly electronegative oxygen atom for the alkoxides; whereas, the charge is located on the C-11/C-10 carbon atoms indicating an ambident soft nucleophilic character for the carbanions and the distribution of the anionic charge into the ring is represented in terms of the resonance structures I and IV.
[11,12], where v(r) is the external potential associated to the nucleus. The amount of charge in the carbanions and alkoxides was examined specifically from the Fukui function for electrophilic attack f−(r) (Figure 2, Figure 3 and Figure 4) and the local HSAB principle [13,14]. Figure 2 shows that the largest values of f−(r) for 1a and 2a are located on the O and the C-11/C-10 atoms for the alkoxides and carbanions respectively. They are associated with softer nucleophilic regions (s−(r) = Sf−(r)), by giving up electronic charge and are especially reactive toward soft electrophiles [13]. Therefore, charge localization occurs on the strongly electronegative oxygen atom for the alkoxides; whereas, the charge is located on the C-11/C-10 carbon atoms indicating an ambident soft nucleophilic character for the carbanions and the distribution of the anionic charge into the ring is represented in terms of the resonance structures I and IV.


 calculated previously for a set of p-substituted phenols XC6H4OH [7]. From Table 2 we can observe the negative slope values; the acidity increases (ΔacidG° decreases) when
calculated previously for a set of p-substituted phenols XC6H4OH [7]. From Table 2 we can observe the negative slope values; the acidity increases (ΔacidG° decreases) when  increases, validating the proposal that the central ring exhibits more contribution to ΔacidG° than the edge rings and it behaves as a rather separated benzenoid ring or localized entity. This is a nice coincidence with the ambident nucleophilic character experimentally observed for the 9-anthrylmethyl carbanion and related intermediates [26], and the calculated aromaticity of the central ring of anthracene [27,28,29].
increases, validating the proposal that the central ring exhibits more contribution to ΔacidG° than the edge rings and it behaves as a rather separated benzenoid ring or localized entity. This is a nice coincidence with the ambident nucleophilic character experimentally observed for the 9-anthrylmethyl carbanion and related intermediates [26], and the calculated aromaticity of the central ring of anthracene [27,28,29].


3. Theoretical Methodology
4. Conclusions
Supplementary Materials
Acknowledgments
Conflicts of Interest
References and Notes
- Wenzel, T.J.; Wilcox, J.D. Chiral reagents for the determination of enantiomeric excess and absolute configuration using NMR spectroscopy. Chirality 2003, 15, 256–270. [Google Scholar] [CrossRef]
- García-Martínez, C.; Cervantes, H.; Méndez, F.; Escalante, J. Stoichiometry, association constant, and solvation model of chiral hydroxyfuranones in the presence of Pirkle’s alcohols. J. Spectrosc. Lett. 2011, 44, 168–175. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Finn, J.M. Chiral high-pressure liquid chromatographic stationary phases. 3. General resolution of arylalkylcarbinols. J. Org. Chem. 1981, 46, 2935–2938. [Google Scholar] [CrossRef]
- Pirkle, W.H.; Hoover, D.J. Topics in Stereochemistry; Allinger, N.L., Eliel, E.L., Wilen, S.H., Eds.; John Wiley& Sons: New York, NY, USA, 1982; Volume 13, pp. 263–331. [Google Scholar]
- Stewart, R.; van der Linden, R. The acidity of some aromatic fluoro alcohols and ketones. Can. J. Chem. 1960, 38, 399–406. [Google Scholar] [CrossRef]
- Ramírez, E.R.; García-Martínez, C.; Méndez, F. Influence of fluorine atoms and aromatic rings on the acidity of ethanol. J. Phys. Chem. A 2009, 113, 10753–10758. [Google Scholar] [CrossRef]
- Romero, M.L.; Méndez, F. Is the hydrogen atomic charge representative of the acidity of parasubstituted phenols? J. Phys. Chem. A 2003, 107, 4526–4530. [Google Scholar] [CrossRef]
- Romero, M.L.; Méndez, F. The local HSAB principle and bond dissociation energy of p-substituted phenol. J. Phys. Chem. A 2003, 107, 5874–5875. [Google Scholar] [CrossRef]
- Engler, T.A.; Shechter, H. Generation and the ambident character of 9-anthrylmethyl carbanions. Tetrahedron Lett. 1983, 24, 4645–4648. [Google Scholar] [CrossRef]
- Takagi, M.; Nojima, M.; Kusabayashi, S. Protonation and alkylation of ambident (9-anthryl)arylmethyl anions. J. Am. Chem. Soc. 1983, 105, 4676–4684. [Google Scholar] [CrossRef]
- Parr, R.G.; Yang, W. Density Functional Theory of Atoms and Molecules; Oxford University Press: New York, NY, USA, 1989. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Local softness and chemical reactivity in the molecules CO, SCN− and H2CO. J. Mol. Struct. 1988, 163, 305–313. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and soft acids and bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Méndez, F.; Gázquez, J.L. Chemical reactivity of enolate ions: The local hard and soft acids and bases principle viewpoint. J. Am. Chem. Soc. 1994, 116, 9298–9301. [Google Scholar] [CrossRef]
- Levine, I. Quantum Chemistry, 5th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2001; pp. 493–495. [Google Scholar]
- Politzer, P.; Murray, J.S.; Lipkowitz, K.B.; Boyd, D.B. Molecular Electrostatic Potentials and Chemical Reactivity. In Reviews in Computational Chemistry; Wiley-VCH: Hoboken, NJ, USA, 1991; Volume 2, Chapter 7. [Google Scholar]
- Berger, S.T.A.; Ofial, A.R.; Mayr, H. Inverse solvent effects in carbocation carbanion combination reactions: The unique behavior of trifluoromethylsulfonyl stabilized carbanions. J. Am. Chem. Soc. 2007, 129, 9753–9761. [Google Scholar] [CrossRef]
- Hansch, C.; Leo, A.; Taft, R.W. A survey of Hammett substituent constants and resonance and field parameters. Chem. Rev. 1991, 91, 165–195. [Google Scholar] [CrossRef]
- Taft, R.W.; Bordwell, F.G. Structural and solvent effects evaluated from acidities measured in dimethyl sulfoxide and in the gas phase. Acc. Chem. Res. 1988, 21, 463–469. [Google Scholar] [CrossRef]
- Smith, M.B.; March, J. March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure; John Wiley & Sons, Inc.: New York, NY, USA, 2001; pp. 32–97. [Google Scholar]
- Shaik, S.; Zilberg, S.; Haas, Y. A kekulé-crossing model for the “anomalous” behavior of the b2u modes of aromatic hydrocarbons in the lowest excited 1B2u state. Acc. Chem. Res. 1996, 29, 211–218. [Google Scholar] [CrossRef]
- López, P.; Méndez, F. Fukui function as a descriptor of the imidazolium protonated cation resonance hybrid structure. Org. Lett. 2004, 6, 1781–1783. [Google Scholar] [CrossRef]
- Gázquez, J.L.; Méndez, F. The hard and soft acids and bases principle: An atoms in molecules viewpoint. J. Phys. Chem. 1994, 98, 4591–4593. [Google Scholar] [CrossRef]
- Chattaraj, P.-K. Chemical reactivity and selectivity: Local HSAB principle versus frontier orbital theory. J. Phys. Chem. A 2001, 105, 511–513. [Google Scholar] [CrossRef]
- Méndez, F.; Galván, M.; Garritz, A.; Vela, A.; Gázquez, J.L. Local softness and chemical reactivity of maleimide: nucleophilic addition. J. Mol. Struct. 1992, 277, 81–86. [Google Scholar] [CrossRef]
- Experimental results suggested the same= effects for NO2 and SO2CF3 in nitro and triflinate stabilized 9-anthrylmethyl carbanions
- Wheland, G.W. Resonance in Organic Chemistry; Wiley: New York, NY, USA, 1955; p. 517. [Google Scholar]
- Schleyer, P.R.; Manoharan, M.; Jiao, H.; Stahl, F. The acenes: Is there a relationship between aromatic stabilization and reactivity? Org. Lett. 2001, 3, 3643–3646. [Google Scholar] [CrossRef]
- Aihara, J.; Kanno, H. Local aromaticities in large polyacene molecules. J. Phys. Chem. A 2005, 109, 3717–3721. [Google Scholar] [CrossRef]
- Chigo-Anota, E.; Ramírez-Gutierrez, R.E.; Escobedo-Morales, A.; Hernández-Cocoletzi, G. Influence of point defects on the electronic properties of boron nitride nanosheets. J. Mol. Model. 2012, 18, 2175–2184. [Google Scholar] [CrossRef]
- M., J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Montgomery, J.A., Jr.; Vreven, T.; Kudin, K.N.; Burant, J.C.; et al. Gaussian 03, Revision C.02; Frisch, Gaussian, Inc.: Wallingford, CT, USA, 2004. [Google Scholar]
- Although there are no experimental ΔacidG° values for 1a–1g and 2a–2g reported in the literature, the B3LYP/6–31+G(d,p) calculated ΔacidG° values for ethanol, its fluorine derivatives, phenylethanol and p-substituted phenols showed a good agreement with the experimental values reported in the literature
- Solca, N.; Dopfer, O. Spectroscopic identification of oxonium and carbenium ions of protonated phenol in the gas phase: IR spectra of weakly bound C6H7O+-L dimers (L = Ne, Ar, N2). J. Am. Chem. Soc. 2004, 126, 1716–1725. [Google Scholar] [CrossRef]
- Fujio, M.; McIver, R.T., Jr.; Taft, R.W. Effects of the acidities of phenols from specific substituent-solvent interactions. Inherent substituent parameters from gas-phase acidities. J. Am. Chem. Soc. 1981, 103, 4017–4029. [Google Scholar] [CrossRef]
- McQuarrie, D.A.; Simon, J.D. Molecular Thermodynamics; University Science Books: Sausalito, CA, USA, 1999; pp. 273–285. [Google Scholar]
- The g0penMol. Available online: http://www.csc.fi/english/pages/g0penMol (accessed on 22 February 2013).
- Lum, R.C.; Grabowski, J.J. Intrinsic competition between elimination and substitution mechanisms controlled by nucleophile structure. J. Am. Chem. Soc. 1992, 114, 9663–9665. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 1a and 2a are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Ramírez, R.E.; García-Martínez, C.; Méndez, F. Understanding the Nucleophilic Character and Stability of the Carbanions and Alkoxides of 1-(9-Anthryl)ethanol and Derivatives. Molecules 2013, 18, 10254-10265. https://doi.org/10.3390/molecules180910254
Ramírez RE, García-Martínez C, Méndez F. Understanding the Nucleophilic Character and Stability of the Carbanions and Alkoxides of 1-(9-Anthryl)ethanol and Derivatives. Molecules. 2013; 18(9):10254-10265. https://doi.org/10.3390/molecules180910254
Chicago/Turabian StyleRamírez, Ramsés E., Cirilo García-Martínez, and Francisco Méndez. 2013. "Understanding the Nucleophilic Character and Stability of the Carbanions and Alkoxides of 1-(9-Anthryl)ethanol and Derivatives" Molecules 18, no. 9: 10254-10265. https://doi.org/10.3390/molecules180910254
APA StyleRamírez, R. E., García-Martínez, C., & Méndez, F. (2013). Understanding the Nucleophilic Character and Stability of the Carbanions and Alkoxides of 1-(9-Anthryl)ethanol and Derivatives. Molecules, 18(9), 10254-10265. https://doi.org/10.3390/molecules180910254