A Recyclable Palladium-Catalyzed Synthesis of 2-Methylene-2,3-Dihydrobenzofuran-3-ols by Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols in Ionic Liquids
Abstract
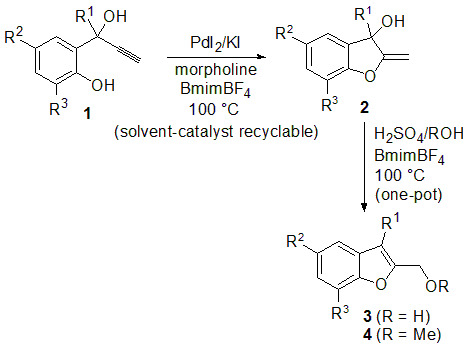
:1. Introduction



2. Results and Discussion

| Entry | Ionic Liquid | T (°C) | Conversion of 1a b (%) | Yield of 2a c (%) |
|---|---|---|---|---|
| 1 | BmimBF4 | 70 | 80 | 60 |
| 2 d | BmimBF4 | 70 | 100 | 82 |
| 3 | BmimBF4 | 100 | 100 | 86 |
| 4 e | BmimBF4 | 100 | 100 | 80 |
| 5 f | BmimBF4 | 100 | 100 | 63 |
| 6 g | BmimBF4 | 100 | 100 | 65 |
| 7 | BmimCl | 100 | 100 | 51 |
| 8 | BmimPF6 | 100 | 100 | 58 |
| 9 | BmimN(CN)2 | 100 | 100 | 63 |
| 10 | BmimOTf | 100 | 60 | 23 |

| Entry | 1 | 2 | Yield of 2 (%) b | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Run 1 c | Run 2 c | Run 3 c | Run 4 c | Run 5 c | Run 6 c | Run 7 c | |||
| 1 |  |  | 86 | 85 | 87 | 86 | 85 | 86 | 87 |
| 2 |  |  | 84 | 86 | 82 | 75 | 72 | 73 | 72 |
| 3 |  |  | 70 | 71 | 71 | 68 | 70 | 69 | 70 |
| 4 |  |  | 78 | 80 | 78 | 80 | 81 | 81 | 80 |
| 5 |  |  | 80 | 81 | 79 | 80 | 78 | 81 | 79 |
| 6 |  |  | 75 | 77 | 76 | 77 | 75 | 76 | 78 |
| 7 |  |  | 76 | 76 | 77 | 75 | 78 | 74 | 77 |

| Entry | 1 | R | t (h) | 3 or 4 | Yield of 3 or 4 b (%) |
|---|---|---|---|---|---|
| 1 |  | H | 3 |  | 65 |
| 2 |  | H | 3 |  | 55 |
| 3 |  | H | 3 |  | 54 |
| 4 |  | H | 3 |  | 71 |
| 5 |  | H | 3 |  | 56 |
| 6 |  | H | 3 |  | 52 |
| 7 |  | H | 3 |  | 54 |
| 8 | 1a | Me | 18 |  | 80 |
| 9 | 1b | Me | 18 |  | 65 |
| 10 | 1c | Me | 18 |  | 52 |
| 11 | 1d | Me | 15 |  | 70 |
| 12 | 1e | Me | 18 |  | 52 |
| 13 | 1f | Me | 18 |  | 55 |
| 14 | 1g | Me | 18 |  | 60 |
3. Experimental
3.1. General
3.2. Preparation of Substrates
3.3. Preparation of Ionic Liquids
3.4. General Procedure for the PdI2/KI-Catalyzed Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols 1 to Give 2-Methylene-2,3-Dihydrobenzofuran-3-ols 2
3.5. General Procedure for the One-Pot Synthesis of 2-Hydroxymethylbenzofurans 3 and 2-Methoxy- methylbenzofurans 4 Starting From 2-(1-Hydroxyprop-2-ynyl)phenols 1
3.6. Characterization of Products
4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Adcock, H.V.; Davies, P.W. π-Acid mediated insertion of alkynes into carbon-heteroatom σ-bonds. Synthesis 2012, 44, 3401–3420. [Google Scholar] [CrossRef]
- Yamamoto, Y. Transition-metal-catalyzed cycloisomerizations of α,ω-dienes. Chem. Rev. 2012, 112, 4736–4769. [Google Scholar] [CrossRef]
- Watson, I.D.G.; Toste, F.D. Catalytic enantioselective carbon-carbon bond formation using cycloisomerization reactions. Chem. Sci. 2012, 3, 2899–2919. [Google Scholar] [CrossRef]
- Marinetti, A.; Jullien, H.; Voituriez, A. Enantioselective, transition metal catalyzed cycloisomerizations. Chem. Soc. Rev. 2012, 41, 4884–4908. [Google Scholar] [CrossRef]
- Herndon, J.W. The Chemistry of the carbon-transition metal double and triple bond: Annual survey covering the year 2009. Coord. Chem. Rev. 2011, 255, 3–100. [Google Scholar] [CrossRef]
- Krause, N.; Winter, C. Gold-catalyzed nucleophilic cyclization of functionalized allenes: A powerful access to carbo- and heterocycles. Chem. Rev. 2011, 111, 1994–2009. [Google Scholar] [CrossRef]
- Belmont, P.; Parker, E. Silver and gold catalysis for cycloisomerization reactions. Eur. J. Org. Chem. 2009, 2009, 6075–6089. [Google Scholar] [CrossRef]
- Soriano, E.; Marco-Contelles, J. Mechanistic insights on the cycloisomerization of polyunsaturated precursors catalyzed by platinum and gold complexes. Acc. Chem. Res. 2009, 42, 1026–1036. [Google Scholar] [CrossRef]
- Majumdar, K.C.; Debnath, P.; Roy, B. Metal-catalyzed heterocyclization: Formation of five- and six-membered oxygen heterocycles through carbon-oxygen bond forming reactions. Heterocycles 2009, 78, 2661–2728. [Google Scholar] [CrossRef]
- Zeni, G.; Larock, R.C. Synthesis of heterocycles via palladium π-olefin and π-alkyne chemistry. Chem. Rev. 2004, 104, 2285–2309. [Google Scholar] [CrossRef]
- Gabriele, B.; Salerno, G.; Costa, M. PdI2-catalyzed synthesis of heterocycles. Synlett 2004, 14, 2468–2483. [Google Scholar] [CrossRef]
- Gabriele, B.; Veltri, L.; Plastina, P.; Mancuso, R.; Vetere, M.V.; Maltese, V. Copper-catalyzed synthesis of substituted furans and pyrroles by heterocyclodehydration and tandem heterocyclodehydration-hydration of 3-yne-1,2-diols and 1-amino-3-yn-2-ol derivatives. J. Org. Chem. 2013, 78, 4919–4928. [Google Scholar] [CrossRef]
- Spina, R.; Colacino, E.; Gabriele, B.; Salerno, G.; Martinez, J.; Lamaty, F. Synthesis of pyrrolin-4-ones by Pt-catalyzed cycloisomerization in PEG under microwaves. J. Org. Chem. 2013, 78, 2698–2702. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Veltri, L.; Maltese, V.; Salerno, G. Synthesis of substituted thiophenes by palladium-catalyzed heterocyclodehydration of 1-mercapto-3-yn-2-ols in conventional and nonconventional solvents. J. Org. Chem. 2012, 77, 9905–9909. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Lupinacci, E.; Veltri, L.; Salerno, G.; Carfagna, C. Synthesis of benzothiophene derivatives by Pd-catalyzed or radical-promoted heterocyclodehydration of 1-(2-mercaptophenyl)-2-yn-1-ols. J. Org. Chem. 2011, 76, 8277–8286. [Google Scholar] [CrossRef]
- Gabriele, B.; Plastina, P.; Vetere, M.V.; Veltri, L.; Mancuso, R.; Salerno, G. A simple and convenient synthesis of substituted furans and pyrroles by CuCl2-Catalyzed heterocyclodehydration of 3-yne-1,2-diols and N-Boc- or N-Tosyl-1-amino-3-yn-2-ols. Tetrahedron Lett. 2010, 51, 3565–3567. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Lupinacci, E.; Spina, R.; Salerno, G.; Veltri, L.; Dibenedetto, A. Recyclable catalytic synthesis of substituted quinolines: Copper-catalyzed heterocyclization of 1-(2-aminoaryl)-2-yn-1-ols in ionic liquids. Tetrahedron 2009, 65, 8507–8512. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G. A novel synthesis of 2-functionalized benzofurans by palladium-catalyzed cycloisomerization of 2-(1-hydroxyprop-2-ynyl)phenols followed by acid-catalyzed allylic isomerization or allylic nucleophilic substitution. J. Org. Chem. 2008, 73, 7336–7341. [Google Scholar] [CrossRef]
- Plastina, P.; Gabriele, B.; Salerno, G. Palladium-catalyzed oxidative aminocarbonylation of alkynols. Synthesis 2007, 3083–3087. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Ruffolo, G.; Plastina, P. Novel and convenient synthesis of substituted quinolines by copper- or palladium-catalyzed cyclodehydration of 1-(2-aminoaryl)-2-yn-1-ols. J. Org. Chem. 2007, 72, 6873–6877. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Solvent nanostructure, the solvophobic effect and amphiphile self-assembly in ionic liquids. Chem. Soc. Rev. 2013, 42, 1096–1120. [Google Scholar] [CrossRef]
- Rehman, A.; Zeng, X. Ionic liquids as green solvents and electrolytes for robust chemical sensor development. Acc. Chem. Res. 2012, 45, 1667–1677. [Google Scholar] [CrossRef]
- Patel, D.D.; Lee, J.-M. Applications of ionic liquids. Chem. Rec. 2012, 12, 329–355. [Google Scholar] [CrossRef]
- Wong, W.-L.; Wong, K.-Y. Recent development in functionalized ionic liquids as reaction media and promoters. Can. J. Chem. 2012, 90, 1–16. [Google Scholar] [CrossRef]
- Payagala, T.; Armstrong, D.W. Chiral ionic liquids: A compendium of syntheses and applications (2005–2012). Chirality 2012, 24, 17–53. [Google Scholar] [CrossRef]
- de Maria, P.D.; Yang, Z.; Kohlmann, C.; Greiner, L.; Lozano, P.; García-Verdugo, E.; Zhao, H.; Gamenara, D.; Saenz Méndez, P.; Dennewald, D.; et al. Ionic Liquids in Biotransformations and Organocatalysis: Solvents and Beyond; Domínguez de Maria, P., Ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Dupont, J. From molten salts to ionic liquids: A “nano” journey. Acc. Chem. Res. 2011, 44, 1223–1231. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Hubbard, C.D.; Illner, P.; van Eldik, R. Understanding chemical reaction mechanisms in ionic liquids: Successes and challenges. Chem. Soc. Rev. 2011, 40, 272–290. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, S.; Deng, Y. Recent advances in ionic liquid catalysis. Green Chem. 2011, 13, 2619–2637. [Google Scholar] [CrossRef]
- Wilkes, J.S.; Wasserscheid, P.; Welton, T.; Gordon, C.M.; Muldoon, M.J.; Wagner, M.; Hilgers, C.; Davis, J.H.; Holbrey, J.D.; Rogers, R.D.; et al. Ionic Liquids in Synthesis, 2nd ed.; Wasserscheid, P., Welton, T., Eds.; Wiley-VCH: Weinheim, Germany, 2008. [Google Scholar]
- Ohtaka, A. Recyclable polymer-supported nanometal catalysts in water. Chem. Rec. 2013, 13, 274–285. [Google Scholar] [CrossRef]
- Gawande, M.B.; Branco, P.S.; Varma, R.S. Nano-magnetite (Fe3O4) as a support for recyclable catalysts in the development of sustainable methodologies. Chem. Soc. Rev. 2013, 42, 3371–3393. [Google Scholar] [CrossRef]
- Colacino, E.; Martinez, J.; Lamaty, F.; Petrikeeva, L.S.; Khemchyan, L.L.; Ananikov, V.P.; Beletskaya, I.P. PEG as an alternative reaction medium in metal-mediated transformations. Coord. Chem. Rev. 2012, 256, 2893–2920. [Google Scholar] [CrossRef]
- Yuan, D.; Huang, B. Progress in organic synthesis reactions catalyzed by palladium supported on magnetic nanoparticles. Chin. J. Org. Chem. 2012, 32, 1368–1379. [Google Scholar] [CrossRef]
- Liu, H.; Jia, Z.; Ji, S. Research progress in supported catalysts for Heck reaction. Chin. J. Catal. 2012, 33, 757–767. [Google Scholar]
- Gu, Y. Multicomponent reactions in unconventional solvents: State of the art. Green Chem. 2012, 14, 2091–2128. [Google Scholar] [CrossRef]
- Arpad, M. Efficient, selective, and recyclable palladium catalysts in carbon-carbon coupling reactions. Chem. Rev. 2011, 111, 2251–2320. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Tyurin, V. Recyclable nanostructured catalytic systems in modern environmentally friendly organic synthesis. Molecules 2010, 15, 4792–4814. [Google Scholar] [CrossRef]
- Bellina, F.; Chiappe, C. The Heck reaction in ionic liquids: Progress and challenges. Molecules 2010, 15, 2211–2245. [Google Scholar] [CrossRef]
- Lim, C.W.; Lee, I.S. Magnetically recyclable nanocatalyst systems for the organic reactions. Nano Today 2010, 5, 412–434. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G. Acid-catalysed or radical-promoted allylic substitution of 2-methylene-2,3-dihydrobenzofuran-3-ols with thiol derivatives: A novel and expedient synthesis of 2-(thiomethyl)benzofurans. Eur. J. Org. Chem. 2010, 2010, 3459–3464. [Google Scholar] [CrossRef]
- Gabriele, B.; Mancuso, R.; Salerno, G.; Plastina, P. A novel palladium-catalyzed dicarbonylation process leading to coumarins. J. Org. Chem. 2008, 73, 756–759. [Google Scholar] [CrossRef]
- Crosthwaite, J.M.; Aki, S.N.V.K.; Maginn, E.J.; Brennecke, J.F. Liquid phase behavior of imidazolium-based ionic liquids with alcohols. J. Phys. Chem. B 2004, 108, 5113–5119. [Google Scholar]
- Sample Availability: Samples of the compounds 2a–j, 3a–j, and 4a–j are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Mancuso, R.; Gabriele, B. A Recyclable Palladium-Catalyzed Synthesis of 2-Methylene-2,3-Dihydrobenzofuran-3-ols by Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols in Ionic Liquids. Molecules 2013, 18, 10901-10911. https://doi.org/10.3390/molecules180910901
Mancuso R, Gabriele B. A Recyclable Palladium-Catalyzed Synthesis of 2-Methylene-2,3-Dihydrobenzofuran-3-ols by Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols in Ionic Liquids. Molecules. 2013; 18(9):10901-10911. https://doi.org/10.3390/molecules180910901
Chicago/Turabian StyleMancuso, Raffaella, and Bartolo Gabriele. 2013. "A Recyclable Palladium-Catalyzed Synthesis of 2-Methylene-2,3-Dihydrobenzofuran-3-ols by Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols in Ionic Liquids" Molecules 18, no. 9: 10901-10911. https://doi.org/10.3390/molecules180910901
APA StyleMancuso, R., & Gabriele, B. (2013). A Recyclable Palladium-Catalyzed Synthesis of 2-Methylene-2,3-Dihydrobenzofuran-3-ols by Cycloisomerization of 2-(1-Hydroxyprop-2-ynyl)phenols in Ionic Liquids. Molecules, 18(9), 10901-10911. https://doi.org/10.3390/molecules180910901