Abstract
A modern approach in the search for new bioactive molecules is the synthesis of novel chemical entities combining molecules of different biosynthetic origin presenting biological effects as single compounds. Gastroprotective compounds from South American medicinal plants, namely quinones and diterpenes, were used as building blocks to obtain hybrid diterpenylquinones. Starting from the labdane diterpene junicedric acid and two isomers, as well as from three quinones, including lapachol, 18 hybrid molecules were synthesized. Six of them are described for the first time. The potential gastroprotective mechanisms of action of the compounds were assessed in dose-response experiments using human gastric epithelial cells (AGS) and human lung fibroblasts (MRC-5). The following studies were carried out: stimulation of cell proliferation, cytoprotection against sodium taurocholate (NaT)-induced damage, synthesis of PGE2 and total reduced sulfhydryl (GSH) content. The antioxidant capacity of the compounds was determined on the inhibition of the lipoperoxidation in human erythrocyte membranes. Hybrid compounds presented activities different from those shown by the starting compounds, supporting the potential of this approach in the search for new bioactive molecules. The effects might be modulated by selective modification in the terpene or quinone moieties of the new molecules. Structure-activity relationships are discussed.
1. Introduction
South American traditional medicine uses plants in therapeutic practice. Several herbal crude drugs have been shown to contain bioactive constituents that can be clearly related to their beneficial effects. The resin of the “araucaria” tree (Araucaria araucana (Mol.) K. Koch, Araucariaceae) was used by the Mapuche amerindians in Chile to treat gastric ulcers and to relieve stomach pain [1]. The stem bark of the crude drug “lapacho” or “taheebo” (Tabebuia spp., Bignoniaceae) was formerly used by the Guarani as an anti-inflammatory agent and then incorporated into Paraguayan folk medicine, to treat cancer and wounds [2]. The naphthoquinone lapachol from Tabebuia species [3] and labdane diterpenes from A. araucana, display gastroprotective activity in vivo [4] supporting the ethnobotanical information. The bioactive compounds from those crude drugs (quinones and diterpenes) exert their effect as single chemical entities. A new approach in drug design is to combine two different molecules with individual intrinsic effects into a single new hybrid compound [5]. Little has been done on the synthesis of products combining naturally occurring moieties arising from different biosynthetic pathways into such hybrid molecules. Recent studies presented the synthesis, gastroprotective effect and cytotoxicity of diterpenylnaphthoquinones [6] and the potential gastroprotective effect of novel sesquiterpene quinone derivatives in human cells [7]. Terpenylquinones have been described from some marine organisms and display several biological activities, including cytotoxicity and anti-inflammatory effect [8]. New hybrid compounds containing terpene and quinone moieties have been prepared and some of them present relevant biological effects including cytotoxicity [9,10] and antifungal effect [11].
The study of gastroprotective activity of compounds has been traditionally carried out using laboratory animals. However, due to social and economic pressures, the international trend has been a gradual reduction in the use of experimental animals. The use of primary cell culture as well as immortalized cell lines allows in vitro testing and studies of possible mechanisms of action. There are several advantages in using cell cultures as biological models, namely: the amount of compound is reduced to a minimum, variables are much better controlled than when working with entire animals and the costs are substantially reduced. More advanced techniques consider the use of gastric epithelial cells [7,12,13].
The aim of this work was to prepare new hybrid compounds using as starting molecules gastroprotective diterpenes with the labdane skeleton and quinones. Human cell cultures were used to assess their potential mechanisms of gastroprotective activity and compare it to that of the starting substances. To draw some structure-activity trends, the hybrids included variations both in the quinone as well as in the diterpene moieties.
2. Results and Discussion
2.1. Hybrid Compounds
A series of hybrid compounds was synthesized using as building blocks diterpenes and quinones. The quinones included lapachol (1) and its hydrogenation products 2 and 3. The quinone part of the molecule included an isoprenyl side chain, hydrogenated side chains with aromatic rings in the naphthoquinone moiety or hydrogenated side chains and aromatic rings. The diterpene junicedric acid (4) with an exocyclic double bond, its isomer 5 with an Δ8(9) double bond and the hydrogenation product 6 were used as the terpene part of the hybrid molecules. Eighteen hybrids, including six new compounds, were synthesized (Scheme 1), differing in some structural features while maintaining common characteristics to evaluate structure-activity trends. All compounds were characterized by spectroscopic and spectrometric means and are in agreement with the proposed structures. The structures of compounds 1–24 are shown in Figure 1, Figure 2 and Figure 3.
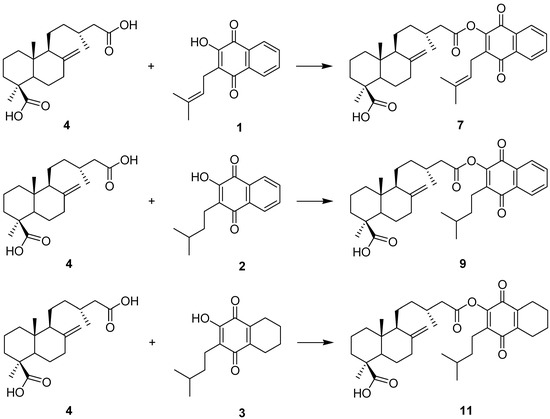
Scheme 1.
General synthetic procedures for preparation of the hybrid compounds. Quinones: compounds 1, 2 and 3. Diterpenes: compounds 4, 5 and 6.
Scheme 1.
General synthetic procedures for preparation of the hybrid compounds. Quinones: compounds 1, 2 and 3. Diterpenes: compounds 4, 5 and 6.
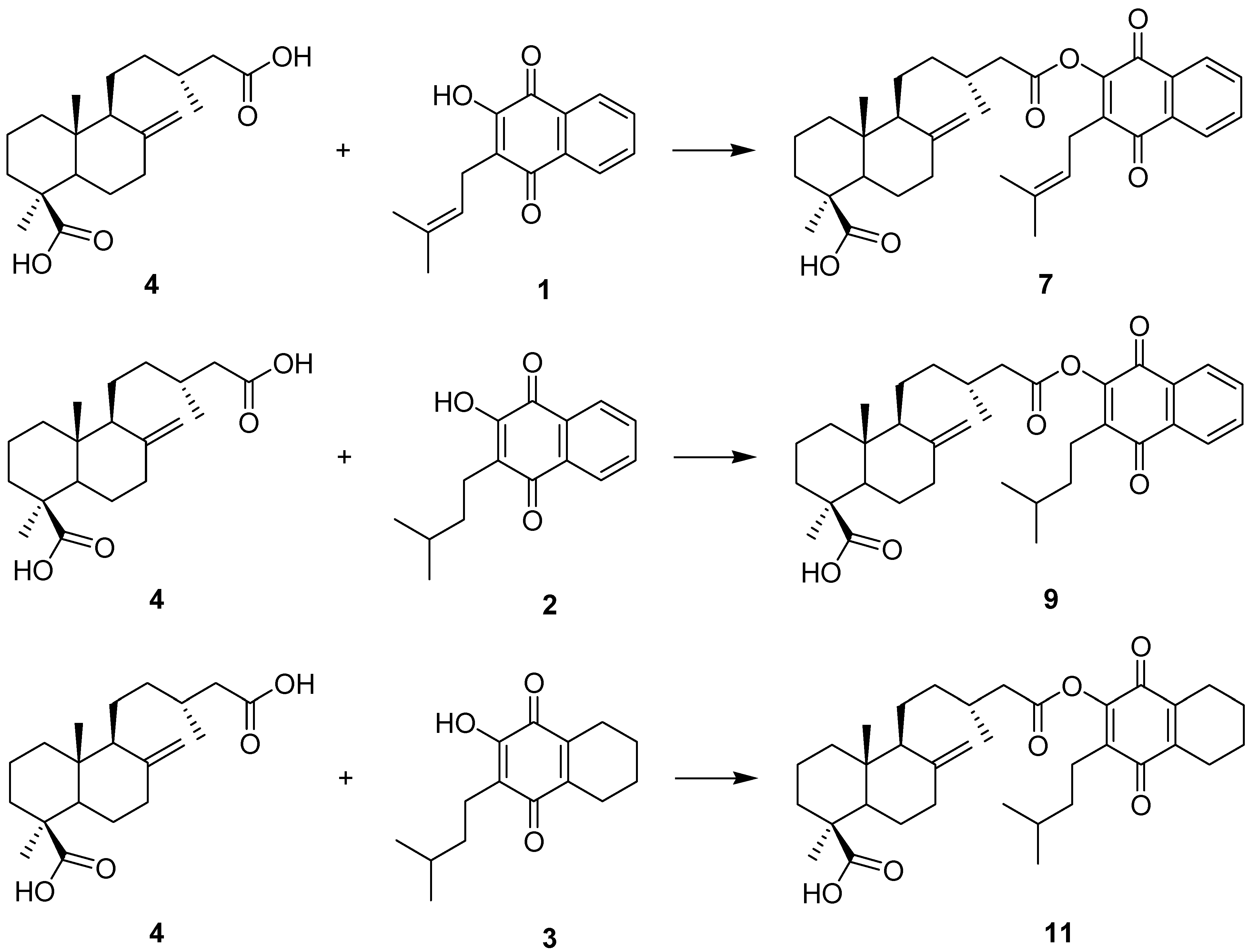
Reagents and conditions: (a): DCC, DMAP, CH2Cl2, rt; or (b): oxalyl chloride, CH2Cl2, quinone, TEA.
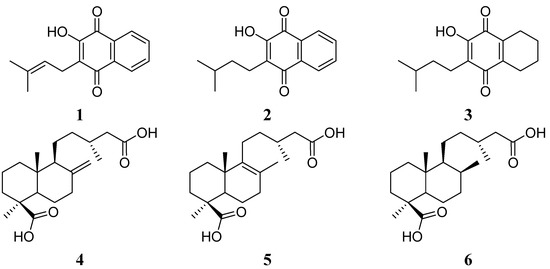
Figure 1.
Structure of the starting quinones 1–3 and diterpenes 4–6.
Figure 1.
Structure of the starting quinones 1–3 and diterpenes 4–6.
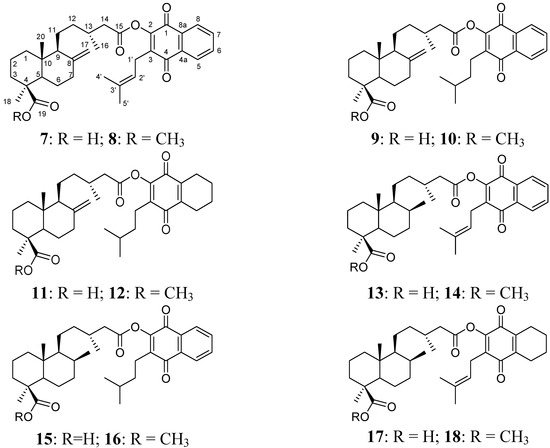
Figure 2.
Diterpenylquinone derivatives of junicedric acid 7–12 and 17β-dihydrojunicedric acid 13–18.
Figure 2.
Diterpenylquinone derivatives of junicedric acid 7–12 and 17β-dihydrojunicedric acid 13–18.
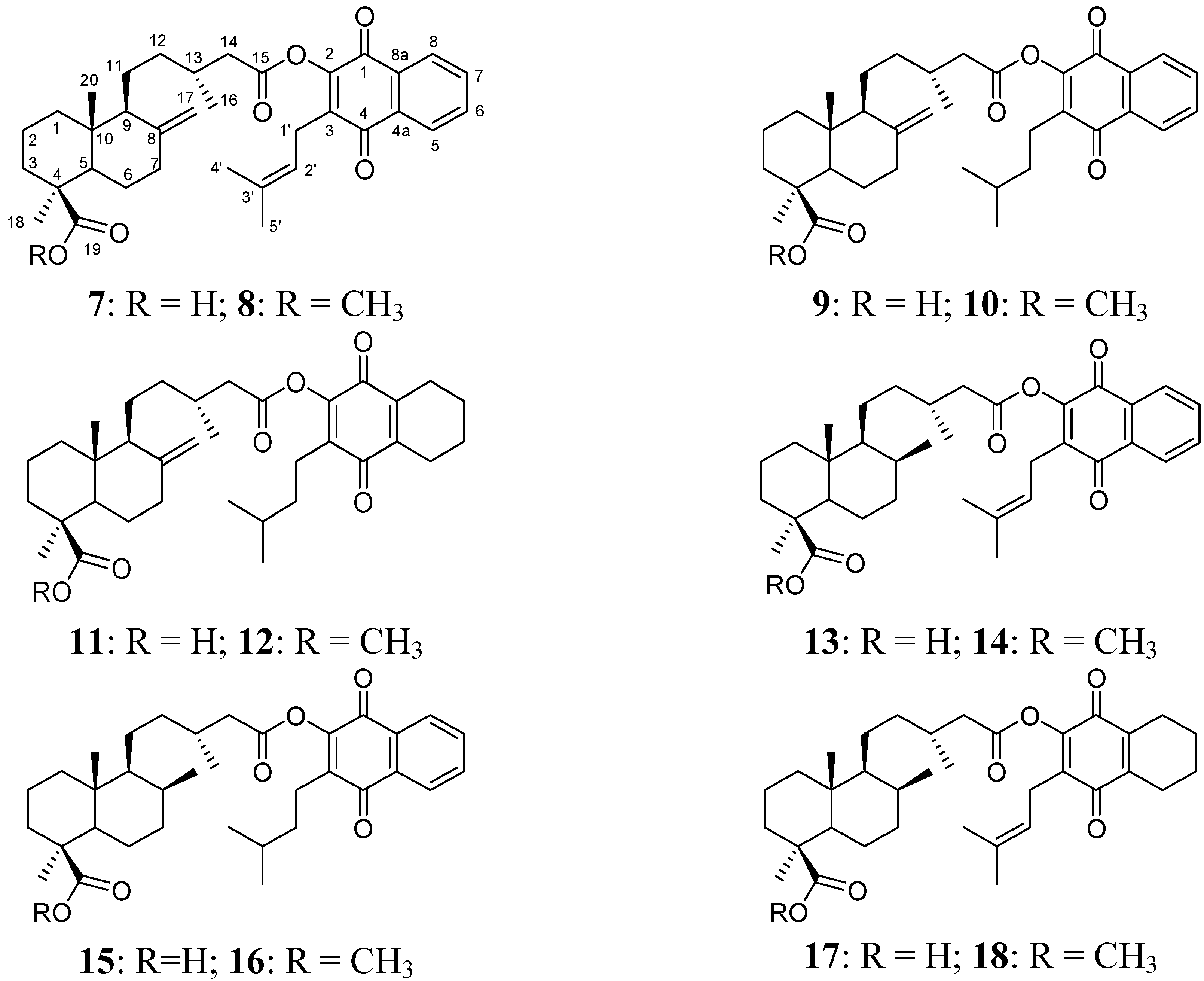
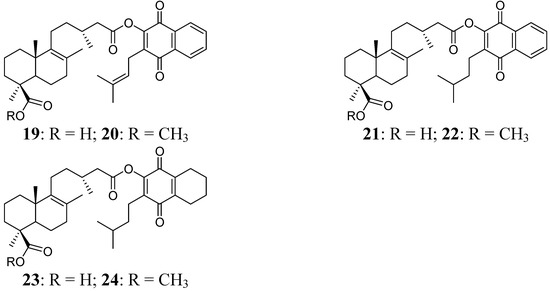
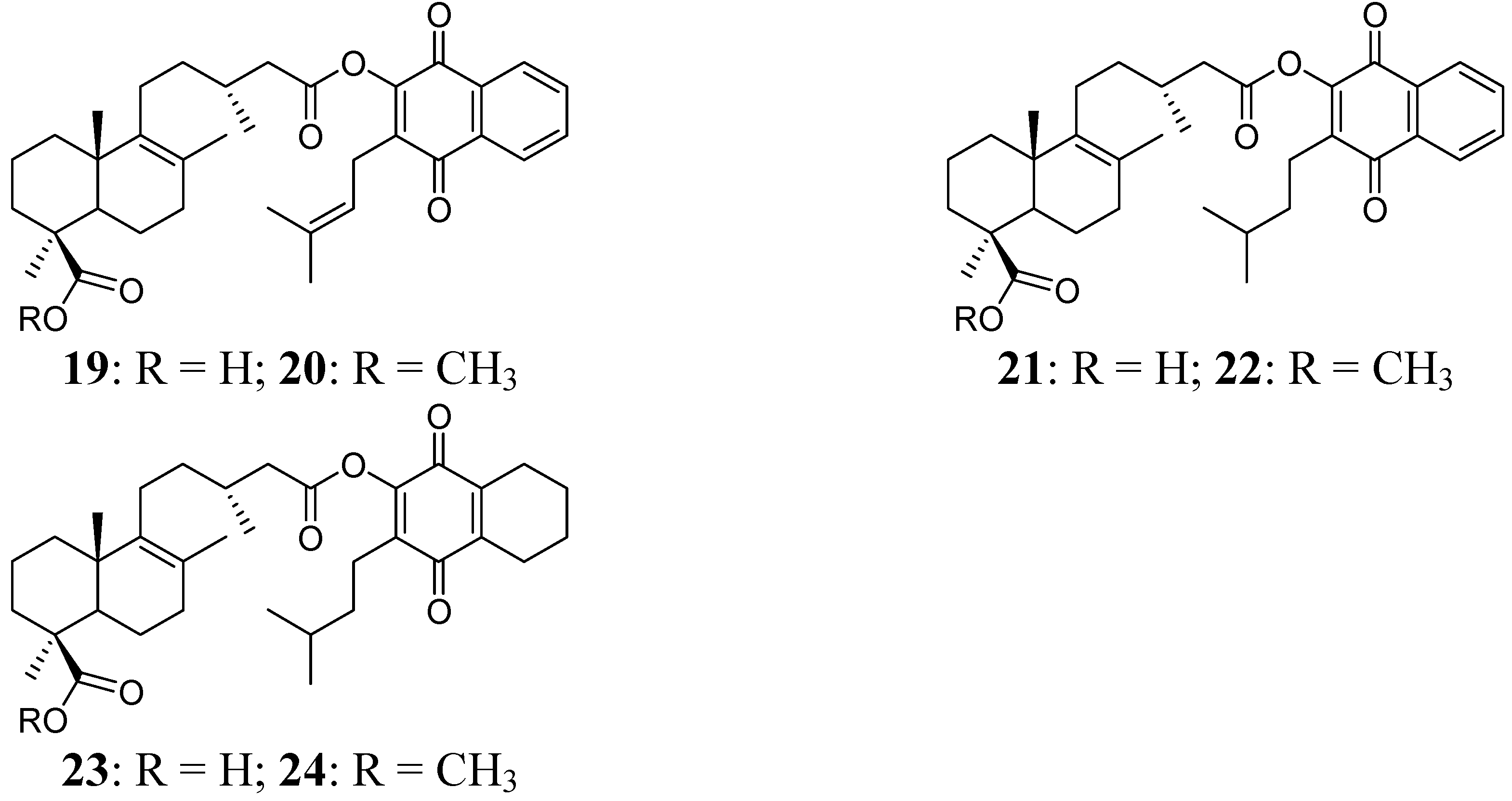
Figure 3.
Diterpenylquinone derivatives of Δ8(9) junicedric acid 19–24.
Figure 3.
Diterpenylquinone derivatives of Δ8(9) junicedric acid 19–24.
2.2. Cytotoxicity
The cytotoxicity of all compounds was evaluated on confluent cultures of human lung fibroblasts (MRC-5) and human gastric epithelial cells (AGS) to determine the working concentrations in the subsequent mechanism of action experiments. Cell viability was determined by means of the neutral red uptake assay and results are expressed as IC50 values (µM) in Table 1. Hydrogenation increases cytotoxicity for the lapachol derivatives 2 and 3. From the starting diterpenes, Δ8(9) junicedric acid (5) presented lower toxicity towards MRC-5 and AGS cells, with IC50 values of 834 and 666 µM, respectively. The diterpene with higher cytotoxicity was compound 6 (IC50 values of 163 and 283 µM for MRC-5 and AGS cells, respectively).
Among the six hybrid compounds derived from diterpene 4 (compounds 7–12), the carboxylic acid derivative 7 resulted more cytotoxic than the parent quinone and diterpene 1 and 4, respectively. A similar trend was observed for hybrids 9 and 11, prepared from quinones 2 and 3, and diterpene 4, respectively. Methylation of 7, 9 and 11 led to the less cytotoxic derivatives of the series 8, 10 and 12, indicating that the free C-19 carboxylic acid function is relevant for the cytotoxicity of this series. The highest cytotoxicity value was found for compound 11, in which both, the prenylated side chain and the benzene ring of the lapachol were reduced by hydrogenation.
The diterpene 5 differs from 4 in the position of the double bond, leading to different configurations for both compounds and their hybrid derivatives. Hybrid compounds containing diterpene 5 (compounds 19–24), showed the same trend than derivatives from diterpene 4 when presenting a free C-19 COOH function. Therefore, compounds 19, 21 and 23 were more toxic than their corresponding C-19 COOMe derivatives 20, 22 and 24. As for the series from diterpene 4, the most cytotoxic compound for the derivative series of 5 resulted compound 23, with the prenylated side chain and the benzene ring of the lapachol completely reduced.
Among the hybrid compounds obtained from diterpene 6 (compounds 13–18), it was observed that hybrids 13 and 17 bearing a carboxylic acid function at C-19, were more toxic than the corresponding methylester derivatives 14 and 18. However, when only hydrogenation of the prenylated side chain of the lapachol was present in the hybrid compounds (15 and 16), the ester derivative 16 showed higher cytotoxicity that the corresponding carboxylic acid derivative 15.

Table 1.
.Cytotoxicity expressed as IC50 values (µM) towards confluent cultures of human lung fibroblasts (MRC-5) and gastric epithelial cells (AGS) treated with the compounds 1–24 and lansoprazole.
| Compound | IC50 ± SD (µM) a | |
|---|---|---|
| MRC-5 | AGS | |
| Lapachol (1) | >1000 | 571 ± 28 |
| Dihydroprenyl lapachol (2) | 611 ± 42 | 292 ± 12 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachol (3) | 307 ± 22 | 87 ± 4 |
| Junicedric acid (4) | 439 ± 26 | 383 ± 23 |
| Δ8(9) Junicedric acid (5) | 834 ± 51 | 666 ± 35 |
| 17β-Dihydrojunicedric acid (6) | 163 ± 5 | 283 ± 17 |
| Lapachoyl junicedrate (7) | 221 ± 13 | 143 ± 6 |
| Lapachoyl junicedrate methyl ester (8) | >1000 | >1000 |
| Dihydroprenyl lapachol junicedrate (9) | 449 ± 27 | 49 ± 10 |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | >1000 | 659 ± 42 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate(11) | 42 ± 2 | 63 ± 4 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (12) | 667 ± 40 | >1000 |
| Lapachoyl 17β-dihydrojunicedrate (13) | 355 ± 21 | 351 ± 29 |
| Lapachoyl 17β-dihydrojunicedrate methyl ester (14) | 727 ± 48 | 916 ± 54 |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | >1000 | >1000 |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16) | 596 ± 44 | 643 ± 39 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17) | 44 ± 4 | 60 ± 5 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 369 ± 29 | 884 ± 62 |
| Lapachoyl Δ8(9) junicedrate (19) | 129 ± 9 | 148 ± 9 |
| Lapachoyl Δ8(9) junicedrate methyl ester (20) | 483 ± 31 | 321 ± 24 |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | 601 ± 26 | 278 ± 14 |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 848 ± 51 | 340 ± 20 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23) | 20 ± 2 | 29 ± 2 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (24) | >1000 | >1000 |
| Lansoprazole b | 316 ± 11 | 168 ± 8 |
Cells were treated during 24 h with the compounds. Cell viability was determined by means of the neutral red uptake assay. a Results are expressed as mean values ± SD. Each concentration was tested in quadruplicate together with the control and repeated three times in different experiments; b Reference compound.
2.3. MRC-5 Fibroblast Proliferation
A key factor in the renewal and repair of gastric mucosa is the proliferative capacity of fibroblasts [14]. The ability of the compounds to accelerate cell proliferation and hence gastric wound healing was determined using MRC-5 fibroblasts. The compounds were evaluated at six concentrations lower than the IC50 values, being IC50/2 the highest concentration tested. The positive results are presented in Figure 4. Percentual proliferative increase compared to untreated cells is shown in parentheses. Stimulation of fibroblast proliferation was observed for the diterpene 5 (3.6%), the hybrid 11 (3.6%), obtained by the combination of 4 and 3 and the derivatives from diterpene 6: 13 (7.3%), 17 (5.5 to 14.5%) and 18 (3.6%). The active compounds 11, 17 and 18 have in common the quinone 3 in their structures. These compounds differ from the inactive derivatives 23 and 24 (also containing the quinone 3) by the more planar configuration of the diterpene moiety of compounds 23 and 24. None of the hybrid derivatives that showed activity contain the diterpene moiety 5.
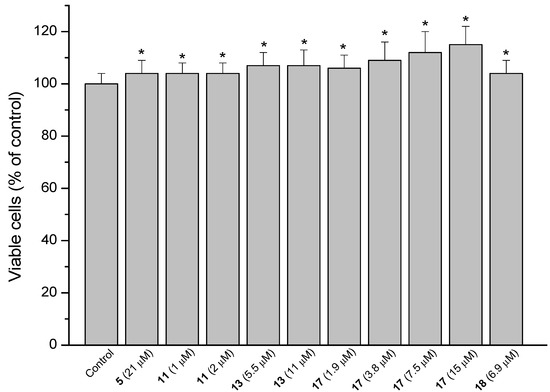
Figure 4.
Stimulating effect of the diterpene 5 and derivatives 11, 13, 17 and 18 on the proliferation of MRC-5 cells. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett´s test. * p < 0.05 compared to the control group.
Figure 4.
Stimulating effect of the diterpene 5 and derivatives 11, 13, 17 and 18 on the proliferation of MRC-5 cells. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett´s test. * p < 0.05 compared to the control group.
2.4. Sodium Taurocholate (NaT)-Induced Damage
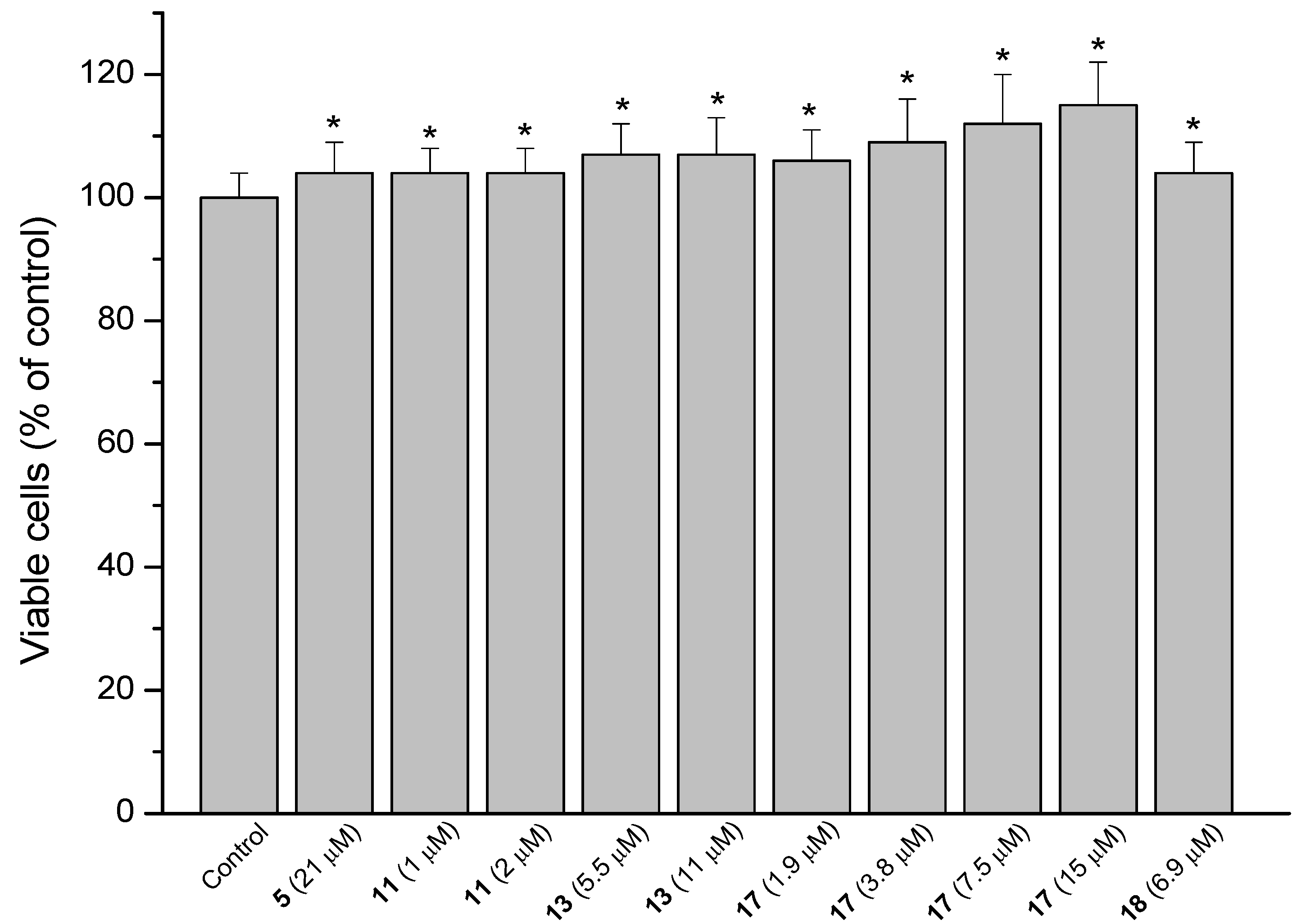
The model of AGS cells damaged by NaT (bile salt) was used to assess the gastroprotective effect of compounds against the gastric ulcer induced by bile reflux [15]. Results are presented as a reduction in cell viability. Treatment of the cells with 10 mM NaT during 30 min caused a 48% reduction of cell viability compared with untreated controls. Cells were pre-treated during 60 min with the compounds at 1/2, 1/4 and 1/8 of the respective IC50 values and then 10 mM NaT was added to all wells. The reference compound sucralfate at 4 mg/mL (580 µM) showed a reduction of 35% in cell viability. The positive results are shown in Figure 5, Figure 6 and Figure 7.
Figure 5.
Effect of pre-treatment during 60 min with the reference compound sucralfate (Suc), the quinones 1 and 2 and the diterpenes 5 and 6 followed by an incubation during 30 min with 10 mM NaT on the viability of AGS cells determined by the neutral red uptake assay. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett´s test. * p < 0.05 compared to NaT group.
Figure 5.
Effect of pre-treatment during 60 min with the reference compound sucralfate (Suc), the quinones 1 and 2 and the diterpenes 5 and 6 followed by an incubation during 30 min with 10 mM NaT on the viability of AGS cells determined by the neutral red uptake assay. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett´s test. * p < 0.05 compared to NaT group.
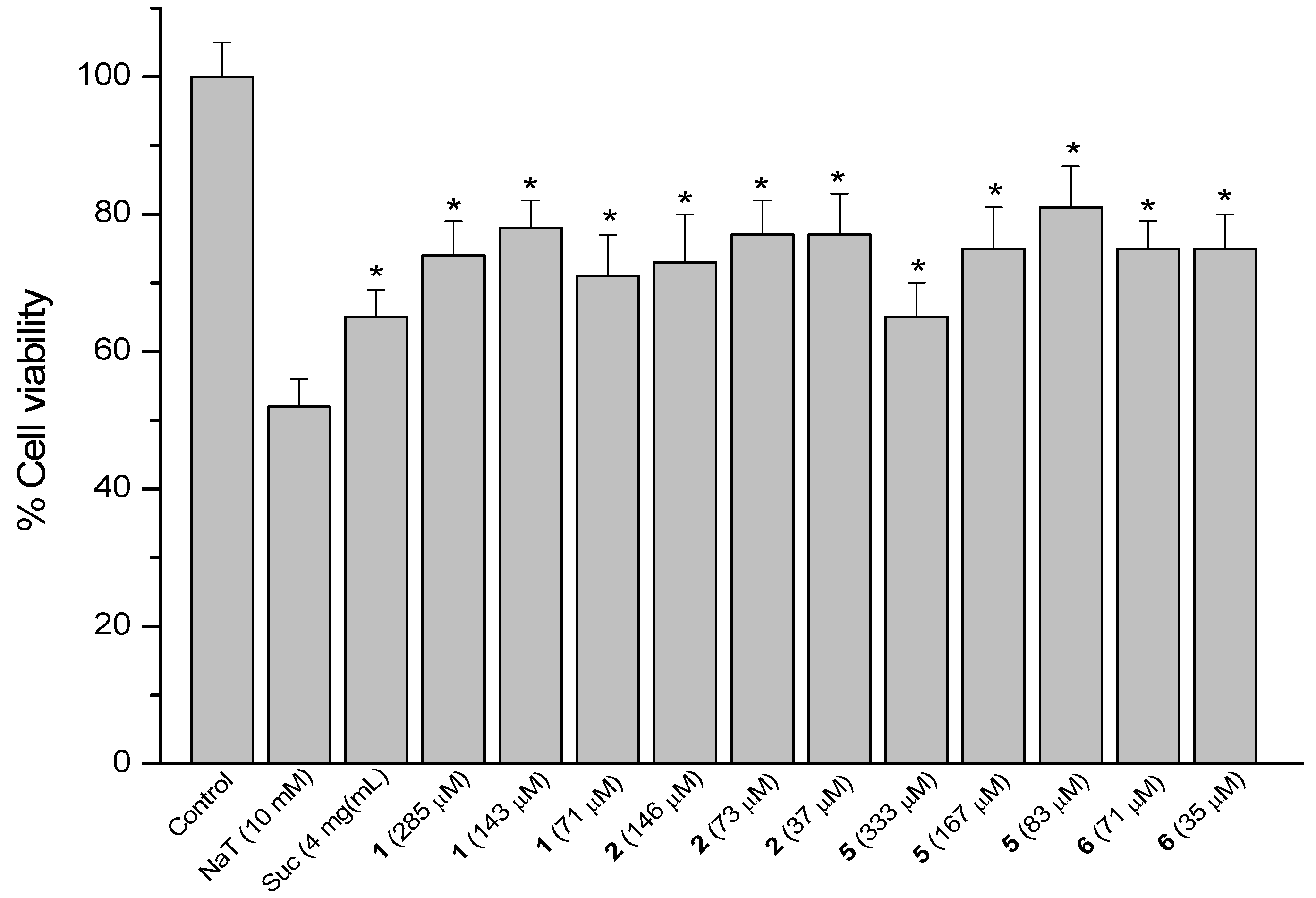
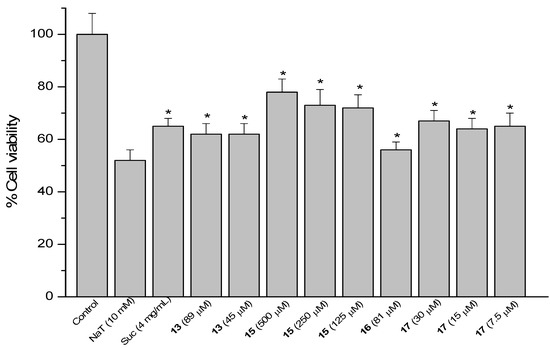
Figure 6.
Effect of pre-treatment during 60 min with the reference compound sucralfate (Suc) and derivatives 13, 15, 16 and 17 from 17β-dihydrojunicedric acid, followed by an incubation during 30 min with 10 mM NaT on the viability of AGS cells determined by the neutral red uptake assay. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett’s test. * p < 0.05 compared to NaT group.
Figure 6.
Effect of pre-treatment during 60 min with the reference compound sucralfate (Suc) and derivatives 13, 15, 16 and 17 from 17β-dihydrojunicedric acid, followed by an incubation during 30 min with 10 mM NaT on the viability of AGS cells determined by the neutral red uptake assay. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett’s test. * p < 0.05 compared to NaT group.
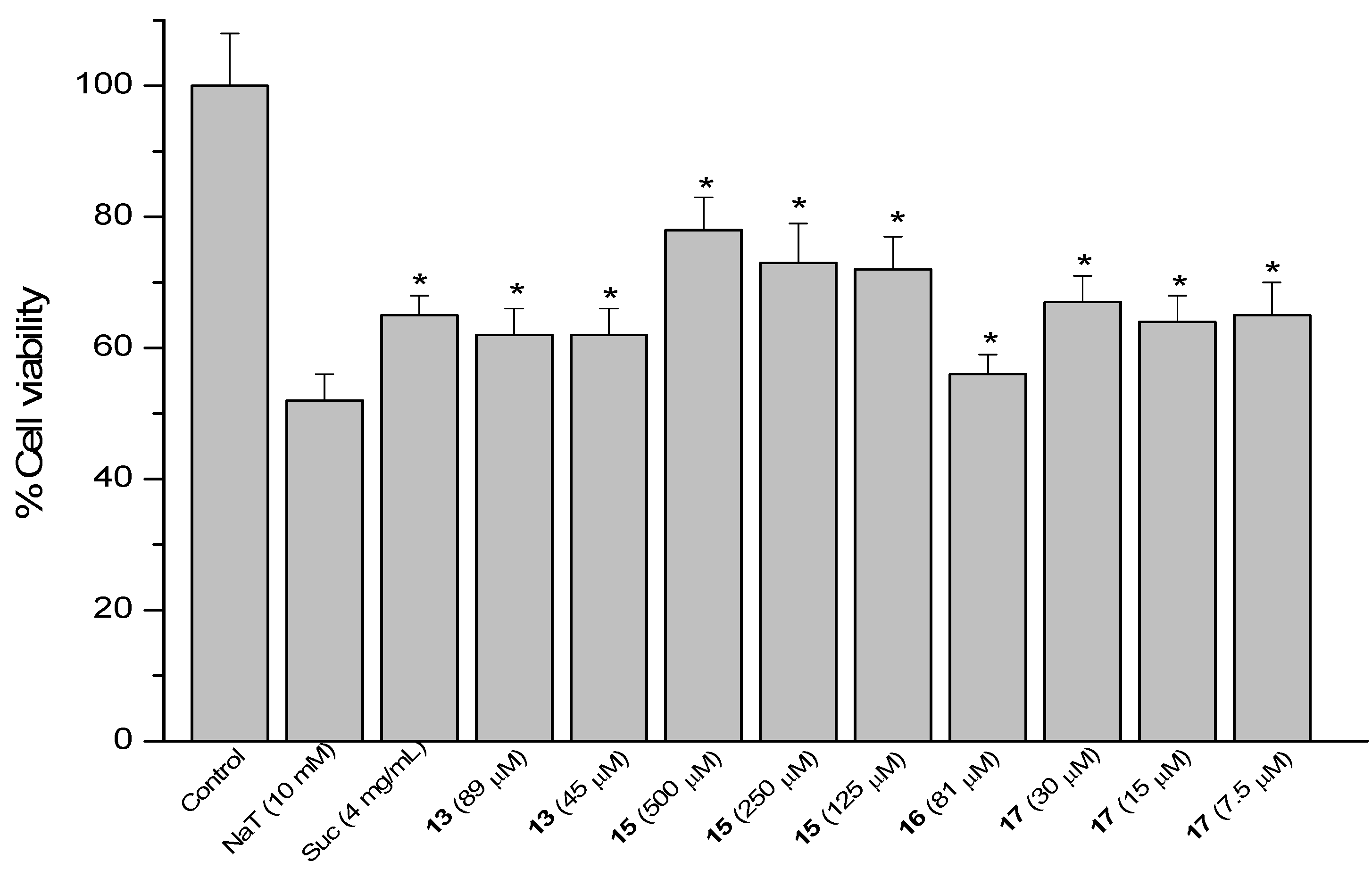
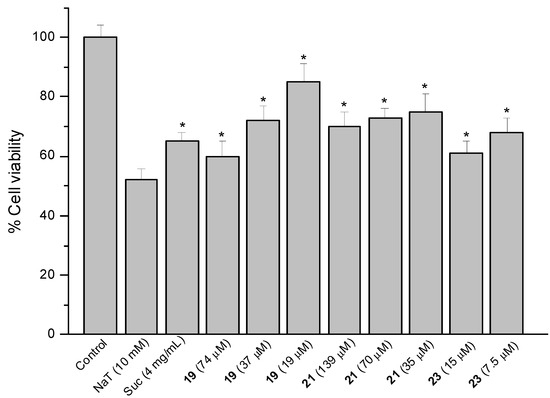
Figure 7.
Effect of pre-treatment during 60 min with the reference compound sucralfate (Suc) and Δ8(9) junicedric acid derivatives 19, 21 and 23 followed by an incubation during 30 min with 10 mM NaT on the viability of AGS cells determined by the neutral red uptake assay. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett’s test. * p < 0.05 compared to NaT group.
Figure 7.
Effect of pre-treatment during 60 min with the reference compound sucralfate (Suc) and Δ8(9) junicedric acid derivatives 19, 21 and 23 followed by an incubation during 30 min with 10 mM NaT on the viability of AGS cells determined by the neutral red uptake assay. Each value represents the mean ± SD of three different experiments in quadruplicate. ANOVA followed by Dunnett’s test. * p < 0.05 compared to NaT group.
The results showed significant cytoprotective effect (p < 0.05) for the starting quinones 1 (25, 22 and 29% cell protection at 285, 143 and 71 µM, respectively) and 2 (27, 23 and 23%, at 146, 73 and 37 µM respectively), the diterpenes 5 (35, 25 and 19% reduction in cell viability at 333, 167 and 83 µM, respectively) and 6 (25% cell viability reduction at 71 and 35 µM) (Figure 5).
Cytoprotection was observed for the diterpene 6 hybrids 13, 15–17 (Figure 6). The effect of compound 17 at 30, 15 and 7.5 µM was in the same range as sucralfate (34%–36%), while the activity of 13 at 89 and 45 µM was 38% and the effect of 16 at 81 µM was 44%, compared to NaT controls. Best effect, even higher than that of sucralfate, was found for the hybrid 15 at 500, 250 and 125 µM, reducing cell viability by 21, 27 and 27%, respectively.
The hybrids of the diterpene 5 with 1, 2 and 3 (compounds 19, 21 and 23) were active (Figure 7). However, the corresponding methyl esters were devoid of effect, suggesting that the free COOH at C-19 is needed for cytoprotection. Even if COOH is needed for cytoprotection, the carboxylic acid derivatives were the most toxic compounds. The cytoprotection effect might be due to the implication of OH residues in radical mechanisms with ROS species, and that might be the reason why the methyl ester derivatives, lacking of OH, do not present cytoprotective effects. Compound 19 reduced cell viability by 40, 27 and 15% at 74, 37 and 19 µM. Compound 21 at 139, 70 and 35 µM improved viability by 30, 27 and 25%, while the effect of compound 23 at 15 and 7.5 µM was 39 and 32%, respectively. The inverse dose-response effect of compounds 5 and 19 and in a less extent for 21 might be related to the solubility of the compounds.
Cytoprotective compounds may protect against NaT-induced damage forming a physical barrier or binding bile salts, interacting with cell membranes or modifying the expression of trefoil factor family 2 mRNA and c-fos protein [16,17].
2.5. Effect on Prostaglandin E2 (PGE2) Content
A relevant gastroprotective mechanism involves the prostaglandins that accelerate ulcer healing, epithelial cell proliferation, synthesis of growth factors and inhibition of inflammatory cell infiltration [18]. The prostaglandin E2 (PGE2) is considered to be the major humoral factor providing resistance to the gastric mucosa [19]. Some terpenes and their derivatives exert their gastroprotective effect increasing the gastric PGE2 synthesis in vitro and in vivo [7,20]. Experiments were carried out using post-confluent AGS cells. The results are presented in Table 2. Among the starting quinones and diterpenes 1–6, only lapachol 1 stimulated PGE2 synthesis in AGS, cells showing a 2.5- and 2.3-fold increase in PGE2 content at 286 and 143 µM, respectively. Compound 8 at 500 and 250 µM elicited a 15.2- and 5.2-fold increase of PGE2 content, respectively.

Table 2.
Effect of lapachol 1 and the diterpenyl naphthoquinones 8–10, 15, 18, 21, 22 and 24 on the total PGE2 content of post-confluent AGS cells treated during 1 h with the compounds at 1/2 and 1/4 of IC50.
| Compound | Concentration (µM) | PGE2 (pg/mL) |
|---|---|---|
| Control | - | 52 ± 5 |
| Indomethacin a | 100 | Not detected |
| Lapachol (1) | 286 | 132 ± 12 * |
| 143 | 117 ± 10 * | |
| Lapachoyl junicedrate methyl ester (8) | 500 | 787 ± 56 * |
| 250 | 272 ± 30 * | |
| Dihydroprenyl lapachol junicedrate (9) | 12.5 | 309 ± 29 * |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | 323 | 465 ± 39 * |
| 162 | 306 ± 32 * | |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | 500 | 372 ± 25 * |
| 250 | 279 ± 20 * | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 221 | 156 ± 13 * |
| 111 | 123 ± 9 * | |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | 85 | 162 ± 18 * |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 170 | 114 ± 11 * |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (24) | 250 | 123 ± 12 * |
Each value represents the mean ± SD of two different experiments in triplicate. ANOVA followed by Dunnett’s multiple comparison test. * p < 0.01 compared to control group. a Reference compound.
The dihydroprenyl lapachol hybrids 9–10, 15, 21–22, bearing the same quinone moiety but differing in the identity of the terpene, increased PGE2 synthesis as follows: 9 (12.5 µM, 5.9-fold), 10 (323 and 162 µM, 8.9- and 5.9-fold), 15 (500 and 250 µM, 7.2 and 5.4-fold), 21 (85 µM, 3.1-fold) and 22 (170 µM, 2.2-fold), respectively.
PGE2 synthesis was also stimulated by compounds 18 (221 and 111 µM, 3.0 and 2.4-fold, respectively) and 24 (250 µM, 2.4-fold). The hybrids 18 and 24 have in common the quinone moiety 3 and differ in the identity of the diterpene part of the hybrid molecule.
2.6. Effect on Cellular Reduced Glutathione (GSH) Content
Generation of gastric ulcers has been related to the effect of free radicals. This fact explains the ulcerogenic effect of ethanol on the gastric mucosa. Intracellular GSH content is a determining factor that contributes to the protection of the gastric mucosa against oxidative stress [21]. Experiments were carried out using post-confluent AGS cells. The known stimulant of GSH synthesis, N-acetyl-L-cysteine (NAC) was used as positive control. Under our experimental conditions, NAC (750 µM) increased GSH content by 30% compared to untreated controls. Results are summarized in Table 3. Among the starting diterpenes, junicedric acid (4) and 17β-dihydrojunicedric acid (6) significantly (p < 0.05 and p < 0.01) stimulated GSH production. Best activity was observed for compound 6 (60 and 43% increase at 71 and 37 µM, respectively). Among the hybrid compounds, a good effect was found for the junicedric acid-lapachol derivatives 7 (23 and 18% at 36 and 18 µM, respectively) and 8 (24 and 19% at 250 and 125 µM, respectively) as well as for dihydroprenyl lapachol junicedrate 9 (16 and 15% at 12 and 6 µM, respectively).
Considering the diterpene 6 hybrids, best activity was found for the derivative 15 (35 and 31% at 250 and 125 µM, respectively), 17 (51 and 89% at 15 and 7.5 µM, respectively) and 18 (26% both at 221 and 111 µM). Compounds 17 and 18 differ in the free COOH (17) or COOCH3 function (18) in the diterpene moiety.
Most of the Δ8(9) junicedric acid (diterpene 5) hybrids presented activity. However, the starting diterpene was inactive. In this group of compounds, effect was observed for the derivatives with lapachol (compound 20, 13 and 19% at 80 and 40 µM, respectively), dihydroprenyl lapachol (compound 21, 33% at 70 µM) and dihydroprenyl-5,6,7-8-tetrahydrolapachol (compound 23, 26% at 7.3 µM). Inverse dose-response effect was observed for the hybrids 17, 20 and 24. This effect might be related to the solubility of the compounds.

Table 3.
Total reduced sulfhydril (GSH) content in post-confluent AGS cells treated during 4 h with N-acetyl-L-cysteine (NAC) and the compounds 1–24 at 1/4 and 1/8 of IC50.
| Compound | Concentration (µM) | GSH (nmol/106 cells) |
|---|---|---|
| Control | - | 13.6 ± 0.7 |
| NAC a | 750 | * 17.7 ± 1.4 |
| Lapachol(1) | 143 | 14.1 ± 0.8 |
| 72 | 13.9 ± 0.9 | |
| Dihydroprenyl lapachol(2) | 73 | 13.8 ± 0.7 |
| 37 | 13.6 ± 0.7 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachol (3) | 22 | 13.0 ± 0.5 |
| 11 | 14.3 ± 0.7 | |
| Junicedric acid (4) | 96 | * 17.2 ± 1.0 |
| 48 | * 18.4 ± 1.1 | |
| Δ8(9) Junicedric acid (5) | 166 | 14.1 ± 0.6 |
| 83 | 13.6 ± 0.5 | |
| 17β-Dihydrojunicedric acid (6) | 71 | ** 21.7 ± 1.3 |
| 37 | * 19.5 ± 0.9 | |
| Lapachoyl junicedrate (7) | 36 | * 16.7 ± 0.7 |
| 18 | 16.1 ± 0.9 | |
| Lapachoyl junicedrate methyl ester (8) | 250 | * 16.8 ± 0.9 |
| 125 | * 16.2 ± 0.6 | |
| Dihydroprenyl lapachol junicedrate (9) | 12 | * 15.8 ± 0.6 |
| 6 | * 15.6 ± 0.6 | |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | 165 | 13.0 ± 0.7 |
| 83 | 13.4 ± 0.7 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate (11) | 16 | 13.2 ± 0.5 |
| 8 | 13.1 ± 0.6 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (12) | 250 | 13.9 ± 0.8 |
| 125 | 13.7 ± 1.0 | |
| Lapachoyl 17β-dihydrojunicedrate (13) | 88 | 11.4 ± 0.6 |
| 44 | 11.9 ± 0.4 | |
| Lapachoyl 17β-dihydrojunicedrate methyl ester (14) | 229 | 12.7 ± 0.4 |
| 115 | 14.4 ± 0.5 | |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | 250 | * 18.4 ± 1.2 |
| 125 | * 17.8 ± 0.9 | |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16) | 160 | 14.1 ± 0.6 |
| 80 | 14.9 ± 0.7 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17) | 15 | ** 20.5 ± 1.0 |
| 7.5 | ** 25.8 ± 1.6 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 221 | * 17.2 ± 1.0 |
| 111 | * 17.1 ± 1.1 | |
| Lapachoyl Δ8(9) junicedrate (19) | 37 | 13.5 ± 0.5 |
| 19 | 13.1 ± 0.4 | |
| Lapachoyl Δ8(9) junicedrate methyl ester (20) | 80 | * 15.4 ± 1.3 |
| 40 | * 16.2 ± 1.0 | |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | 70 | * 18.1 ± 1.1 |
| 35 | 13.3 ± 0.7 | |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 85 | 14.2 ± 0.6 |
| 43 | 13.6 ± 0.9 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23) | 7.3 | * 17.1 ± 1.3 |
| 3.6 | 12.6 ± 0.8 | |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester(24) | 250 | * 18.0 ± 0.9 |
| 125 | * 18.7 ± 1.0 |
a NAC: reference compound. Each value represents the mean ± SD of three different experiments in triplicate. ANOVA followed by Dunnet’s test. * p < 0.05; ** p < 0.01.
2.7. Inhibition of Lipoperoxidation
Antioxidant effect of the compounds was assessed using human erythrocyte membranes. The products were tested at 100 µg/mL and results are presented in Table 4. Among the starting quinones, the hydrogenation products of lapachol 2 and 3 were more active than lapachol (1), with IC50 values of 25.4 and 33.3 µg/mL, respectively. From the diterpene moieties, only Δ8(9)-junicedric acid (5) was active with an IC50 of 43.8 µg/mL. The inhibition of lipoperoxidation for the hybrids prepared from the diterpene 4 (compounds 7–12) was moderate. Better effects were found for the diterpene 4 derivative with lapachol and a COOCH3 at C-19 (8, IC50: 81.4 µg/mL), and the hybrids containing 4 and the quinone 2, either with a free C-19 carboxylic acid (9, IC50: 40.1 µg/mL) or as the methyl ester (10, IC50: 99.4 µg/mL). Among the hybrids of diterpene 6 (compounds 13–18), best antioxidant activity was found for: 13, 14 and 15 with IC50 values of 61.1, 19.7 and 25.3 µg/mL, respectively. The three compounds contain the quinone 1 or 2 in the hybrid structure. The compounds 19–24 were prepared using Δ8(9) junicedric acid (5) as the diterpene moiety and presented moderate antioxidant effect, best activity was displayed by 20 (IC50: 58.1 µg/mL) and 23 (IC50: 59.5 µg/mL). In the same assay, the reference compound catechin presented an IC50 value of 75.4 µg/mL.
Structural diversity is a requirement to draw structure-activity relationships/trends in compounds series. The mechanisms of action of the starting diterpenes and quinones were compared with those of the new hybrids. The starting compounds showed activity in one or two (exceptionally three) out of the mechanisms of action investigated. Diterpene 5 stimulates cell proliferation, while none of its hybrids presented effect. From the four hybrids of diterpenes 4 and 6 with the quinone 3, three were active.
The quinones 1–2 and diterpenes 5–6 were active in the NaT assay. Hybrids from diterpenes 5 and 6 with quinones show effects on NaT when the COOH group at C-19 of the diterpene is free (compounds 13, 15, 17, 19, 21 and 23). The corresponding methyl esters (except compound 16) were devoid of effect. The products prepared from the diterpene 4, with a 8(17) double bond were inactive.
Among the parent molecules, only lapachol increased PGE2 synthesis. Relevant changes in PGE2 levels were observed for hybrid compounds, with several products stimulating synthesis. Regardless of the diterpene moiety, hybrids with dihydroprenyl lapachol 2 and two methyl esters from the hybrids with 3 were active.
In the GSH assay, quinones were inactive, but the diterpenes 4 and 6 presented effect. For the hybrids of diterpene 4, the activity is observed for the products with a planar naphthoquinone (7–9) but it is lost when the aromatic ring of the quinone is hydrogenated. The diterpene 5, with a double bond Δ8(9) has a more planar configuration and was devoid of activity. However, four out of the six hybrids from 5 proved to be active. Most of them presented the free COOH function at C-19.

Table 4.
Effect of compounds 1–24 on the inhibition of the lipoperoxidation in human erythrocyte membranes. a Percent effect at 100 µg/mL or IC50 values (µg/mL).
| Compound | % Inhibition of the lipoperoxidation a |
|---|---|
| Lapachol (1) | 38 |
| Dihydroprenyl lapachol (2) | IC50 25.4 ± 2.4 µg/mL |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachol (3) | IC50 33.3 ± 2.3 µg/mL |
| Junicedric acid (4) | 25 |
| Δ8(9) Junicedric acid (5) | IC50 43.8 ± 2.8 µg/mL |
| 17β-dihydrojunicedric acid (6) | 30 |
| Lapachoyl junicedrate (7) | 10 |
| Lapachoyl junicedrate methyl ester (8) | IC50 81.4 ± 11.0 µg/mL |
| Dihydroprenyl lapachol junicedrate (9) | IC50 40.1 ± 5.3 µg/mL |
| Dihydroprenyl lapachoyl junicedrate methyl ester (10) | IC50 99.4 ± 9.1 µg/mL |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate (11) | 44 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (12) | 37 |
| Lapachoyl 17β-dihydrojunicedrate (13) | IC50 61.1 ± 5.9 µg/mL |
| Lapachoyl 17β-dihydrojunicedrate methyl ester (14) | IC50 19.7 ± 1.4 µg/mL |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate (15) | IC50 25.3 ± 4.1 µg/mL |
| Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16) | 46 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17) | 48 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18) | 28 |
| Lapachoyl Δ8(9) junicedrate (19) | 49 |
| Lapachoyl Δ8(9) junicedrate methyl ester (20) | IC50 58.1 ± 4.1 µg/mL |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate (21) | Nd |
| Dihydroprenyl lapachoyl Δ8(9) junicedrate methyl ester (22) | 46 |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23) | IC50 59.5 ± 4.3 µg/mL |
| Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl junicedrate methyl ester (24) | 27 |
| Catechin b | IC50 75.4 ± 6.0 µg/mL |
Results are expressed as mean values ± SD of three different experiments in triplicate. b Reference compound. Nd: not determined due to turbidity.
Among the starting compounds, the quinones 2 and 3 as well as the diterpene 5, displayed inhibitory activity in the lipoperoxidation assay. Best antioxidant effect was found for hybrids from the diterpene 6 either with the quinone 1 or 2 or with weaker effect for diterpene 6 hybrids with the same quinones.
In a previous report, part of the compounds presented in this study, were evaluated for gastroprotective effect by the HCl-EtOH-induced gastric lesions model in mice [6].The compounds were administered orally at a single dose of 5 mg/kg. Compounds 9–10 and 21–22 displayed significant activity in vivo [6]. In the present study, these compounds stimulated the PGE2 synthesis in AGS cells. Our results show that compounds 8 and 20, active in vivo, increased GSH content in AGS cells and protected erythrocyte membranes against induced lipoperoxidation. These in vitro findings sustain the in vivo observations suggesting that different and complementary mechanisms may be underlying the gastroprotective effect of the compounds.
Gastric lesions are a consequence of an imbalance between defensive and aggressive factors in the gastric mucosa. Increase in the defensive factors, namely: restitution of gastric epithelium, protection against damage induced by bile salts and free radicals, increase in PGE2 levels, GSH, bicarbonate and others, may reduce the appearance of gastric lesions in animals [7,16,17]. It has been shown that gastroprotective compounds exert their effects mainly stimulating the defensive factors in the gastric mucosa rather than inhibiting the aggressive agents (gastric acid or pepsin) [16,17,22].
In this work the possible mechanisms of gastroprotective effect of diterpenylquinones were assessed using human lung fibroblasts (MRC-5) and gastric epithelial cells (AGS). The evaluation of the compound activity on some defensive factors depending on gastric epithelial cells has been carried out using AGS cells. This cell line has been successfully used in the study of different aspects related to the gastric mucosa [7,23,24]. The hybrid compounds investigated stimulated one or more of the defensive factors described as relevant to protect the gastric mucosa. To obtain hybrid molecules with better activity than the parent compounds, structural modifications should be undertaken, followed by a suitable combination of substituents placed at the appropriate positions of hybrid compounds [25]. To understand the relationship between in vitro and in vivo activity, further research should be carried out, since factors such as absorption, bioavailability, location of the targets in different tissues or organs, receptor and enzyme densities will influence in effect [26].
3. Experimental
3.1. General Experimental Procedures
Optical rotations were obtained for solutions in CHCl3 (concentrations expressed in g/100 mL) on a Jasco DIP 370 polarimeter (Jasco Analytical Instruments, Easton, MD, USA). IR spectra were recorded on a Nicolet Nexus FT-IR instrument (Thermo Electron Corporation, Waltham, MA, USA).1H-NMR spectra were recorded at 400 MHz and 13C-NMR data were obtained at 100 MHz on a Bruker Avance spectrometer (Bruker, Rheinstetten, Germany). Chemical shifts are given in δ (ppm) with TMS as the internal standard. Mass spectra were measured in a LC/MSD-TOF (Agilent Technologies) by the electrospray technique. Positive or negative ion mode was used according to the samples. As internal reference in the ESI (+) mode, purine (m/z 121.0509) and HP-0921 (m/z 922.0098) were used. In the negative mode: ESI (−), purine (m/z 112.9856) and HP-0921 (m/z 1033.9881) were used as internal references. Silica gel 60 (Merck, 63-200 µm particle size) was used for column chromatography, precoated silica gel plates (Merck, Kieselgel 60 F254, 0.25 mm) were used for thin layer chromatography (TLC). TLC spots were visualized by spraying the chromatograms with p-anisaldehyde-ethanol-acetic acid-H2SO4(2:170:20:10 v/v) and heating at 110 °C for 3 min. 1,3-Dicyclohexylcarbodiimide (DCC) and dimethylaminopyridine (DMAP) were from Merck (Schuchardt, Germany). Oxalyl chloride was from Sigma-Aldrich (St. Louis, MO, USA).
3.2. General Procedure for the Synthesis of Hybrid Compounds
3.2.1. Quinones
The naphthoquinone lapachol (1) was isolated from the wood of “lapacho” tree (Tabebuia sp). The hydrogenation products of lapachol (compounds 2 and 3) were prepared trating (1) in EtOAc with Pd/C [6]. The synthetic scheme for the synthesis is presented in [6].
3.2.2. Diterpenes
The diterpene 4 was obtained from the resin of Araucaria araucana as previously reported [6]. The isomers 5 and 6 were prepared from 4. Briefly, treatment of 4 with HBr in acetic acid afforded Δ8(9) junicedric acid (5) while catalytic hydrogenation of 4 in ethyl acetate with 10% Pd/C in a 1:10 molar ratio yielded the hydrogenation product 6. The synthetic scheme for the synthesis is shown in [6].
3.2.3. Hybrid Compounds
The compounds 7–14 and 19–22 were synthesized according to [6]. The new compounds 15 and 17 were prepared by treating the diacid 6 (1 mEq) in dry CH2Cl2 (DCM) with 1,3-dicyclohexylcarbodiimide (DCC) (1 mEq) at room temperature under constant stirring. After 10 min, the quinones 2 and 3 (1 mEq) dissolved in dry DCM, were added together with a catalytic amount of dimethylaminopyridine (DMAP). After 2–4 h, the reaction was stopped by adding water and extracted with DCM. Compound 23 was synthesized from the diacid 5 under an inert (N2) atmosphere. Briefly, the diterpene 5 was dissolved in dry CH2Cl2 (DCM) and ice cooled under nitrogen flow. To this solution, oxalyl chloride in dry DCM was added drop wise with stirring. The diterpene: oxalyl chloride molar ratio was 1:40. The mixture was stirred at room temperature overnight, then the DCM was evaporated and the residue vacuum dried. The dry residue was dissolved in dry DCM and the quinone 3 was added as well as triethylamine (TEA) under constant N2 flow. The diterpene acyl chloride-quinone-TEA molar ratio was 1:1:2. The reaction mixture was left at room temperature under stirring and inert atmosphere for two days. Then, the mixture was washed two times with water, and the aqueous phase extracted with DCM to obtain the crude reaction mixture.
The reaction mixtures were dried over Na2SO4. Purification was carried out using a combination of gel permeation in Sephadex LH-20 with a MeOH:DCM 1:1 (v/v) mixture, or silica gel column chromatography using PE:EtOAc mixtures (90:10, 85:15, 80:20) (v/v), to afford compounds 15, 17 and 23 in 14.3, 13.7 and 17% w/w yields, respectively. The methyl esters 16, 18, and 24 were obtained treating the compounds 15, 17 and 23 with diazomethane in diethyl ether, with 92%–98% w/w yield. The purity of all the derivatives was over 95%, as determined by 1H-NMR spectroscopy. The new compounds 15–18, 23 and 24 are described below.
Dihydroprenyl lapachol 17β-dihydrojunicedrate (15): 17β-Dihydrojunicedric acid (6, 246.8 mg, 0.732 mmol), DCC (302 mg, 1.464 mmol), a catalytic amount of DMAP and dihydroprenyl lapachol (2, 177.9 mg, 0.732 mmol), were stirred at room temperature in dry CH2Cl2 (20 mL) for 2–4 h. The reaction mixture was cooled in an ice bath. After addition of water, the aqueous phase was extracted with EtOAc (3 × 20 mL). The extract was dried over anhydrous Na2SO4 and taken to dryness under reduced pressure. Purification was carried out using a combination of gel permeation in Sephadex LH-20 with a MeOH-DCM 1:1 (v/v) mixture and silica gel column chromatography using PE-EtOAc mixtures 90:10 (v/v), to afford compound 15 (60.7 mg, 14.3%): yellow resin; 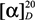 +2.35 (c 0.085 CHCl3); IR νmax (film) 2957, 2931, 2854, 1725, 1675, 1463, 1386, 1287, 1074, 998 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.75 (2H, m, quinone H-6 and H-7), 2.74 (1H, dd, J = 16, 4 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.42 (1H, m, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.27 (3H, s, H-18), 1.13 (3H, d, J = 6.8 Hz, H-16), 0.93 (6H, d, J = 6.4 Hz, H-4' and H-5'), 0.90 (3H, d, J = 6.4 Hz, H-17), 0.84 (3H, s, H-20); 13C-NMR (CDCl3): 39.61 (C-1), 19.43 (C-2), 37.45 (C-3), 45.46 (C-4), 52.82 (C-5), 23.00 (C-6), 34.94 (C-7), 29.00 (C-8), 57.64 (C-9), 38.84 (C-10), 18.84 (C-11), 35.12 (C-12), 30.79 (C-13), 40.72 (C-14), 170.29 (C-15), 20.10 (C-16), 15.17 (C-17), 28.04 (C-18), 178.08 (C-19), 14.89 (C-20); Quinone: 173.42, 184.49 (C-1 and C-4), 151.04 (C-2), 140.09 (C-3), 130.89, 132.08 (C-4a, C-8a), 126.61, 126.50 (C-5 and C-8), 133.95, 133.72 (C-6 and C-7), 22.40 (C-1'), 37.00 (C-2'), 28.34 (C-3'), 22.28 (C-4'), 22.28 (C-5'); EIMS m/z 576 [M]+ (2), 293 (17), 244 (20), 243 (20), 242 (100), 227 (27), 123 (28), 109 (14), 95 (10), 81 (11), 55 (11).
+2.35 (c 0.085 CHCl3); IR νmax (film) 2957, 2931, 2854, 1725, 1675, 1463, 1386, 1287, 1074, 998 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.75 (2H, m, quinone H-6 and H-7), 2.74 (1H, dd, J = 16, 4 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.42 (1H, m, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.27 (3H, s, H-18), 1.13 (3H, d, J = 6.8 Hz, H-16), 0.93 (6H, d, J = 6.4 Hz, H-4' and H-5'), 0.90 (3H, d, J = 6.4 Hz, H-17), 0.84 (3H, s, H-20); 13C-NMR (CDCl3): 39.61 (C-1), 19.43 (C-2), 37.45 (C-3), 45.46 (C-4), 52.82 (C-5), 23.00 (C-6), 34.94 (C-7), 29.00 (C-8), 57.64 (C-9), 38.84 (C-10), 18.84 (C-11), 35.12 (C-12), 30.79 (C-13), 40.72 (C-14), 170.29 (C-15), 20.10 (C-16), 15.17 (C-17), 28.04 (C-18), 178.08 (C-19), 14.89 (C-20); Quinone: 173.42, 184.49 (C-1 and C-4), 151.04 (C-2), 140.09 (C-3), 130.89, 132.08 (C-4a, C-8a), 126.61, 126.50 (C-5 and C-8), 133.95, 133.72 (C-6 and C-7), 22.40 (C-1'), 37.00 (C-2'), 28.34 (C-3'), 22.28 (C-4'), 22.28 (C-5'); EIMS m/z 576 [M]+ (2), 293 (17), 244 (20), 243 (20), 242 (100), 227 (27), 123 (28), 109 (14), 95 (10), 81 (11), 55 (11).
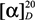 +2.35 (c 0.085 CHCl3); IR νmax (film) 2957, 2931, 2854, 1725, 1675, 1463, 1386, 1287, 1074, 998 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.75 (2H, m, quinone H-6 and H-7), 2.74 (1H, dd, J = 16, 4 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.42 (1H, m, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.27 (3H, s, H-18), 1.13 (3H, d, J = 6.8 Hz, H-16), 0.93 (6H, d, J = 6.4 Hz, H-4' and H-5'), 0.90 (3H, d, J = 6.4 Hz, H-17), 0.84 (3H, s, H-20); 13C-NMR (CDCl3): 39.61 (C-1), 19.43 (C-2), 37.45 (C-3), 45.46 (C-4), 52.82 (C-5), 23.00 (C-6), 34.94 (C-7), 29.00 (C-8), 57.64 (C-9), 38.84 (C-10), 18.84 (C-11), 35.12 (C-12), 30.79 (C-13), 40.72 (C-14), 170.29 (C-15), 20.10 (C-16), 15.17 (C-17), 28.04 (C-18), 178.08 (C-19), 14.89 (C-20); Quinone: 173.42, 184.49 (C-1 and C-4), 151.04 (C-2), 140.09 (C-3), 130.89, 132.08 (C-4a, C-8a), 126.61, 126.50 (C-5 and C-8), 133.95, 133.72 (C-6 and C-7), 22.40 (C-1'), 37.00 (C-2'), 28.34 (C-3'), 22.28 (C-4'), 22.28 (C-5'); EIMS m/z 576 [M]+ (2), 293 (17), 244 (20), 243 (20), 242 (100), 227 (27), 123 (28), 109 (14), 95 (10), 81 (11), 55 (11).
+2.35 (c 0.085 CHCl3); IR νmax (film) 2957, 2931, 2854, 1725, 1675, 1463, 1386, 1287, 1074, 998 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.75 (2H, m, quinone H-6 and H-7), 2.74 (1H, dd, J = 16, 4 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.42 (1H, m, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.27 (3H, s, H-18), 1.13 (3H, d, J = 6.8 Hz, H-16), 0.93 (6H, d, J = 6.4 Hz, H-4' and H-5'), 0.90 (3H, d, J = 6.4 Hz, H-17), 0.84 (3H, s, H-20); 13C-NMR (CDCl3): 39.61 (C-1), 19.43 (C-2), 37.45 (C-3), 45.46 (C-4), 52.82 (C-5), 23.00 (C-6), 34.94 (C-7), 29.00 (C-8), 57.64 (C-9), 38.84 (C-10), 18.84 (C-11), 35.12 (C-12), 30.79 (C-13), 40.72 (C-14), 170.29 (C-15), 20.10 (C-16), 15.17 (C-17), 28.04 (C-18), 178.08 (C-19), 14.89 (C-20); Quinone: 173.42, 184.49 (C-1 and C-4), 151.04 (C-2), 140.09 (C-3), 130.89, 132.08 (C-4a, C-8a), 126.61, 126.50 (C-5 and C-8), 133.95, 133.72 (C-6 and C-7), 22.40 (C-1'), 37.00 (C-2'), 28.34 (C-3'), 22.28 (C-4'), 22.28 (C-5'); EIMS m/z 576 [M]+ (2), 293 (17), 244 (20), 243 (20), 242 (100), 227 (27), 123 (28), 109 (14), 95 (10), 81 (11), 55 (11).Dihydroprenyl lapachol 17β-dihydrojunicedrate methyl ester (16). Yellow resin; 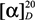 +20 (c 0.056, CHCl3); IR νmax (film) 2951, 2868, 2844, 1772, 1725, 1679, 1463, 1380, 1300, 1151, 1081, 941, 712 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.74 (2H, m, quinone H-6 and H-7), 3.65 (3H, s, OCH3), 2.75 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.44 (1H, dd, J = 15.2, 8.8 Hz, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.19 (3H, s, H-18), 1.13 (3H, d, J = 6.4 Hz, H-16), 0.95 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 7.6 Hz, H-17), 0.70 (3H, s, H-20); 13C-NMR (CDCl3): 40.23 (C-1), 19.47 (C-2), 38.55 (C-3), 44.31 (C-4), 53.31 (C-5), 23.43 (C-6), 35.35 (C-7), 29.66 (C-8), 57.89 (C-9), 39.08 (C-10), 19.28 (C-11), 35.62 (C-12), 31.27 (C-13), 41.18 (C-14), 170.79 (C-15), 20.57 (C-16), 15.34 (C-17), 29.26 (C-18), 178.51 (C-19), 14.68 (C-20), 51.55 (OCH3); Quinone: 173.26, 184.95 (C-1 and C-4), 151.5 (C-2), 140.54 (C-3), 131.32, 132.52 (C-4a, C-8a), 127.06, 126.95 (C-5 and C-8), 134.41, 134.18 (C-6 and C-7), 22.86 (C-1'), 37.90 (C-2'), 28.79 (C-3'), 22.74 (C-4'), 22.74 (C-5'); molecular formula: C36H50O6 (578.3607). m/z 596.3947 [M+NH4]+, error: 0.20 ppm with the empirical formula C36H54NO6; m/z 601.3499 [M+Na]+, error: 0.08 ppm with the empirical formula C36H50O6Na.
+20 (c 0.056, CHCl3); IR νmax (film) 2951, 2868, 2844, 1772, 1725, 1679, 1463, 1380, 1300, 1151, 1081, 941, 712 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.74 (2H, m, quinone H-6 and H-7), 3.65 (3H, s, OCH3), 2.75 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.44 (1H, dd, J = 15.2, 8.8 Hz, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.19 (3H, s, H-18), 1.13 (3H, d, J = 6.4 Hz, H-16), 0.95 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 7.6 Hz, H-17), 0.70 (3H, s, H-20); 13C-NMR (CDCl3): 40.23 (C-1), 19.47 (C-2), 38.55 (C-3), 44.31 (C-4), 53.31 (C-5), 23.43 (C-6), 35.35 (C-7), 29.66 (C-8), 57.89 (C-9), 39.08 (C-10), 19.28 (C-11), 35.62 (C-12), 31.27 (C-13), 41.18 (C-14), 170.79 (C-15), 20.57 (C-16), 15.34 (C-17), 29.26 (C-18), 178.51 (C-19), 14.68 (C-20), 51.55 (OCH3); Quinone: 173.26, 184.95 (C-1 and C-4), 151.5 (C-2), 140.54 (C-3), 131.32, 132.52 (C-4a, C-8a), 127.06, 126.95 (C-5 and C-8), 134.41, 134.18 (C-6 and C-7), 22.86 (C-1'), 37.90 (C-2'), 28.79 (C-3'), 22.74 (C-4'), 22.74 (C-5'); molecular formula: C36H50O6 (578.3607). m/z 596.3947 [M+NH4]+, error: 0.20 ppm with the empirical formula C36H54NO6; m/z 601.3499 [M+Na]+, error: 0.08 ppm with the empirical formula C36H50O6Na.
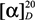 +20 (c 0.056, CHCl3); IR νmax (film) 2951, 2868, 2844, 1772, 1725, 1679, 1463, 1380, 1300, 1151, 1081, 941, 712 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.74 (2H, m, quinone H-6 and H-7), 3.65 (3H, s, OCH3), 2.75 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.44 (1H, dd, J = 15.2, 8.8 Hz, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.19 (3H, s, H-18), 1.13 (3H, d, J = 6.4 Hz, H-16), 0.95 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 7.6 Hz, H-17), 0.70 (3H, s, H-20); 13C-NMR (CDCl3): 40.23 (C-1), 19.47 (C-2), 38.55 (C-3), 44.31 (C-4), 53.31 (C-5), 23.43 (C-6), 35.35 (C-7), 29.66 (C-8), 57.89 (C-9), 39.08 (C-10), 19.28 (C-11), 35.62 (C-12), 31.27 (C-13), 41.18 (C-14), 170.79 (C-15), 20.57 (C-16), 15.34 (C-17), 29.26 (C-18), 178.51 (C-19), 14.68 (C-20), 51.55 (OCH3); Quinone: 173.26, 184.95 (C-1 and C-4), 151.5 (C-2), 140.54 (C-3), 131.32, 132.52 (C-4a, C-8a), 127.06, 126.95 (C-5 and C-8), 134.41, 134.18 (C-6 and C-7), 22.86 (C-1'), 37.90 (C-2'), 28.79 (C-3'), 22.74 (C-4'), 22.74 (C-5'); molecular formula: C36H50O6 (578.3607). m/z 596.3947 [M+NH4]+, error: 0.20 ppm with the empirical formula C36H54NO6; m/z 601.3499 [M+Na]+, error: 0.08 ppm with the empirical formula C36H50O6Na.
+20 (c 0.056, CHCl3); IR νmax (film) 2951, 2868, 2844, 1772, 1725, 1679, 1463, 1380, 1300, 1151, 1081, 941, 712 cm−1; 1H-NMR (CDCl3): δ 8.11 (2H, m, quinone H-5 and H-8), 7.74 (2H, m, quinone H-6 and H-7), 3.65 (3H, s, OCH3), 2.75 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.55 (2H, t, J = 8 Hz, H-1'), 2.44 (1H, dd, J = 15.2, 8.8 Hz, H-14 β), 1.61 (1H, m, H-3'), 1.45-1.52 (2H, m, H-2'), 1.19 (3H, s, H-18), 1.13 (3H, d, J = 6.4 Hz, H-16), 0.95 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 7.6 Hz, H-17), 0.70 (3H, s, H-20); 13C-NMR (CDCl3): 40.23 (C-1), 19.47 (C-2), 38.55 (C-3), 44.31 (C-4), 53.31 (C-5), 23.43 (C-6), 35.35 (C-7), 29.66 (C-8), 57.89 (C-9), 39.08 (C-10), 19.28 (C-11), 35.62 (C-12), 31.27 (C-13), 41.18 (C-14), 170.79 (C-15), 20.57 (C-16), 15.34 (C-17), 29.26 (C-18), 178.51 (C-19), 14.68 (C-20), 51.55 (OCH3); Quinone: 173.26, 184.95 (C-1 and C-4), 151.5 (C-2), 140.54 (C-3), 131.32, 132.52 (C-4a, C-8a), 127.06, 126.95 (C-5 and C-8), 134.41, 134.18 (C-6 and C-7), 22.86 (C-1'), 37.90 (C-2'), 28.79 (C-3'), 22.74 (C-4'), 22.74 (C-5'); molecular formula: C36H50O6 (578.3607). m/z 596.3947 [M+NH4]+, error: 0.20 ppm with the empirical formula C36H54NO6; m/z 601.3499 [M+Na]+, error: 0.08 ppm with the empirical formula C36H50O6Na.Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate (17). 17β-Dihydrojunicedric acid (6, 229.2 mg, 0.679 mmol), DCC (280.2 mg, 1.358 mmol), a catalytic amount of DMAP and dihydroprenyl-5,6,7,8-tetrahydrolapachol (3, 168.4 mg, 0.679 mmol), were stirred at room temperature in dry CH2Cl2 (20 ml) for 2–4 h. The reaction mixture was cooled in an ice bath. After addition of water, the aqueous phase was extracted with EtOAc (3 × 20 mL). The extract was dried over anhydrous Na2SO4 and taken to dryness under reduced pressure. The residue was purified by silica gel column chromatography, eluting with an hexane/EtOAc gradient (95:5, 91:9, 87:13), yielding 17 (52.9 mg, 13.7%): amber resin; 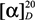 +23 (c 0.065, CHCl3); IR νmax (film) 2959, 1769, 1699, 1662, 1625, 1549, 1467, 1455, 1382, 1269, 1205, 1077, 913, 755 cm−1; 1H-NMR (CDCl3): δ 2.68 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.40 (2H, m, quinone H-5 and H-8), 2.28 (1H, m, H-14 β), 1.70 (2H, m, quinone H-6 and H-7), 1.59 (2H, m, H-1'), 1.48 (2H, m, H-2'), 1.25 (3H, s, H-18), 1.10 (3H, d, J = 6.8 Hz, H-16), 0.92 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 6.4 Hz, H-17), 0.80 (3H, s, H-20); 13C-NMR (CDCl3): 40.14 (C-1), 19.22 (C-2), 38.31 (C-3), 44.20 (C-4), 53.34 (C-5), 23.41 (C-6), 35.36 (C-7), 29.67 (C-8), 57.86 (C-9), 39.30 (C-10), 18.33 (C-11), 35.60 (C-12), 31.24 (C-13), 41.13 (C-14), 170.97 (C-15), 20.51 (C-16), 15.36 (C-17), 29.45 (C-18), 180.70 (C-19), 14.79 (C-20); Quinone: 184.36, 187.37 (C-1 and C-4), 149.02 (C-2), 141.29 (C-3), 137.32, 143.20 (C-4a, C-8a), 23.22, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.41 (C-1'), 37.90 (C-2'), 28.67 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H52O6 (568.3764). m/z 586.4086 [M+NH4]+, error: 0.20 ppm with the empirical formula C35H56NO6.
+23 (c 0.065, CHCl3); IR νmax (film) 2959, 1769, 1699, 1662, 1625, 1549, 1467, 1455, 1382, 1269, 1205, 1077, 913, 755 cm−1; 1H-NMR (CDCl3): δ 2.68 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.40 (2H, m, quinone H-5 and H-8), 2.28 (1H, m, H-14 β), 1.70 (2H, m, quinone H-6 and H-7), 1.59 (2H, m, H-1'), 1.48 (2H, m, H-2'), 1.25 (3H, s, H-18), 1.10 (3H, d, J = 6.8 Hz, H-16), 0.92 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 6.4 Hz, H-17), 0.80 (3H, s, H-20); 13C-NMR (CDCl3): 40.14 (C-1), 19.22 (C-2), 38.31 (C-3), 44.20 (C-4), 53.34 (C-5), 23.41 (C-6), 35.36 (C-7), 29.67 (C-8), 57.86 (C-9), 39.30 (C-10), 18.33 (C-11), 35.60 (C-12), 31.24 (C-13), 41.13 (C-14), 170.97 (C-15), 20.51 (C-16), 15.36 (C-17), 29.45 (C-18), 180.70 (C-19), 14.79 (C-20); Quinone: 184.36, 187.37 (C-1 and C-4), 149.02 (C-2), 141.29 (C-3), 137.32, 143.20 (C-4a, C-8a), 23.22, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.41 (C-1'), 37.90 (C-2'), 28.67 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H52O6 (568.3764). m/z 586.4086 [M+NH4]+, error: 0.20 ppm with the empirical formula C35H56NO6.
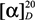 +23 (c 0.065, CHCl3); IR νmax (film) 2959, 1769, 1699, 1662, 1625, 1549, 1467, 1455, 1382, 1269, 1205, 1077, 913, 755 cm−1; 1H-NMR (CDCl3): δ 2.68 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.40 (2H, m, quinone H-5 and H-8), 2.28 (1H, m, H-14 β), 1.70 (2H, m, quinone H-6 and H-7), 1.59 (2H, m, H-1'), 1.48 (2H, m, H-2'), 1.25 (3H, s, H-18), 1.10 (3H, d, J = 6.8 Hz, H-16), 0.92 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 6.4 Hz, H-17), 0.80 (3H, s, H-20); 13C-NMR (CDCl3): 40.14 (C-1), 19.22 (C-2), 38.31 (C-3), 44.20 (C-4), 53.34 (C-5), 23.41 (C-6), 35.36 (C-7), 29.67 (C-8), 57.86 (C-9), 39.30 (C-10), 18.33 (C-11), 35.60 (C-12), 31.24 (C-13), 41.13 (C-14), 170.97 (C-15), 20.51 (C-16), 15.36 (C-17), 29.45 (C-18), 180.70 (C-19), 14.79 (C-20); Quinone: 184.36, 187.37 (C-1 and C-4), 149.02 (C-2), 141.29 (C-3), 137.32, 143.20 (C-4a, C-8a), 23.22, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.41 (C-1'), 37.90 (C-2'), 28.67 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H52O6 (568.3764). m/z 586.4086 [M+NH4]+, error: 0.20 ppm with the empirical formula C35H56NO6.
+23 (c 0.065, CHCl3); IR νmax (film) 2959, 1769, 1699, 1662, 1625, 1549, 1467, 1455, 1382, 1269, 1205, 1077, 913, 755 cm−1; 1H-NMR (CDCl3): δ 2.68 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.40 (2H, m, quinone H-5 and H-8), 2.28 (1H, m, H-14 β), 1.70 (2H, m, quinone H-6 and H-7), 1.59 (2H, m, H-1'), 1.48 (2H, m, H-2'), 1.25 (3H, s, H-18), 1.10 (3H, d, J = 6.8 Hz, H-16), 0.92 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.92 (3H, d, J = 6.4 Hz, H-17), 0.80 (3H, s, H-20); 13C-NMR (CDCl3): 40.14 (C-1), 19.22 (C-2), 38.31 (C-3), 44.20 (C-4), 53.34 (C-5), 23.41 (C-6), 35.36 (C-7), 29.67 (C-8), 57.86 (C-9), 39.30 (C-10), 18.33 (C-11), 35.60 (C-12), 31.24 (C-13), 41.13 (C-14), 170.97 (C-15), 20.51 (C-16), 15.36 (C-17), 29.45 (C-18), 180.70 (C-19), 14.79 (C-20); Quinone: 184.36, 187.37 (C-1 and C-4), 149.02 (C-2), 141.29 (C-3), 137.32, 143.20 (C-4a, C-8a), 23.22, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.41 (C-1'), 37.90 (C-2'), 28.67 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H52O6 (568.3764). m/z 586.4086 [M+NH4]+, error: 0.20 ppm with the empirical formula C35H56NO6.Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl 17β-dihydrojunicedrate methyl ester (18). Yellow resin; 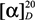 +43 (c 0.014 g/100 mL CHCl3); IR νmax (film) 2956, 2846, 1772, 1729, 1662, 1464, 1452, 1385, 1208, 1193, 1151, 1077, 910 cm−1; 1H-NMR (CDCl3): 21 δ 3.57 (3H, s, OCH3), 2.60 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.31 (2H, m, quinone H-5 and H-8), 2.30 (1H, m, H-14 β), 2.07 (1H, d, J = 12.4 Hz, H-13), 1.62 (2H, m, quinone H-6 and H-7), 1.53 (2H, m, H-1'), 1.10 (3H, s, H-18), 1.01 (3H, d, J = 6.8 Hz, H-16), 0.84 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.82 (3H, d, J = 7.2 Hz, H-17), 0.60 (3H, s, H-20); 13C-NMR (CDCl3): 40.22 (C-1), 19.46 (C-2), 38.55 (C-3), 44.32 (C-4), 53.31 (C-5), 23.42 (C-6), 35.34 (C-7), 29.65 (C-8), 57.89 (C-9), 39.08 (C-10), 19.27 (C-11), 35.62 (C-12), 31.26 (C-13), 41.13 (C-14), 170.97 (C-15), 20.52 (C-16), 15.33 (C-17), 29.26 (C-18), 178.53 (C-19), 14.66 (C-20), 51.54 (OCH3); Quinone: 180.73, 187.37 (C-1 and C-4), 149.03 (C-2), 141.30 (C-3), 137.33, 143.21 (C-4a, C-8a), 23.07, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.23 (C-1'), 37.91 (C-2'), 28.68 (C-3'), 22.70 (C-4'), 22.70 (C-5'); molecular formula: C36H54O6 (582.3920). m/z 600.4242 [M+NH4]+, error: 2.85 ppm with the empirical formula C36H58NO6.
+43 (c 0.014 g/100 mL CHCl3); IR νmax (film) 2956, 2846, 1772, 1729, 1662, 1464, 1452, 1385, 1208, 1193, 1151, 1077, 910 cm−1; 1H-NMR (CDCl3): 21 δ 3.57 (3H, s, OCH3), 2.60 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.31 (2H, m, quinone H-5 and H-8), 2.30 (1H, m, H-14 β), 2.07 (1H, d, J = 12.4 Hz, H-13), 1.62 (2H, m, quinone H-6 and H-7), 1.53 (2H, m, H-1'), 1.10 (3H, s, H-18), 1.01 (3H, d, J = 6.8 Hz, H-16), 0.84 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.82 (3H, d, J = 7.2 Hz, H-17), 0.60 (3H, s, H-20); 13C-NMR (CDCl3): 40.22 (C-1), 19.46 (C-2), 38.55 (C-3), 44.32 (C-4), 53.31 (C-5), 23.42 (C-6), 35.34 (C-7), 29.65 (C-8), 57.89 (C-9), 39.08 (C-10), 19.27 (C-11), 35.62 (C-12), 31.26 (C-13), 41.13 (C-14), 170.97 (C-15), 20.52 (C-16), 15.33 (C-17), 29.26 (C-18), 178.53 (C-19), 14.66 (C-20), 51.54 (OCH3); Quinone: 180.73, 187.37 (C-1 and C-4), 149.03 (C-2), 141.30 (C-3), 137.33, 143.21 (C-4a, C-8a), 23.07, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.23 (C-1'), 37.91 (C-2'), 28.68 (C-3'), 22.70 (C-4'), 22.70 (C-5'); molecular formula: C36H54O6 (582.3920). m/z 600.4242 [M+NH4]+, error: 2.85 ppm with the empirical formula C36H58NO6.
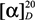 +43 (c 0.014 g/100 mL CHCl3); IR νmax (film) 2956, 2846, 1772, 1729, 1662, 1464, 1452, 1385, 1208, 1193, 1151, 1077, 910 cm−1; 1H-NMR (CDCl3): 21 δ 3.57 (3H, s, OCH3), 2.60 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.31 (2H, m, quinone H-5 and H-8), 2.30 (1H, m, H-14 β), 2.07 (1H, d, J = 12.4 Hz, H-13), 1.62 (2H, m, quinone H-6 and H-7), 1.53 (2H, m, H-1'), 1.10 (3H, s, H-18), 1.01 (3H, d, J = 6.8 Hz, H-16), 0.84 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.82 (3H, d, J = 7.2 Hz, H-17), 0.60 (3H, s, H-20); 13C-NMR (CDCl3): 40.22 (C-1), 19.46 (C-2), 38.55 (C-3), 44.32 (C-4), 53.31 (C-5), 23.42 (C-6), 35.34 (C-7), 29.65 (C-8), 57.89 (C-9), 39.08 (C-10), 19.27 (C-11), 35.62 (C-12), 31.26 (C-13), 41.13 (C-14), 170.97 (C-15), 20.52 (C-16), 15.33 (C-17), 29.26 (C-18), 178.53 (C-19), 14.66 (C-20), 51.54 (OCH3); Quinone: 180.73, 187.37 (C-1 and C-4), 149.03 (C-2), 141.30 (C-3), 137.33, 143.21 (C-4a, C-8a), 23.07, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.23 (C-1'), 37.91 (C-2'), 28.68 (C-3'), 22.70 (C-4'), 22.70 (C-5'); molecular formula: C36H54O6 (582.3920). m/z 600.4242 [M+NH4]+, error: 2.85 ppm with the empirical formula C36H58NO6.
+43 (c 0.014 g/100 mL CHCl3); IR νmax (film) 2956, 2846, 1772, 1729, 1662, 1464, 1452, 1385, 1208, 1193, 1151, 1077, 910 cm−1; 1H-NMR (CDCl3): 21 δ 3.57 (3H, s, OCH3), 2.60 (1H, dd, J = 14.8, 5.2 Hz, H-14 α), 2.31 (2H, m, quinone H-5 and H-8), 2.30 (1H, m, H-14 β), 2.07 (1H, d, J = 12.4 Hz, H-13), 1.62 (2H, m, quinone H-6 and H-7), 1.53 (2H, m, H-1'), 1.10 (3H, s, H-18), 1.01 (3H, d, J = 6.8 Hz, H-16), 0.84 (6H, d, J = 6.8 Hz, H-4' and H-5'), 0.82 (3H, d, J = 7.2 Hz, H-17), 0.60 (3H, s, H-20); 13C-NMR (CDCl3): 40.22 (C-1), 19.46 (C-2), 38.55 (C-3), 44.32 (C-4), 53.31 (C-5), 23.42 (C-6), 35.34 (C-7), 29.65 (C-8), 57.89 (C-9), 39.08 (C-10), 19.27 (C-11), 35.62 (C-12), 31.26 (C-13), 41.13 (C-14), 170.97 (C-15), 20.52 (C-16), 15.33 (C-17), 29.26 (C-18), 178.53 (C-19), 14.66 (C-20), 51.54 (OCH3); Quinone: 180.73, 187.37 (C-1 and C-4), 149.03 (C-2), 141.30 (C-3), 137.33, 143.21 (C-4a, C-8a), 23.07, 21.45 (C-5 and C-8), 22.30, 21.45 (C-6 and C-7), 23.23 (C-1'), 37.91 (C-2'), 28.68 (C-3'), 22.70 (C-4'), 22.70 (C-5'); molecular formula: C36H54O6 (582.3920). m/z 600.4242 [M+NH4]+, error: 2.85 ppm with the empirical formula C36H58NO6.Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate (23). Δ8(9) Junicedric acid (5, 228 mg, 0.678 mmol), oxalyl chloride (3.44 mg, 27.12 mmol), dihydroprenyl lapachol (2, 0.168 mg, 0.678 mmol) and triethylamine (TEA) (0.137 mg, 1.356 mmol) were dissolved in dry CH2Cl2 (DCM) under nitrogen flow. The reaction mixture was left at room temperature under stirring and inert atmosphere for two days. Then, the mixture was washed two times with water, and the aqueous phase extracted with DCM to obtain the crude reaction mixture. The extracts were dried over Na2SO4. Purification was carried out using silica gel column chromatography using PE:EtOAc 90:10 (v/v), to afford compound 23 (65.4 mg, 17%): orange resin; 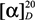 +31 (c 0.036, CHCl3); IR νmax (film) 2954, 2864, 1772, 1695, 1662, 1619, 1469, 1380 1263, 1207, 1134, 1114, 1077, 911 cm−1; 1H-NMR (CDCl3): δ 2.61 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.41 (4H, m, quinone H-5 and H-8), 2.40–2.35 (1H, m, 14 β), 2.40-2.33 (2H, m, H-1'), 2.08 (1H, m, H-13), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.30 (2H, m, H-2'), 1.24 (3H, s, H-18), 1.08 (3H, d, J = 6.6 Hz, H-16), 0.89 (3H, d, J = 6.6 Hz, H-4'), 0.89 (3H, d, J = 6.6, H-5'), 0.85 (3H, s, H-20), 13C-NMR (CDCl3): 37.22 (C-1), 19.89 (C-2), 37.38 (C-3), 44.18 (C-4), 53.92 (C-5), 21.45 (C-6), 37.16 (C-7), 127.18 (C-8), 139.35 (C-9), 40.13 (C-10), 26.13 (C-11), 34.62 (C-12), 31.75 (C-13), 41.35 (C-14), 170.87 (C-15), 19.88 (C-16), 20.11 (C-17), 29.04 (C-18), 180.68 (C-19), 18.29 (C-20); Quinone: 184.73, 187.36 (C-1 and C-4), 149.01 (C-2), 141.29 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.86 (C-5 and C-8), 21.45, 21.11 (C-6 and C-7), 22.28 (C-1'), 37.49 (C-2'), 28.66 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H50O6 (566.3607), m/z 567.3687 [M+H]+, error: 1.11 ppm with the empirical formula C35H51O6; m/z 584.3944 [M+NH4]+, error: 0.23 ppm with the empirical formula C35H54NO6.
+31 (c 0.036, CHCl3); IR νmax (film) 2954, 2864, 1772, 1695, 1662, 1619, 1469, 1380 1263, 1207, 1134, 1114, 1077, 911 cm−1; 1H-NMR (CDCl3): δ 2.61 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.41 (4H, m, quinone H-5 and H-8), 2.40–2.35 (1H, m, 14 β), 2.40-2.33 (2H, m, H-1'), 2.08 (1H, m, H-13), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.30 (2H, m, H-2'), 1.24 (3H, s, H-18), 1.08 (3H, d, J = 6.6 Hz, H-16), 0.89 (3H, d, J = 6.6 Hz, H-4'), 0.89 (3H, d, J = 6.6, H-5'), 0.85 (3H, s, H-20), 13C-NMR (CDCl3): 37.22 (C-1), 19.89 (C-2), 37.38 (C-3), 44.18 (C-4), 53.92 (C-5), 21.45 (C-6), 37.16 (C-7), 127.18 (C-8), 139.35 (C-9), 40.13 (C-10), 26.13 (C-11), 34.62 (C-12), 31.75 (C-13), 41.35 (C-14), 170.87 (C-15), 19.88 (C-16), 20.11 (C-17), 29.04 (C-18), 180.68 (C-19), 18.29 (C-20); Quinone: 184.73, 187.36 (C-1 and C-4), 149.01 (C-2), 141.29 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.86 (C-5 and C-8), 21.45, 21.11 (C-6 and C-7), 22.28 (C-1'), 37.49 (C-2'), 28.66 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H50O6 (566.3607), m/z 567.3687 [M+H]+, error: 1.11 ppm with the empirical formula C35H51O6; m/z 584.3944 [M+NH4]+, error: 0.23 ppm with the empirical formula C35H54NO6.
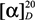 +31 (c 0.036, CHCl3); IR νmax (film) 2954, 2864, 1772, 1695, 1662, 1619, 1469, 1380 1263, 1207, 1134, 1114, 1077, 911 cm−1; 1H-NMR (CDCl3): δ 2.61 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.41 (4H, m, quinone H-5 and H-8), 2.40–2.35 (1H, m, 14 β), 2.40-2.33 (2H, m, H-1'), 2.08 (1H, m, H-13), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.30 (2H, m, H-2'), 1.24 (3H, s, H-18), 1.08 (3H, d, J = 6.6 Hz, H-16), 0.89 (3H, d, J = 6.6 Hz, H-4'), 0.89 (3H, d, J = 6.6, H-5'), 0.85 (3H, s, H-20), 13C-NMR (CDCl3): 37.22 (C-1), 19.89 (C-2), 37.38 (C-3), 44.18 (C-4), 53.92 (C-5), 21.45 (C-6), 37.16 (C-7), 127.18 (C-8), 139.35 (C-9), 40.13 (C-10), 26.13 (C-11), 34.62 (C-12), 31.75 (C-13), 41.35 (C-14), 170.87 (C-15), 19.88 (C-16), 20.11 (C-17), 29.04 (C-18), 180.68 (C-19), 18.29 (C-20); Quinone: 184.73, 187.36 (C-1 and C-4), 149.01 (C-2), 141.29 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.86 (C-5 and C-8), 21.45, 21.11 (C-6 and C-7), 22.28 (C-1'), 37.49 (C-2'), 28.66 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H50O6 (566.3607), m/z 567.3687 [M+H]+, error: 1.11 ppm with the empirical formula C35H51O6; m/z 584.3944 [M+NH4]+, error: 0.23 ppm with the empirical formula C35H54NO6.
+31 (c 0.036, CHCl3); IR νmax (film) 2954, 2864, 1772, 1695, 1662, 1619, 1469, 1380 1263, 1207, 1134, 1114, 1077, 911 cm−1; 1H-NMR (CDCl3): δ 2.61 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.41 (4H, m, quinone H-5 and H-8), 2.40–2.35 (1H, m, 14 β), 2.40-2.33 (2H, m, H-1'), 2.08 (1H, m, H-13), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.30 (2H, m, H-2'), 1.24 (3H, s, H-18), 1.08 (3H, d, J = 6.6 Hz, H-16), 0.89 (3H, d, J = 6.6 Hz, H-4'), 0.89 (3H, d, J = 6.6, H-5'), 0.85 (3H, s, H-20), 13C-NMR (CDCl3): 37.22 (C-1), 19.89 (C-2), 37.38 (C-3), 44.18 (C-4), 53.92 (C-5), 21.45 (C-6), 37.16 (C-7), 127.18 (C-8), 139.35 (C-9), 40.13 (C-10), 26.13 (C-11), 34.62 (C-12), 31.75 (C-13), 41.35 (C-14), 170.87 (C-15), 19.88 (C-16), 20.11 (C-17), 29.04 (C-18), 180.68 (C-19), 18.29 (C-20); Quinone: 184.73, 187.36 (C-1 and C-4), 149.01 (C-2), 141.29 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.86 (C-5 and C-8), 21.45, 21.11 (C-6 and C-7), 22.28 (C-1'), 37.49 (C-2'), 28.66 (C-3'), 22.71 (C-4'), 22.71 (C-5'); molecular formula: C35H50O6 (566.3607), m/z 567.3687 [M+H]+, error: 1.11 ppm with the empirical formula C35H51O6; m/z 584.3944 [M+NH4]+, error: 0.23 ppm with the empirical formula C35H54NO6.Dihydroprenyl-5,6,7,8-tetrahydrolapachoyl Δ8(9) junicedrate methyl ester (24). Yellow solid; 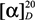 +22 (c 0.027, CHCl3); IR νmax (film) 2953, 2868, 1769, 1726, 1659, 1470, 1449, 1379, 1138, 1081, 916, 761 cm−1; 1H-NMR (CDCl3): δ 3.61 (3H, s, OCH3), 2.76 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.62 (1H, dd, J = 15.3, 8.1 Hz, H-14 β), 2.06 (1H, m, H-13), 2.40 (4H, m, quinone H-5 and H-8), 2.40-2.33 (2H, m, H-1'), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.27 (2H, m, H-2'), 1.18 (3H, s, H-18), 1.07 (3H, d, J = 6.8 Hz, H-16), 0.89 (3H, d, J = 6.4 Hz, H-4'), 0.89 (3H, d, J = 6.4, H-5'), 0.75 (3H, s, H-20), 13C-NMR (CDCl3): 37.63 (C-1), 19.98 (C-2), 37.89 (C-3), 44.28 (C-4), 53.93 (C-5), 21.25 (C-6), 37.62 (C-7), 127.18 (C-8), 139.42 (C-9), 39.95 (C-10), 26.12 (C-11), 34.71 (C-12), 31.75 (C-13), 41.36 (C-14), 170.88 (C-15), 19.89 (C-16), 20.14 (C-17), 28.86 (C-18), 178.55 (C-19), 18.14 (C-20), 51.51 (OCH3); Quinone: 180.69, 187.36 (C-1 and C-4), 149.00 (C-2), 141.28 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.71 (C-5 and C-8), 21.44, 21.44 (C-6 and C-7), 22.28 (C-1'), 38.13 (C-2'), 28.66 (C-3'), 22.84 (C-4'), 22.84 (C-5'); molecular formula: C36H52O6 (580.3764). m/z 581.3832 [M+H]+, error: 0.65 ppm with the empirical formula C36H53O6; m/z 598.4102 [M+NH4]+, error: 0.13 ppm with the empirical formula C36H56NO6.
+22 (c 0.027, CHCl3); IR νmax (film) 2953, 2868, 1769, 1726, 1659, 1470, 1449, 1379, 1138, 1081, 916, 761 cm−1; 1H-NMR (CDCl3): δ 3.61 (3H, s, OCH3), 2.76 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.62 (1H, dd, J = 15.3, 8.1 Hz, H-14 β), 2.06 (1H, m, H-13), 2.40 (4H, m, quinone H-5 and H-8), 2.40-2.33 (2H, m, H-1'), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.27 (2H, m, H-2'), 1.18 (3H, s, H-18), 1.07 (3H, d, J = 6.8 Hz, H-16), 0.89 (3H, d, J = 6.4 Hz, H-4'), 0.89 (3H, d, J = 6.4, H-5'), 0.75 (3H, s, H-20), 13C-NMR (CDCl3): 37.63 (C-1), 19.98 (C-2), 37.89 (C-3), 44.28 (C-4), 53.93 (C-5), 21.25 (C-6), 37.62 (C-7), 127.18 (C-8), 139.42 (C-9), 39.95 (C-10), 26.12 (C-11), 34.71 (C-12), 31.75 (C-13), 41.36 (C-14), 170.88 (C-15), 19.89 (C-16), 20.14 (C-17), 28.86 (C-18), 178.55 (C-19), 18.14 (C-20), 51.51 (OCH3); Quinone: 180.69, 187.36 (C-1 and C-4), 149.00 (C-2), 141.28 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.71 (C-5 and C-8), 21.44, 21.44 (C-6 and C-7), 22.28 (C-1'), 38.13 (C-2'), 28.66 (C-3'), 22.84 (C-4'), 22.84 (C-5'); molecular formula: C36H52O6 (580.3764). m/z 581.3832 [M+H]+, error: 0.65 ppm with the empirical formula C36H53O6; m/z 598.4102 [M+NH4]+, error: 0.13 ppm with the empirical formula C36H56NO6.
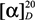 +22 (c 0.027, CHCl3); IR νmax (film) 2953, 2868, 1769, 1726, 1659, 1470, 1449, 1379, 1138, 1081, 916, 761 cm−1; 1H-NMR (CDCl3): δ 3.61 (3H, s, OCH3), 2.76 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.62 (1H, dd, J = 15.3, 8.1 Hz, H-14 β), 2.06 (1H, m, H-13), 2.40 (4H, m, quinone H-5 and H-8), 2.40-2.33 (2H, m, H-1'), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.27 (2H, m, H-2'), 1.18 (3H, s, H-18), 1.07 (3H, d, J = 6.8 Hz, H-16), 0.89 (3H, d, J = 6.4 Hz, H-4'), 0.89 (3H, d, J = 6.4, H-5'), 0.75 (3H, s, H-20), 13C-NMR (CDCl3): 37.63 (C-1), 19.98 (C-2), 37.89 (C-3), 44.28 (C-4), 53.93 (C-5), 21.25 (C-6), 37.62 (C-7), 127.18 (C-8), 139.42 (C-9), 39.95 (C-10), 26.12 (C-11), 34.71 (C-12), 31.75 (C-13), 41.36 (C-14), 170.88 (C-15), 19.89 (C-16), 20.14 (C-17), 28.86 (C-18), 178.55 (C-19), 18.14 (C-20), 51.51 (OCH3); Quinone: 180.69, 187.36 (C-1 and C-4), 149.00 (C-2), 141.28 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.71 (C-5 and C-8), 21.44, 21.44 (C-6 and C-7), 22.28 (C-1'), 38.13 (C-2'), 28.66 (C-3'), 22.84 (C-4'), 22.84 (C-5'); molecular formula: C36H52O6 (580.3764). m/z 581.3832 [M+H]+, error: 0.65 ppm with the empirical formula C36H53O6; m/z 598.4102 [M+NH4]+, error: 0.13 ppm with the empirical formula C36H56NO6.
+22 (c 0.027, CHCl3); IR νmax (film) 2953, 2868, 1769, 1726, 1659, 1470, 1449, 1379, 1138, 1081, 916, 761 cm−1; 1H-NMR (CDCl3): δ 3.61 (3H, s, OCH3), 2.76 (1H, dd, J = 15.3, 5.5 Hz, H-14 α), 2.62 (1H, dd, J = 15.3, 8.1 Hz, H-14 β), 2.06 (1H, m, H-13), 2.40 (4H, m, quinone H-5 and H-8), 2.40-2.33 (2H, m, H-1'), 1.67 (4H, m, quinone H-6 and H-7), 1.56 (3H, s, H-17), 1.53 (1H, m, H-3'), 1.27 (2H, m, H-2'), 1.18 (3H, s, H-18), 1.07 (3H, d, J = 6.8 Hz, H-16), 0.89 (3H, d, J = 6.4 Hz, H-4'), 0.89 (3H, d, J = 6.4, H-5'), 0.75 (3H, s, H-20), 13C-NMR (CDCl3): 37.63 (C-1), 19.98 (C-2), 37.89 (C-3), 44.28 (C-4), 53.93 (C-5), 21.25 (C-6), 37.62 (C-7), 127.18 (C-8), 139.42 (C-9), 39.95 (C-10), 26.12 (C-11), 34.71 (C-12), 31.75 (C-13), 41.36 (C-14), 170.88 (C-15), 19.89 (C-16), 20.14 (C-17), 28.86 (C-18), 178.55 (C-19), 18.14 (C-20), 51.51 (OCH3); Quinone: 180.69, 187.36 (C-1 and C-4), 149.00 (C-2), 141.28 (C-3), 137.32, 143.21 (C-4a, C-8a), 23.22, 22.71 (C-5 and C-8), 21.44, 21.44 (C-6 and C-7), 22.28 (C-1'), 38.13 (C-2'), 28.66 (C-3'), 22.84 (C-4'), 22.84 (C-5'); molecular formula: C36H52O6 (580.3764). m/z 581.3832 [M+H]+, error: 0.65 ppm with the empirical formula C36H53O6; m/z 598.4102 [M+NH4]+, error: 0.13 ppm with the empirical formula C36H56NO6.3.3. Cell Culture
Human lung fibroblasts MRC-5 (ATCC CCL-171) were grown in minimum essential Eagle medium (MEM) with Earles’s salts and 2 mM L-glutamine (Sigma-Aldrich Co.). Human epithelial gastric cells AGS (ATCC CRL-1739) were grown in Ham F-12 medium containing 1 mM l-glutamine. Both media were supplemented with 1.5 g/L sodium bicarbonate (Sigma-Aldrich Co.), 10% heat-inactivated fetal bovine serum (FBS), 100 IU/mL penicillin and 100 µg/mL streptomycin.Both cell lines were grown as monolayers in a humidified incubator with 5% CO2 in air at 37 °C. Culture media, antibiotics and FBS were obtained from Invitrogen Corp.
3.4. Cytotoxicity Assay
Cytotoxicity values were required as a reference to determine the working concentrations in the subsequent experiments. Briefly, confluent cultures of MRC-5 and AGS cells were treated during 24 h with medium containing the compounds at concentrations ranging from 0 up to 1,000 µM. The products were first dissolved in DMSO (1% final concentration) and then in the corresponding culture medium supplemented with 2% FBS. Untreated cells were used as controls. Cell viability was determined at the end of the incubation by means of the neutral red uptake (NRU) assay [27]. Lansoprazole (Sigma-Aldrich Co., min. 98% by TLC) was used as reference compound.
3.5. MRC-5 Fibroblast Proliferation Assay
One day after seeding, cells were treated during 4 days with medium supplemented with 10% FBS and the studied compounds at concentrations ranging from 1/64 up to 1/2 of the respective IC50 values. Untreated cells were used as controls. Cell viability was determined at the end of the incubation by means of the NRU assay [27].
3.6. Sodium Taurocholate-Induced Damage to AGS Cells
The effect of sodium taurocholate (NaT) on cell viability was determined according to [15]. Briefly, one day post-confluent AGS cells were incubated during 60 min with the compounds at 1/2, 1/4 and 1/8 of the respective IC50 values. Then, 10 mM NaT was added to all wells for 30 min. Untreated cells were used as controls. Sucralfate (4 mg/mL; 580 µM) was used as reference compound (Sigma-Aldrich Co., min. 30% sucrose octasulfate by HPLC). After incubation, the NRU assay was carried out [27].
3.7. Determination of Prostaglandin E2 (PGE2) Content
One day after confluence, AGS cells were treated for 1 h with the compounds at 1/2 and 1/4 of the respective IC50 values. A control with medium only was included. Indomethacin (100 μM) was used as standard inhibitor of prostaglandin synthesis (Sigma-Aldrich Co., min. 99% by TLC). After incubation, PGE2 content was determined by means of a specific enzyme immunoassay kit (R & D Systems KGE004B, Minneapolis, MN, USA) and values were calculated according to the manufacturer instructions. Results are expressed as pg/well.
3.8. Determination of Cellular Reduced Glutathione (GSH) Content
One day after confluence, AGS cells were incubated during 4 h with the compounds at 1/4 and 1/8 of their respective IC50 values. Untreated cells were used as controls. The GSH synthesis stimulant N-acetyl-l-cysteine (750 µM) was used as reference substance (Sigma-Aldrich Co., ≥ 90% by HPLC). After incubation, the GSH content was determined using a colorimetric kit (BioAssays Systems, Hayward, CA, USA). Results are expressed as nanomol of soluble reduced sulfhydryls/106 cells.
3.9. Inhibition of Lipoperoxidation in Erythrocyte Membranes
The inhibition of lipid peroxidation was determined using human erythrocyte membranes [28]. The compounds were tested at 100 µg/mL. Catechin was used as the reference compound (Sigma-Aldrich Co., min 98% by TLC).
3.10. Statistical Analysis
Results were expressed as mean values ± SD. Experiments with MRC-5 and AGS cells were carried out three times using different cell preparations. Each concentration was tested in quadruplicate. Statistical differences between different treatments and their respective control were determined by one-way analysis of variance (ANOVA) followed by the Dunnett’s multiple comparison test. The level of significance was set at p < 0.05 and p < 0.01. All statistical analyses were carried out using the software SPSS 12.0 for Windows.
4. Conclusions
The aim of this work was to synthesize new diterpenylquinones, to assess the possible mechanisms of the gastroprotective effects of the hybrid compounds using cell cultures and to establish some structure-activity trends. The hybrid compounds displayed activities different from those shown by the starting compounds, supporting the potential of this approach in the search for new bioactive molecules. These effects were modulated by selective modification in the terpene or quinone moieties of the hybrids. The changes in effect associated to the stereochemistry of the diterpenes (i.e., a more flexible configuration for diterpenes with a 8(17) double bond or the 17-β-dihydroderivative vs. the Δ8(9) isomer) or with modifications in the planar configuration of the napththoquinones, suggest that the different mechanisms involved might include interactions with active sites of enzymes and/or receptors. Molecular modeling can be a useful tool for better understanding the clues behind the observed effects.
Acknowledgments
Financial support by FONDECYT (Project N 1110054) is kindly acknowledged. I.B. is grateful to CONICYT for a doctoral grant (NAC-Doctorado N 21120593). We are indebted to Irene Manriquez for her skilful technical work.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Schmeda-Hirschmann, G.; Astudillo, L.; Rodriguez, J.A.; Theoduloz, C.; Yañez, T. Gastroprotective effect of the Mapuche crud drug Araucaria araucana resin and its main constituents. J. Ethnopharmacol. 2005, 101, 271–276. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Papastergiou, F. Naphthoquinone derivatives and lignans from the Paraguayan crude drug “tayï pytá” (Tabebuia heptaphylla, Bignoniaceae). Z. Naturforsch. C 2003, 58, 495–501. [Google Scholar]
- Twardowschy, A.; Freitas, C.S.; Baggio, C.H.; Mayer, B.; dos Santos, A.C.; Pizzolatti, M.G.; Zacarias, A.A.; Pereira dos Santos, E.; Otuki, M.F.; Marques, M.C.A. Antiulcerogenic activity of bark extract of Tabebuia avellanedae, Lorentz ex Griseb. J. Ethnopharmacol. 2008, 118, 455–459. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Astudillo, L.; Sepulveda, B.; Rodriguez, J.A.; Theoduloz, C.; Yañez, T.; Palenzuela, J.A. Gastroprotective effect and cytotoxicity of natural and semisynthetic labdane diterpenes from Araucaria araucana. Z. Naturforsch. C 2005, 60, 511–522. [Google Scholar]
- Muregi, F.W.; Ishih, A. Next-generation antimalarial drugs: hybrid molecules as a new strategy in drug design. Drug. Develop. Res. 2010, 71, 20–32. [Google Scholar]
- Pertino, M.W.; Theoduloz, C.; Palenzuela, J.A.; Afonso, M.; Yesilada, E.; Monsalve, F.; González, P.; Droguett, D.; Schmeda-Hirschmann, G. Synthesis and pharmacological activity of diterpenylnaphthoquinone derivatives. Molecules 2011, 16, 8614–8628. [Google Scholar] [CrossRef]
- Theoduloz, C.; Carrión, I.B.; Pertino, M.W.; Schmeda-Hirschmann, G. Potential gastroprotective effect of novel cyperenoic acid/quinone derivatives in human cell cultures. Planta Med. 2012, 78, 1807–1812. [Google Scholar] [CrossRef]
- Dictionary of Natural Products [DVD]; CRC Press: Boca Raton, FL, USA, 2013.
- Miguel del Corral, J.M.; Castro, M.A.; Rodriguez, M.L.; Chamorro, P.; Cuevas, C.; Feliciano, A.S. New cytotoxic diterpenylnaphthohydroquinone derivatives obtained from a natural diterpenoid. Bioorg. Med. Chem. 2007, 15, 5760–5774. [Google Scholar] [CrossRef]
- Miguel del Corral, J.M; Castro, M.A.; Oliveira, A.B.; Gualberto, S.A.; Carmen, C.; Feliciano, A.S. New cytotoxic furoquinones obtained from terpenyl-1,4-naphthoquinones and 1,4-anthracenediones. Bioorg. Med. Chem. 2006, 14, 7231–7240. [Google Scholar] [CrossRef]
- Castro, M.A.; Gamito, A.M.; Tangarife-Castaño, V.; Zapata, B.; del Corral, J.M.; Mesa-Arango, A.C.; Betancur-Galvis, L.; San Feliciano, A. Synthesis and antifungal activity of terpenyl-1,4-naphthoquinone and 1,4-anthracenedione derivatives. Eur. J. Med. Chem. 2013, 67, 19–27. [Google Scholar] [CrossRef]
- Kokoska, E.; Smith, G.; Deshpande, Y.; Wolff, A.; Miller, T. Indomethacin increasessusceptibility to injury in human gastric cells independent of PG synthesis inhibition. Am. J. Physiol. 1998, 275G, 620–628. [Google Scholar]
- Rodríguez, J.A.; Theoduloz, C.; Sánchez, M.; Razmilic, I.; Schmeda-Hirschmann, G. Gastroprotective and ulcer-healing effect of new solidagenone derivatives in human cell cultures. Life Sci. 2005, 77, 2193–205. [Google Scholar] [CrossRef]
- Ye, Y.N.; Liu, E.S.; Koo, M.W.; Li, Y.; Matsui, H.; Ch, C.H. A mechanistic study of proliferation induced by Angelica sinensis in a normal gastric epithelial cell line. Biochem. Pharmacol. 2001, 61, 1439–1448. [Google Scholar] [CrossRef]
- Romano, M.; Razandi, M.; Ivey, K. Effect of sucralfate and its components on taurocholate induced damage to rat gastric mucosal cells in tissue culture. Digest. Dis. Sci. 1990, 35, 467–476. [Google Scholar] [CrossRef]
- Ham, M.; Akiba, Y.; Takeuchi, K.; Montrose, M.H.; Kaunitz, J.D. Gastroduodenal Mucosal Defense. In Physiology of the Gastrointestinal Tract, 5th ed.; Johnson, L., Ed.; Academic Press: New York, USA, 2012; Volume 1, pp. 1169–1208. [Google Scholar]
- de-Faria, F.M.; Alves Almeida, A.C.; Luiz-Ferreira, A.; Dunder, R.J.; Takayama, C.; da Silva, M.S.; da Silva, M.A.; Vilegas, W.; Leite Rozza, A.; Pellizzon, C.H.; et al. Mechanisms of action underlying the gastric antiulcer activity of the Rhizophora mangle L. J. Ethnopharmacol. 2012, 139, 234–243. [Google Scholar] [CrossRef]
- Arakawa, T.; Higuchi, K.; Takashi, F. Prostaglandins in the stomach: an update. J. Clin. Gastroenterol. 1998, 27, S1–S11. [Google Scholar] [CrossRef]
- Kinoshita, Y.; Ishihara, S. Role of growth factors in the repair of gastric mucosal injury. J. Gastroenterol. 2004, 39, 202–203. [Google Scholar] [CrossRef]
- Rodríguez, J.A.; Theoduloz, C.; Yáñez, T.; Becerra, J.; Schmeda-Hirschmann, G. Gastroprotective effect of the diterpene ferruginol in mice: Protection against membrane lipid peroxidation and involvement of prostaglandins. Life Sci. 2006, 78, 2503–2509. [Google Scholar] [CrossRef]
- Mutoh, H.; Hiraishi, H.; Ota, H.; Yoshida, H.; Ivey, K.; Terano, A.; Sugimoto, T. Protective role of extracellular glutathione against ethanol-induced damage in cultured rat gastric mucosal cells. Gastroenterology 1990, 98, 1452–1459. [Google Scholar]
- Matsuda, H.; Pongpiriyadacha, Y.; Morikawa, T.; Kashima, Y.; Nakano, K.; Yoshikawa, M. Protective effects of polygodial and related compounds on ethanol-induced gastric mucosal lesions in rats: Structural requirements and mode of action. Bioorg. Med. Chem. Lett. 2002, 12, 477–482. [Google Scholar] [CrossRef]
- Kokoska, E.R.; Smith, G.S.; Deshpande, Y.; Wolff, A.B.; Rieckenberg, C.; Miller, T.A. Calcium accentuates injury induced by ethanol in human gastric cells. J. Gastro. Surg. 1999, 3, 308–318. [Google Scholar] [CrossRef]
- Smolka, A.J.; Goldenring, J.R.; Gupta, S.; Hammond, C.E. Inhibition of gastric H,K-ATPase activity and gastric epithelial cell IL-8 secretion by pyrrolizidine derivative ML 3000. BMC Gastroenterol. 2004, 10, 4–5. [Google Scholar]
- Singh, P.; Paul, K.; Holzer, W. Synthesis of pyrazole-based hybrid molecules: Search for potent multidrug resistance modulators. Bioorg. Med. Chem. 2006, 14, 5061–5071. [Google Scholar] [CrossRef]
- Morphy, R.; Rankovic, Z. Multi-target drugs: Strategies and challenges for medicinal chemists. In The Practice of Medicinal Chemistry; Wermuth, C.G., Ed.; Elsevier-Academic Press: San Diego, CA, USA, 2008; pp. 549–571. [Google Scholar]
- Rodríguez, J.A.; Haun, M. Cytotoxicity of trans-dehydrocrotonin from Croton cajucara (Euphorbiaceae) on V79 cells and rat hepatocytes. Planta Med. 1999, 65, 522–526. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Rodríguez, J.A.; Theoduloz, C.; Astudillo, L.; Feresin, G.E.; Tapia, A. Free radical scavengers and antioxidants from Peumus boldus Mol. (“Boldo”). Free Radical Res. 2003, 37, 447–452. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the starting products are available from the authors.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
