3. Experimental
3.1. General
All melting points were taken on an Electro thermal IA9000 series digital melting point apparatus. Elemental analysis data were obtained from the Micro analytical unit, Cairo University, Cairo. Egypt. The IR spectra (KBr) were recorded on the Elmer model 1430 spectrophotometer. 1H- and 13C-NMR spectra were recorded in CDCl3 on a Bruker Avance DRX-500 spectrometer (1H at 500 MHz and 13C at 125 MHz). ESIMS and HRESIMS experiments were performed using a Micromass Q-TOF (Manchester, UK). TLC was carried out on precoated silica gel 60 F254 (Merck, Munich, Germany) and spots were visualized by UV lamb. Column chromatography was carried out on silica gel 60 (63–200 μm, Merck).
3.2. 3-(2-Methylbutyl-3-yn-2-yloxy)phenol (2)
To a solution of resorcinol (0.01 mol) and potassium carbonate (0.01 mol) in acetone (30 mL), 3-chloro-2-methylbut-1-yne (0.01 mol) was added drop wise under nitrogen. The reaction mixture was heated and stirred at 70 °C for 72 h and monitored by TLC. After cooling to room temperature the solution was poured in water (100 mL) then acidified with dilute HCl (5%) to remove the residue of K2CO3 and extracted by diethyl ether (4 × 15 mL). The mixture was dried over anhydrous sodium sulphate, filtered and then the solvent was evaporated and the crude product was purified by column chromatography over silica gel (100–200 mesh) and eluted with mixtures of hexane/ethyl acetate (1:1 v/v) as eluent to give compound 2, which was crystallized from dichloromethane/hexane as solvent system to give pale white crystals (40%) with mp 160–162 °C; IR (KBr) νmax cm−1: 3,540, 3,352, 1,335, 1,119, 921, 849; 1H-NMR (CDCl3): δ 7.18 (Ar-1H, m, J = 9.2 Hz, H-5), 6.58 (Ar-1H, m, J = 9.1 Hz, H-4), 6.51 (Ar-1H, m, J = 8.9 Hz, H-2,6), 3.51 (1H, s, ≡CH), 1.69 (6H, s, 2CH3); 13C-NMR (CDCl3): 160.1 (C-3), 157.4 (C-1), 130.5 (C-5), 107.8 (C-4), 102.7 (C-2), 85.5 (C≡CH), 81.7 [C(CH3)2], 75.4 (≡CH), δ 29.2 (2CH3); EIMS (C11H12O2) (% int.) m/z 176 (100), 205 [M–H]+ (25), 190 [M–OH]+ (21), 176 [M–2CH3]+ (8) and 107 [M–HC≡C−C(CH3)2−O]+ (12); Analysis: calcd. %C, 74.98; %H, 6.83; %O, 18.18. Found: %C 74.88; %H, 6.81; %O 18.09.
3.3. 2,2-Dimethyl-2H-chromen-5-ol (3)
Compound 2 (0.01 mole) was boiled in dry xylene for 8 h under a nitrogen atmosphere, the reaction mixture was cooled, and then extracted with dichloromethane. The residue after removing the solvent was subjected to column chromatography over silica gel (800 g) and eluted with mixtures of hexane/ethyl acetate (1:2 v/v) as eluent to produce compound 3, which was crystallized from ethanol to give white crystals (60%) with mp 155–156 °C; IR (KBr) νmax cm−1: 3,540, 3,352, 1,335, 1,129, 931, 841; 1H-NMR (CDCl3): δ 11.2 (s, OH exchangeable with D2O), 7.13 (Ar-1H, m, H-7), 6.86 (Ar-1H, m, J = 9.2 Hz, H-4), 6.55 (Ar-1H, d, J = 8.9 Hz, H-8), 6.31 (Ar-1H, d, J = 8.9 Hz, H-6), 5.91 (Ar-1H, d, J = 9.1 Hz, H-3), 1.59 (3H, s, 2CH3); 13C-NMR (CDCl3): δ 156.7 (C-5), 155.5 (C-8a), 130.4 (C-7), 128.5 (C-3), 116.2 (C-4), 109.8 (C-4a), 109.7 (C-6), 106.5 (C-8), 85.7 (C-2), 28.5 (2CH3, C-9,10); EIMS (C11H12O2) (% int.) m/z 176 (100), 175 [M–H]+ (32), 160 [M–OH]+ (21) and 146 [M–2CH3]+ (12); Analysis: calcd. %C, 74.96; % H, 6.85; %O, 18.19. Found: %C 74.91; %H, 6.82; %O 18.12.
3.4. 2,2-Dimethyl-2H-chromene-5,8-dione (4)
To a solution of KH2PO4 (10 g) in water (200 mL) was mechanically stirred in a 2 L round bottom flask and ice (200 g) was added compound 3 (0.01 mol). The flask was cooled in an ice-ethanol mixture and (KSO3)2NO (6 g, 22.4 mmol) added, followed by a solution of 4-(methylmercapto)phenol (1.0 g, 7.1 mmol) in diethyl ether (20 cm3). After 1 min the solution turned orange and after 5 min dark red. After 1 h the mixture was evaporated at 20 °C to remove the diethyl ether and extracted with chloroform (3 × 50 cm3). Evaporation gave a crimson solid (0.95 g, 86% yields) with mp 180–181 °C; IR (KBr) νmax cm−1: 1,750, 1,735, 1,343, 1,139, 951, 841; 1H-NMR (CDCl3): δ 6.93 (Ar-1H, d, H-6,7), 6.44 (Ar-1H, s, H-4), 5.81 (Ar-1H, d, J = 9.2 Hz, H-3), 1.42 (3H, s, 2CH3); 13C-NMR (CDCl3): δ 185.5 (C-5), 181.7 (C-8), 150.4 (C-8a), 136.2 (C-6), 135.5 (C-7), 132.8 (C-3), 119.7 (C-4a), 116.5 (C-4), 85.9 (C-2), 28.7 (2CH3, C-9,10); EIMS (C11H10O3) (% int.) m/z 190 (100), 175 [M–1CH3] + (22), 160 [M–2CH3] + (9), 162 [M–CO] (41); Analysis: calcd. %C, 69.46; %H, 5.30; %O, 25.22. Found: %C 69.41; %H, 5.22; %O 25.12.
3.5. 2,2-Dimethyl-2H-chromene-5,7,8-triyltriacetate (5)
Acetylation was carried out following literature method [
5]. In brief, compound
4 (0.01 mol) was dissolved in refluxing
p-dioxane (200 mL) and acetic anhydride (0.03 mol) for 8 h. After cooling, the acetylated compound solution was concentrated and dried under reduced pressure. The crude acetylated lignin was dissolved in chloroform (3 mL) and the solution was added with stirring to diethyl ether. The precipitated acetate was centrifuged, collected, and dried under high vacuum for 24 h. The precipitate formed after cooling the mixture on ice water solution was recrystallized from ethanol give compound
5 as white crystals (0.61 g, 50%) with mp 277–279 °C. IR (KBr)
νmax cm
−1: 1,763, 1,750 and 1,710 (3 C=O ester), 1,576, 1,345, 1,129, 911, 849;
1H-NMR (CDCl
3): δ 6.88 (Ar-1H, d,
J = 9.9 Hz, H-4), 6.42 (Ar-1H, d,
J = 9.1 Hz, H-6), 5.88 (Ar-1H, d,
J = 8.5 Hz, H-3), 2.28, 2.2, 2.1 (s, 9H, 3 C
H3CH
2O), 1.49 (6H, s, 2CH
3);
13C-NMR (CDCl
3): δ 169.1 (3C=O), 153.2 (C-8a), 147.2 (C-5), 144.2 (C-7), 130.1 (C-8), 127.4 (C-3), 114.1 (C-4a), 116.5 (C-4), 103.5 (C-6), 86.7 (C-2), 28.2 (2CH
3, C-9,10), 20.5 (3CH
3-ester); EIMS (C
17H
18O
7) (% int.)
m/z 334 (100), 319 [M–1CH
3]
+ (34), 304 [M–2CH
3]
+ (21) and 199 [M–3OAc] (14)”; Analysis: calcd. %C, 61.08; %H, 5.43; %O, 31.48. Found: %C 60.88; %H, 5.31; %O 31.59.
3.6. 7-Hydroxyl-2,2-dimethyl-2H-chromene-5,8-dione (6)
Compound 6 was obtained by dissolving (0.1 mol) of compound 5 in 10% NaOH solution, then by addition of HCl, we obtain a precipitate, which is filtered off , then transferred into a conical flask and water (30 mL) is added. The mixture is brought to its boiling point, FeCl3 (1.5 g) is added and boiled for 10 min, the hot solution is filtered, washed with boiling water and he precipitate formed dried in air. Recrystallization from toluene gives brown crystals (40%) with mp 254–255 °C; UV (MeOH) λmax: 331 (log ε 3.67), 292 (log ε 4.07), 262 (log ε 4.45); IR (KBr) νmax cm−1: 3,340, 1,752, 1,722, 1,576, 1,345, 1,129, 911, 849; 1H-NMR (CDCl3): δ 16.2 (1H, s, 1OH), 6.83 (Ar-1H, d, J = 9.1 Hz, H-2), 6.42 (Ar-1H, d, J = 9.2 Hz, H-8), 6.81 (Ar-1H, s, H-6), 6.41 (Ar-1H, d, J = 9.2 Hz, H-4), 5.81 (Ar-1H, d, J = 8.4 Hz, H-3), 1.39 (6H, s, H-10,11); 13C-NMR (CDCl3): δ 182.5 (C-5), 196.2 (C-8), 176.5 (C-7), 141.2 (C-8a), 126.5 (C-3), 117.4 (C-4a), 115.5 (C-4), 109.5 (C-6), 86.7 (C-2), 29.5 (2CH3, C-9,10); EIMS (C11H10O4) (% int.) m/z 206 (100), 205 [M–H] + (21), 190 [M–OH] + (24) and 176 [M–2CH3] + (11); Analysis: calcd. %C, 64.08; %H, 4.83; %O, 31.88. Found: %C 64.12; %H, 4.81; %O 31.09.
3.7. 2,2-Dimethyl-2H-chromene-5,7,8(6H)-trione (7)
Compound 7 was obtained by the same procedure described for the synthesis of compound 4 as faint brown crystals (0.59 g, 40% yield) with mp 187–189 °C; IR (KBr) νmax cm−1: 1,750, 1,720, 1,711, 1,581, 1,345, 1,099, 901, 869; 1H-NMR (CDCl3): δ 6.82 (Ar-1H, d, J = 9.2 Hz, H-4), 5.84 (Ar-1H, d, J = 8.2 Hz, H-3), 4.07 (Ar-1H, s, H-6), 1.44(6H, s, 2CH3); 13C-NMR (CDCl3): δ 176.5 (C-8), 196.2 (C-5,7), 141.2 (C-8a), 117.4 (C-4a), 126.5 (C-3), 115.5 (C-4), 45.8(C-6),86.7 (C-2), 29.5 (2CH3, C-9,10); EIMS (C11H10O4) (% int.) m/z 206 (100), 178 [M–CO] + (34) and 176 [M–2CH3] + (27); Analysis: calcd. %C, 64.06; %H, 4.88; %O, 31.04. Found: %C 63.92; %H, 5.81; %O 30.89.
3.8. 4,4,8,8-Tetramethyl-4,8-dihydro-2H-dipyrano-benzo-1,2-dione (8)
Compound 8 was obtained by the same procedures described for the synthesis of compounds 2 and 3, respectively, by refluxing compound 7 (0.01 mol) with 3-chloro-3-methylbut-1-yne (0.01 mol), followed by ring closure to give the di pyrano derivative in dry xylene for 8 h, then the precipitate obtained was filtered off and crystallized from a mixture of cyclohexane and ethanol give compound 8 as faint brown crystals (0.85 g, 40% yields) with mp 292–294 °C; UV (MeOH) λmax: 462 (log ε 3.75), 302 (log ε 3.97), 271 (log ε 4.47), 261 (log ε 4.43); IR (KBr) νmax cm−1: 1,789, 1,754, 1,598, 1,375, 1,049, 911, 859; 1H-NMR (CDCl3): δ 6.75 (d, J = 8.2, Ar-1H, H-10), 6.48 (d, J = 8.3 Hz, Ar-1H, H-6), 5.89 (d, J = 9.2 Hz, Ar-1H, H-9), 5.84 (Ar-1H, d, H-5), 1.34 (12H, s, 4CH3); 13C-NMR (CDCl3): δ 179.4 (C-1), 176.6 (C-2), 173.2 (C-6b), 131.3 (C-9), 130.1 (C-2a), 127.5 (C-6a), 125.5 (C-5), 97.1 (C-10a), 88.9 (C-8), 88.5 (C-4), 29.5 (2CH3, C-13,14), 28.7 (2 CH3; C-11,12); EIMS (C16H16O4) (% int.) m/z 272 (100), 257 [M–1CH3]+ (31), 244 [M–CO]+ (17) and 242 [M–2CH3]+ (14); Analysis: calcd. %C, 70.54; %H, 5.95; %O, 23.50. Found: %C 70.32; %H, 5.81; %O 23.39.
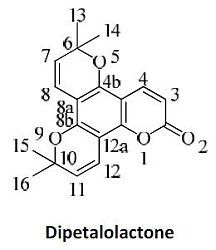
3.9. 6,6,10,10-Tetramethyl-6,10-dihydro-2H-dipyrano[2,3-f:2',3'-h]chromen-2-one (10)
Wittig reaction was carried out as described in the discussion [
6] using 0.01 mol of compound
8. 30% Aqueous NaOH solution (30 mL) was added to the Wittig reaction product and the mixture was boiled for 3 h, cooled, acidified with ice cold hydrochloric acid, and the reaction mixture refluxed for another 2 h. The precipitate obtained after cooling was filtered off, washed with water and crystallized from dil. ethanol to give compound
10 through the intermediate
9 as pale brown crystals (1.24 g, 50% yield) with mp 295–296 °C; UV (MeOH)
λmax: 211 (log ε 4.27), 282 (log ε 3.75) and 232 (log ε 3.97); IR (KBr)
νmax cm
−1: 1,720, 1,711, 1,591, 1,345, 1,089, 901, 869;
1H-NMR (CDCl
3): δ 7.75 (Ar-1H, d,
J = 8.95 Hz, H-4), 6.88 (Ar-1H, d,
J = 8.9 Hz, H-12), 6.84 (Ar-1H, d,
J = 9.2 Hz, H-8), 5.97 (Ar-1H, s, H-3,11), 1.84 (3H, s, H-13,14,15,16);
13C-NMR (CDCl
3): 151.9 (C-12b), 149.8 (C-8b), 148.9 (C-4b), 139.5 (C-4), 128.5 (C-11), 128.2 (C-7,12), 116.4 (C-8), 113.6 (C-3), 108.2 (C-8a), 105.3 (C-4a), 102.5 (C-12a), 86.5 (C-6,10), 28.6 (4 CH
3;C-13,14,15,16) as lit. [
6]; EIMS (C
19H
18O
4) (% int.)
m/z 310. (100), 292 [M–CO]
+ (32), 285 [M–1CH
3]
+ (21) and 250 [M–4CH
3]
+ (7); Analysis: calcd. %C, 73.54; %H, 5.85; %O, 20.64. Found: %C 73.32; %H, 5.81; %O 20.29.
3.10. Biological Activity
3.10.1. Experimental Animals
Young adult (30 ± 5 g) ICR mice (half male and half female) were provided by the Egyptian Holding Company for Biological Products and Vaccines, Cairo, Egypt. Animals were maintained under standard conditions of ventilation, temperature (25 ± 2 °C), humidity (60%–70%) and light/dark condition (12/12 h). The rats were housed in stainless steel cages and provided with free access to food and drinking water ad libitum.
3.10.2. Effect of Compound 10 on the Tumor of S180-Bearing Mice
The effect of compound
10 solution on tumor growth was estimated by evaluating body weight, tumor weight, and percentage of tumor inhibition. S180 tumor cell line was originally obtained from Cairo Institute of Oncology, Cairo, Egypt and maintained as the ascites form by serial passages intraperitoneal in ICR mice. For solid tumor development, S180 cell suspension (0.2 mL, 2 × 10
7 cells/mL) was inoculated subcutaneously into right armpits of mice under sterile condition. The mice were divided into six random groups (10 in each): S180-bearing control, normal control, compound 10 (50, 100, 250 and 500 mg/kg body weight). Test doses were decided on the basis of findings from preliminary studies. Body weight of animals was recorded before the experiment. The doses administrated p.o. daily for 12 days. Normal control and S180-bearing control groups received the same volume of normal saline. On the 13th day, all animals were euthanized. Their body and tumor weights were obtained and documented [
11].
3.10.3. Assessment of Humoral Immune Function: Quantitative Hemolysis of Sheep Red Blood Cells (QHS) Assay
The mice were injected i.p. with 3:5 (v/v) sheep red blood cells (SRBC, 0.2 mL) prepared in normal saline on the 8th day of the experiment. QHS assay was performed in those animals following the immunization. Eyeballs were removed and single cell suspensions of 1 × 10
6/mL were prepared in phosphate buffer solution (PBS). A total of 1.0 mL of 0.4% SRBC and 1.0 mL of 10% guinea pig serum were mixed with cell suspension and incubated for 1 h at 37 °C. After a 3 min centrifugation at 3,000 rpm, the absorbance of the supernatant was measured at 413 nm using a spectrophotometer [
12].
3.10.4. Assessment of Cellular Immune Function
For the assessment of cellular immune function, lymphocyte proliferation and Natural Killer (NK) cell cytotoxicity tests were performed. After the experiment was completed, their spleens were aseptically removed and filtered over a double layer of stainless-steel mesh to obtain single cell suspension. After these washes in Hanks’ balanced salt solution, the spleen cells were finally suspended in 10% FCS RPMI 1640 media supplemented with benzyl penicillin 100 U/mL, streptomycin 100 μg/mL. The cell number was adjusted to 3 × 10
6 cells/mL of culture media for subsequent experiments [
12].
3.10.4.1. Measurement of Lymphocyte Proliferation
For the splenocyte proliferation assay, the spleen cell suspension was added to micro plate wells with 5 μg/mL of concanavalin A (Con A, from
Canavalia ensiformis Type III, Sigma, Munich, Germany) and a polyclonal T cell mitogen. The micro plates were cultured at 37 °C for 72 h in the humidified 5% CO
2 incubator. At 72 h, 1 μ
Ci/well
3H-TdR (thymidine, [methl-
3H]) was added to each well. The cells were harvested 16 h later and the radioactivity incorporated was counted using a liquid scintillation counter [
12].
3.10.4.2. Evaluation of NK Cell Cytotoxicity
The splenocyte prepared as described above were used as effector cells. YAC-1 cells, mice lymphoma sensitive to NK cells were used as target cells. Effectors and target cells resuspended in RPMI-1640 medium supplemented with 3% heat-inactivated fetal bovine serum were added to each well of a 96-well U-bottom micro culture plate in triplicate to obtain an effectors/target (E/T) ratio of 50:1, and incubated at 37 °C in the humidified 5% CO
2 incubator for 8 h. After centrifugation, the culture supernatants were admixed with lactate dehydrogenase (LDH) solution (100 μL/well) and the amount of released LDH was determined. The OD value of each well was measured at 490 nm using a spectrophotometer. The percentage of cytotoxicity generated by NK cells was calculated according to the following formula:
where OD
er (OD
experimental release) was the LDH release from co-cultures at an E/T ratio of 50:1; OD
esr (OD
effector spontaneous release) and OD
tsr (OD
target spontaneous release) were spontaneous LDH releases from effector and target cells incubated with medium alone, respectively; and OD
tmr (OD
target maximum release) was the maximum release from target cells lysed with the lysis solution [
13].
3.10.4.3. Assessment of Nonspecific Immune Function: Phagocytic Activity of Macrophage
Phagocytic activity of macrophages was used to assess the nonspecific immune function. The mice were injected i.p. with 0.5 mL 5% cock red blood cells (CRBC) 10 h prior to the last dose. On the 13th day of the experiment macrophages were obtained from the peritoneal exudates harvested by peritoneal lavage using sterile cold Hanks’ solution. The number of CRBC ingested by macrophages was counted in an optical microscope [
12].
3.11. Statistical Analysis
Data were expressed as the mean ± standard deviation (S.D.) in tables. Statistical analyses were carried out using the analysis of variance (ANOVA) and post-hoc tests for multiple comparisons. Differences were considered statistically significant at
p < 0.05 [
14].