Evolution of the Aroma Volatiles of Pear Fruits Supplemented with Fatty Acid Metabolic Precursors
Abstract
:1. Introduction
2. Results and Discussion
2.1. Contents of Linoleic Acid and Linolenic Acid for Pear Fruits Feeding on Linoleic Acid and Linolenic Acid
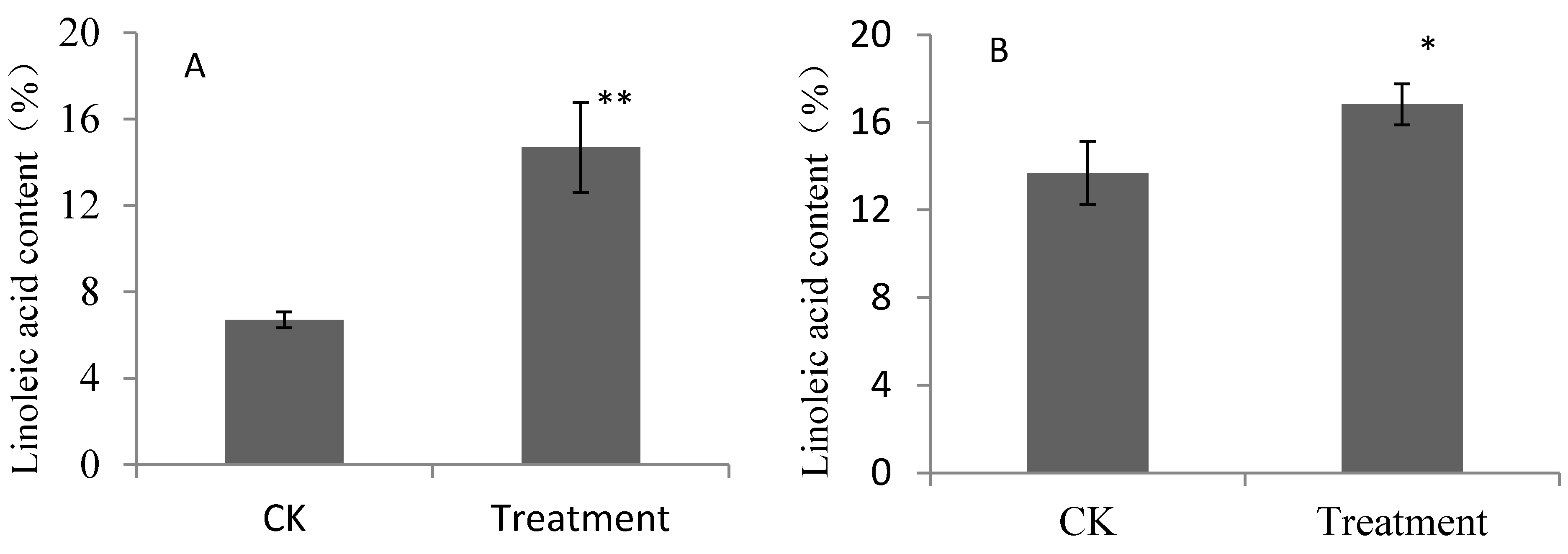

2.2. Composition of Aroma Volatiles Derived from Fatty Acid Metabolic Pathway for Pear Fruits Feeding on Linoleic Acid and Linolenic Acid
| Kinds | Aroma Volatiles | Dangshansuli | Nanguoli | ||||
|---|---|---|---|---|---|---|---|
| CK | Linoleic Acid | Linolenic Acid | CK | Linoleic Acid | Linolenic Acid | ||
| Esters | Methyl acetate | - (a) | 15.7 ± 2.8 (b) | 14.6 ± 4.9 | 5.7 ± 3.1 | 17.6 ± 8.9 | 10.1 ± 3.2 |
| Ethyl acetate | 45.9 ± 3.8 | 50.6 ± 7.1 | 55.6 ± 6.3 | 185.4 ± 27.7 | 324.8 ± 69.6 | 263.3 ± 115.6 | |
| Ethyl propanoate | - | - | - | 4.4 ± 1.1 | 6.8 ± 1.8 | 4.4 ± 1.0 | |
| Methyl butanoate | 0.9 ± 0.1 | 1.8 ± 0.0 | 0.7 ± 0.1 | 7.4 ± 1.3 | 31.5 ± 18.2 | 11.1 ± 2.1 | |
| Methyl 2-butenoate | - | 0.1 ± 0.1 | 0.2 ± 0.0 | - | - | - | |
| Ethyl butanoate | 64.3 ± 8.1 | 18.7 ± 3.7 | 8.8 ± 5.4 | 39.6 ± 13.1 | 132.1 ± 2.1 | 132.8 ± 11.4 | |
| Butyl acetate | - | - | - | - | 33.1 ± 17.2 | 24.2 ± 6.3 | |
| Ethyl 2-butenoate | - | - | - | 6.2 ± 2.0 | 7.0 ± 4.2 | 4.7 ± 2.0 | |
| Ethyl pentanoate | 0.2 ± 0.0 | - | - | 0.8 ± 0.8 | 0.2 ± 0.1 | 2.7 ± 2.4 | |
| Pentyl acetate | 1.3 ± 0.1 | 7.0 ± 0.8 | - | 1.5 ± 0.5 | 1.9 ± 0.8 | 1.3 ± 0.9 | |
| Methyl hexanoate | 1.3 ± 1.0 | 54.5 ± 3.9 | 31.4 ± 12.8 | 33.6 ± 8.5 | 231.4 ± 10.7 | 130.0 ± 3.3 | |
| Methyl 2-hexenoate | 0.5 ± 0.2 | 6.8 ± 0.9 | 6.7 ± 3.0 | 0.3 ± 0.4 | 3.0 ± 1.8 | 1.5 ± 1.1 | |
| Ethyl Hexanoate | 39.2 ± 5.6 | 13.1 ± 11.6 | 17.3 ± 10.2 | 187.2 ± 37.9 | 162.7 ± 15.1 | 391.2 ± 10.0 | |
| Ethyl Hex-3-enoate | - | 21.2 ± 5.5 | 43.7 ± 12.1 | - | - | - | |
| Hexyl acetate | 39.3 ± 14.7 | 165.7 ± 9.4 | 26.6 ± 1.5 | 168.5 ± 28.6 | 118.7 ± 6.7 | 136.2 ± 11.3 | |
| (Z)-2-Hexenyl acetate | - | - | 48.2 ± 1.0 | - | - | - | |
| Methyl heptanoate | - | 8.1 ± 1.1 | 3.5 ± 0.7 | - | - | - | |
| Ethyl 2-hexenoate | 0.2 ± 0.0 | 0.2 ± 0.1 | 0.3 ± 0.1 | 6.7 ± 1.9 | 10.3±6.5 | 10.8 ± 0.1 | |
| Ethyl heptanoate | 1.5 ± 0.5 | - | - | - | - | - | |
| Heptyl acetate | 0.2 ± 0.1 | 0.8 ± 0.5 | - | 0.2 ± 0.0 | - | 1.8 ± 0.2 | |
| 3-Hepten-1-ol,1-acetate | - | 0.5 ± 0.3 | - | - | - | - | |
| Methyl octanoate | - | 28.8 ± 2.6 | 11.3 ± 1.8 | - | 9.6 ± 1.7 | 5.6 ± 0.6 | |
| Methyl (E)-2-octenoate | - | 2.6 ± 0.5 | 4.9 ± 1.2 | - | 1.5 ± 0.1 | - | |
| Ethyl (Z)-4-octenoate | - | - | - | - | - | 0.3 ± 0.1 | |
| Hexyl butanoate | - | - | - | 0.3 ± 0.1 | - | - | |
| Ethyl octanoate | 3.1 ± 0.6 | 0.6 ± 0.0 | - | 2.2 ± 1.1 | 0.9 ± 0.2 | 1.8 ± 0.2 | |
| Methyl nonanoate | - | 0.3 ± 0.1 | 0.4±0.0 | - | - | - | |
| Ethyl (E)-2-octenoate | - | - | - | 1.7 ± 0.8 | 1.4 ± 0.1 | 3.9 ± 0.1 | |
| Methyl 4-decenoate | - | 2.2 ± 0.3 | 1.8 ± 0.8 | - | 1.0 ± 0.2 | - | |
| Methyl decanoate | - | 1.0 ± 0.0 | 1.3 ± 0.4 | - | - | - | |
| Hexyl hexanoate | - | 0.5 ± 0.1 | 0.4 ± 0.1 | - | - | - | |
| Methyl (E,Z)-2,4-decadienoate | - | 5.4 ± 0.9 | 3.6 ± 1.0 | 2.8 ± 1.5 | 3.4 ± 0.2 | 8.5 ± 1.3 | |
| Ethyl (E,Z)-2,4-decadienoate | - | 0.9 ± 0.2 | 1.2 ± 0.4 | 9.6 ± 4.1 | 5.5 ± 1.2 | 19.0 ± 1.2 | |
| Methyl dodecanoate | - | 0.6 ± 0.1 | 0.8 ± 0.1 | - | - | 1.2 ± 0.3 | |
| Methyl tetradecanoate | - | - | 0.1 ± 0.0 | - | - | 0.5 ± 0.1 | |
| Methyl hexadecanoate | - | 1.8 ± 0.7 | 1.7 ± 0.2 | - | - | - | |
| Subtotal | 197.9 cC,(c) | 409.4 aA | 285.1 bB | 664.1 bB | 1104.4 aA | 1163.4 aA | |
| Aldehydes | Hexanal | 16.7 ± 2.1 | 9.2 ± 1.6 | 3.7 ± 4.0 | 131.7 ± 23.1 | 44.1 ± 3.1 | 4.3 ± 0.6 |
| 2-Hexenal | 8.1 ± 2.6 | 2.6 ± 0.1 | 9.0 ± 7.0 | - | - | - | |
| Nonanal | 2.2 ± 0.4 | 0.5 ± 0.1 | 1.6 ± 0.4 | - | - | - | |
| Decanal | 0.9 ± 0.1 | - | - | - | - | - | |
| Subtotal | 27.9 aA | 12.3 bB | 14.3 bAB | 131.7 aA | 44.1 bB | 4.3 cC | |
| Alcohols | Ethanol | 5.5 ± 1.7 | 3.1 ± 0.9 | 5.0 ± 2.0 | 7.8 ± 2.3 | 14.4 ± 0.1 | 18.1 ± 5.5 |
| (E)-2-Hexen-1-ol | 0.2 ± 0.1 | 2.4 ± 0.2 | 10.2 ± 1.7 | - | 2.6 ± 0.2 | 1.7 ± 0.2 | |
| 1-Hexanol | 1.9 ± 1.3 | 86.9 ±1.4 | 23.6 ± 3.3 | 33.3 ± 3.3 | 139.5 ± 1.2 | 4.9 ± 3.2 | |
| 1-Heptanol | - | 3.2 ± 1.6 | - | - | - | - | |
| Octen-2-ol | - | 4.4 ± 0.7 | - | - | - | - | |
| 1-Octanol | 0.3 ± 0.0 | 1.9 ± 0.5 | 0.5 ± 0.1 | 0.7 ± 0.2 | 0.8 ± 0.1 | 1.2 ± 0.1 | |
| (E)-2-Octen-1-ol | - | 1.7 ± 0.0 | - | - | - | - | |
| Subtotal | 7.9 cC | 103.6 aA | 39.3 bB | 41.9 bB | 157.3 aA | 25.9 cB | |
2.3. Contents of Hexanal and Hexanol for Pear Fruits Fed on Hexanal and Hexanol
| Treatments | Dangshansuli | Nanguoli | ||
|---|---|---|---|---|
| Hexanal | Hexanol | Hexanal | Hexanol | |
| CK | 16.7 ± 2.1 (b) | 1.9 ± 1.3 | - (a) | 37.9 ± 10.0 |
| Hexanal | 19.0 ± 0.2 | 1909.3 ± 39.8 **, (c) | 19.7 ± 10.3 ** | 1822.4 ± 748.1 ** |
| Hexanol | 20.3 ± 0.3 | 2193.9 ± 47.8 ** | 11.0 ± 15.6 ** | 3024.8 ± 537.3 ** |
2.4. Hexyl Ester and/or Hexanoate Ester Content in Pear Fruits Fed on Hexanal and Hexanol
| Aroma Volatiles | Dangshansuli | Nanguoli | ||||
|---|---|---|---|---|---|---|
| CK | Hexanal | Hexanol | CK | Hexanal | Hexanol | |
| Methyl hexanoate | 1.3 ± 1.0 bAB (b) | 5.4 ± 4.2 aA | - (a) | 33.6 ± 8.5 bB | 81.0 ± 24.6 aA | 25.8 ± 1.82 bB |
| Ethyl hexanoate | 39.2 ± 5.6 cC | 526.0 ± 22.9 bB | 114.6 ± 4.8 aA | 187.2 ± 37.9 bB | 621.5 ± 226.4 aA | 160.5 ± 76.0 bB |
| Hexyl acetate | 39.3 ± 14.7 bB | 1370.4 ± 162.9 aA | 1350.2 ± 284.8 aA | 168.5 ± 28.6 cB | 594.3 ± 303.8 bAB | 1006.1 ± 71.1 aA |
| Hexyl butanoate | - | 72.4 ± 4.3 aA | 50.0 ± 24.1 bA | 0.3 ± 0.1 cC | 35.0 ± 10.9 bB | 79.7 ± 19.0 aA |
| Hexyl hexanoate | - | 272.6 ± 79.9 aA | 120.7 ± 38.2 bB | - | 137.7 ± 42.9 aA | 41.6 ± 58.9 bB |
| Hexyl benzoate | - | 0.5 ± 0.1 bB | 1.3 ± 0.4 aA | - | - | - |
| Hexyl octanoate | - | 0.9 ± 0.2 bB | 2.2 ± 1.0 aA | - | - | - |
| Total contents | 79.8 ± 21.2 cC (c) | 2248.2 ± 274.6 aA | 1639.0 ± 353.2 bB | 389.60 ± 75.1 bB | 1469.5 ± 608.6 aA | 1313.8 ± 153.9 aA |
3. Experimental Section
3.1. Plant Materials
3.2. Precursor Substrates and Incubation Method
3.3. Extraction of Polar Lipids
3.4. Analysis of Fatty Acid by GC
3.5. Identification and Quantification of Fatty Acids
3.6. Extraction and Concentration of Aroma Volatile of Pear Fruits
3.7. GC-Mass Spectrometry (MS) Analysis of Aroma Volatiles
3.8. Identification and Quantification of Aroma Volatiles
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bartley, I.M.; Stoker, P.G.; Martin, A.D.E.; Hatfield, S.G.S.; Knee, M. Synthesis of aroma compounds by apples supplied with alcohols and methyl esters of fatty acids. J. Sci. Food Agric. 1985, 36, 567–574. [Google Scholar]
- Fellman, J.K.; Miller, T.W.; Mattinson, D.S.; Mattheis, J.P. Factors that influence biosynthesis of volatile flavor compounds in apple fruits. Hortiscience 2000, 35, 1026–1033. [Google Scholar]
- Song, J.; Bangerth, F. Fatty acids as precursors for aroma volatile biosynthesis in pre-climacteric and climacteric apple fruit. Postharvest Biol. Technol. 2003, 30, 113–121. [Google Scholar]
- Wang, C.L.; Xing, J.S.; Chin, C.K.; Ho, C.T.; Martin, C.E. Modification of fatty acids changes the flavor volatiles in tomato leaves. Phytochemistry 2001, 58, 227–232. [Google Scholar]
- Bangerth, F.; Streif, J.; Song, J.; Brackmann, A. Investigations into the physiology of volatile aroma production of apple fruits. Acta Hortic. 1996, 464, 189–194. [Google Scholar]
- Brackmann, A.; Streif, J.; Bangerth, F. Relationship between a reduced aroma production and lipid metabolism of apples after long-term controlled-atmosphere storage. J. Am. Soc. Hortic. Sci. 1993, 118, 243–247. [Google Scholar]
- Harb, J.; Streif, J.; Bangerth, F. Response of controlled atmosphere (CA) stored ‘Golden Delicious’ apples to the treatments with alcohols and aldehydes as aroma precursors. Gartenbauwissenschaft 2000, 65, 154–161. [Google Scholar]
- Kollmannsberger, H.; Berger, R. Precursor atmosphere storage induced flavour changes in apples cv. Red Delicious. Chem. Mikrobiol. Technol. Lebensm. 1992, 14, 81–86. [Google Scholar]
- Chen, J.L.; Zhou, S.; Yan, S.J.; Ma, Y.K.; Hu, X.S. Analysis of Aroma Components of Fengshui Dangshan and Nanguo Pear by SPME/GC/MS. Acta Hortic. Sin. 2005, 32, 301–303. [Google Scholar]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008, 54, 712–732. [Google Scholar]
- Pérez, A.G.; Olías, R.; Olías, J.M.; Sanz, C. Biosynthesis of 4-hydroxy-2,5-dimethyl-3 (2 H)-furanone and derivatives in in vitro grown strawberries. J. Agric. Food Chem. 1999, 47, 655–658. [Google Scholar]
- Roscher, R.; Schreier, P.; Schwab, W. Metabolism of 2,5-dimethyl-4-hydroxy-3 (2 H)-furanone in detached ripening strawberry fruits. J. Agric. Food Chem. 1997, 45, 3202–3205. [Google Scholar]
- Gonda, I.; Bar, E.; Portnoy, V.; Lev, S.; Burger, J.; Schaffer, A.A.; Tadmor, Y.; Gepstein, S.; Giovannoni, J.J.; Katzir, N.; et al. Branched-chain and aromatic amino acid catabolism into aroma volatiles in Cucumis melo L. fruit. J. Exp. Bot. 2010, 61, 1111–1123. [Google Scholar]
- Pérez, A.G.; Sanz, C.; Olías, R.; Ríos, J.J.; Olías, J.M. Evolution of strawberry alcohol acyltransferase activity during fruit development and storage. J. Agric. Food Chem. 1996, 44, 3286–3290. [Google Scholar]
- Perez, A.G.; Olías, R.; Espada, J.; Olías, J.M.; Sanz, C. Rapid determination of sugars, nonvolatile acids, and ascorbic acid in strawberry and other fruits. J. Agric. Food Chem. 1997, 45, 3545–3549. [Google Scholar]
- Altisent, R.; Graell, J.; Lara, I.; López, L.; Echeverría, G. Increased straight-chain esters content after ultra low oxygen storage and its relation to the lipoxygenase system in ‘Golden Reinders®’ apples. Eur. Food. Res. Technol. 2010, 232, 51–61. [Google Scholar]
- Lara, I.; Echeverría, G.; Graell, J.; López, M.L. Volatile emission after controlled atmosphere storage of Mondial Gala apples (Malus domestica): Relationship to some involved enzyme activities. J. Agric. Food Chem. 2007, 55, 6087–6095. [Google Scholar]
- Altisent, R.; Echeverría, G.; Graell, J.; López, L.; Lara, I. Lipoxygenase activity is involved in the regeneration of volatile ester-synthesizing capacity after ultra-low oxygen storage of ‘Fuji’apple. J. Agric. Food Chem. 2009, 57, 4305–4312. [Google Scholar]
- Zhang, B.; Yin, X.R.; Shen, J.Y.; Chen, K.S.; Ferguson, I.B. Volatiles production and lipoxygenase gene expression in kiwifruit peel and flesh during fruit ripening. J. Am. Soc. Hortic. Sci. 2009, 134, 472–477. [Google Scholar]
- Leone, A.; Bleve-Zacheo, T.; Gerardi, C.; Melillo, M.T.; Leo, L.; Zacheo, G. Lipoxygenase involvement in ripening strawberry. J. Agric. Food Chem. 2006, 54, 6835–6844. [Google Scholar]
- Defilippi, B.G.; Dandekar, A.M.; Kader, A.A. Relationship of ethylene biosynthesis to volatile production, related enzymes, and precursor availability in apple peel and flesh tissues. J. Agric. Food Chem. 2005, 53, 3133–3141. [Google Scholar]
- Tholl, D.; Kish, C.M.; Orlova, I.; Sherman, D.; Gershenzon, J.; Pichersky, E.; Dudareva, N. Formation of monoterpenes in Antirrhinum majus and Clarkia breweri flowers involves heterodimeric geranyl diphosphate synthases. Plant. Cell 2004, 16, 977–992. [Google Scholar]
- Kochevenko, A.; Araujo, L.; Maloney, G.S.; Tiemam, T.M.; Do, P.T.; Taylor, M.G.; Klee, H.J.; Fernie, A.R. Catabolism of branched chain amino acids supports respiration but not volatile synthesis in tomato fruits. Mol. Plant. 2012, 5, 366–375. [Google Scholar]
- Buchhaupt, M.; Guder, J.C.; Etschmann, M.M.; Schrader, J. Synthesis of green note aroma compounds by biotransformation of fatty acids using yeast cells coexpressing lipoxygenase and hydroperoxide lyase. Appl. Microbiol. Biotechnol. 2012, 93, 159–168. [Google Scholar]
- Zhang, B.; Yin, X.R.; Li, X.; Yang, S.L.; Ferguson, I.B.; Chen, K.S. Lipoxygenase gene expression in ripening kiwifruit in relation to ethylene and aroma production. J. Agric. Food Chem. 2009, 57, 2875–2881. [Google Scholar]
- Qin, G.H.; Tao, S.T.; Cao, Y.F.; Wu, J.; Zhang, H.P.; Huang, W.J.; Zhang, S.L. Evaluation of the volatile profile of 33 Pyrus ussuriensis cultivars by HS-SPME with GC-MS. Food Chem. 2012, 34, 2367–2382. [Google Scholar]
- Sample Availability: Samples of the fruits are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, G.; Tao, S.; Zhang, H.; Huang, W.; Wu, J.; Xu, Y.; Zhang, S. Evolution of the Aroma Volatiles of Pear Fruits Supplemented with Fatty Acid Metabolic Precursors. Molecules 2014, 19, 20183-20196. https://doi.org/10.3390/molecules191220183
Qin G, Tao S, Zhang H, Huang W, Wu J, Xu Y, Zhang S. Evolution of the Aroma Volatiles of Pear Fruits Supplemented with Fatty Acid Metabolic Precursors. Molecules. 2014; 19(12):20183-20196. https://doi.org/10.3390/molecules191220183
Chicago/Turabian StyleQin, Gaihua, Shutian Tao, Huping Zhang, Wenjiang Huang, Juyou Wu, Yiliu Xu, and Shaoling Zhang. 2014. "Evolution of the Aroma Volatiles of Pear Fruits Supplemented with Fatty Acid Metabolic Precursors" Molecules 19, no. 12: 20183-20196. https://doi.org/10.3390/molecules191220183
APA StyleQin, G., Tao, S., Zhang, H., Huang, W., Wu, J., Xu, Y., & Zhang, S. (2014). Evolution of the Aroma Volatiles of Pear Fruits Supplemented with Fatty Acid Metabolic Precursors. Molecules, 19(12), 20183-20196. https://doi.org/10.3390/molecules191220183




