Au-Nanomaterials as a Superior Choice for Near-Infrared Photothermal Therapy
Abstract
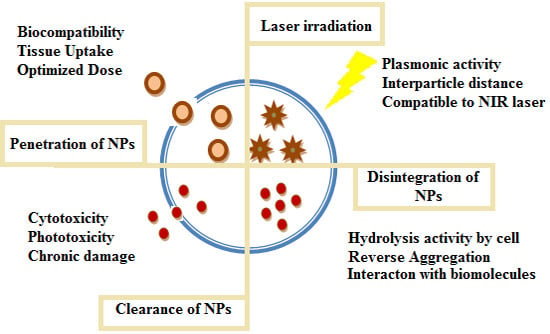
:1. Photothermal Therapy (PTT) and Nanoparticles

2. Hybrid Superparamagnetic Au-Nanoaggregates
| Au-Nanomaterials | Characteristics | Advantages | Limitations |
|---|---|---|---|
| Gold Nanorods | Temperatures can be reached using a high powered LED device |
|
|
| Silica-Coated Au/Fe2O3 Nanoaggregates | Tuning of Au inter-particle distance for better plasmonic activity |
|
|
| Luminescent gold speckled silica (GSS) nanoparticles |
|
|
|
| Thermoresponsive polymer encapsulated Au-nanorods PNIPAM-Au@SiO2 | Simultaneously deliver heat and anticancer drugs |
|
|
| Amphiphilic mixed polymers grafted gold nanoparitcles | Distinct chemical property from amphiphilic polymers, which endowed the Au NPs ability to self-assembly •Potential CT contrast agent |
|
Could not be totally destroyed under NIR irradiation
|
| Au nanomatryoshkas (Au/SiO2/Au) |
|
| No statistical difference in performance of nanomatryoshks and nanoshells |
3. Luminescent Gold Speckled Silica (GSS) Nanoparticles

4. Theranostic Self-Assembly Structured Gold Nanoparticles

5. Photothermal Transducer Au Nanomatryoshkas

6. Gold Nanorods (GNRs)
7. Thermo-Responsive Polymer Encapsulated Gold Nanorods
8. Conclusions and Future Perspectives
Conflicts of Interest
References
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Martín Rodrígueza, E.; García Soléa, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Deng, J.; Yuan, Y.; Flachenecker, C.; Mak, R.; Hornberger, B.; Jin, Q.; Shu, D.; Lai, B.; Maser, J.; et al. The Bionanoprobe: Hard X-ray fluorescence nanoprobe with cryogenic capabilities. J. Synchrotron Radiat. 2014, 21, 66–75. [Google Scholar] [CrossRef]
- Howes, P.D.; Rana, S.; Stevens, M.M. Plasmonic nanomaterials for biodiagnostics. Chem. Soc. Rev. 2014, 43, 3835–3853. [Google Scholar] [CrossRef] [PubMed]
- Khlebtsov, B.N.; Panfilova, E.V.; Terentyuk, G.S.; Maksimova, I.L.; Ivanov, A.V.; Khlebtsov, N.G. Plasmonic nanopowders for photothermal therapy of tumors. Langmuir 2012, 28, 8994–9002. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Wang, H.; Zhao, J.; Lui, H.; Korbelik, M.; Zeng, H. Single-wall carbon nanotubes assisted photothermal cancer therapy: Animal study with a murine model of squamous cell carcinoma. Lasers Surg. Med. 2010, 42, 638–648. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; El-Sayed, M.A. Gold nanoparticles: Optical properties and implementations in cancer diagnosis and photothermal therapy. J. Adv. Res. 2010, 1, 13–28. [Google Scholar] [CrossRef]
- Shi, X.; Gong, H.; Li, Y.; Wang, C.; Cheng, L.; Liu, Z. Graphene-based magnetic plasmonic nanocomposite for dual bioimaging and photothermal therapy. Biomaterials 2013, 34, 4786–4793. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.X.; Huang, Y.Z.; Shi, S.G.; Tang, S.H.; Li, D.H.; Chen, X.L. Cancer therapy improvement with mesoporous silica nanoparticles combining photodynamic and photothermal therapy. Nanotechnology 2014, 25, 285701. [Google Scholar] [CrossRef] [PubMed]
- Melancon, M.P.; Elliott, A.; Ji, X.; Shetty, A.; Yang, Z.; Tian, M.; Taylor, B.; Stafford, R.J.; Li, C. Theranostics with multifunctional magnetic gold nanoshells: Photothermal therapy and t2* magnetic resonance imaging. Investig. Radiol. 2011, 46, 132–140. [Google Scholar] [CrossRef]
- Sailor, M.J.; Park, J.H. Hybrid nanoparticles for detection and treatment of cancer. Adv. Mater. 2012, 24, 3779–3802. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Vardarajan, V.; Koymen, A.R.; Mohanty, S.K. Magnetic-field-assisted photothermal therapy of cancer cells using Fe-doped carbon nanoparticles. J. Biomed. Opt. 2012, 17, 0180031–0180038. [Google Scholar] [CrossRef]
- Li, J.; Han, J.; Xu, T.; Guo, C.; Bu, X.; Zhang, H.; Wang, L.; Sun, H.; Yang, B. Coating Urchinlike gold nanoparticles with polypyrrole thin shells to produce photothermal agents with high stability and photothermal transduction efficiency. Langmuir 2013, 29, 7102–7110. [Google Scholar] [CrossRef] [PubMed]
- Sikdar, D.; Rukhlenko, I.D.; Cheng, W.; Premaratne, M. Effect of number density on optimal design of gold nanoshells for plasmonic photothermal therapy. Biomed. Opt. Express 2013, 4, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.B.; Friman, T.; Pang, H.B.; Pallaoro, A.; de Mendoza, T.H.; Willmore, A.A.; Kotamraju, V.R.; Mann, A.P.; She, Z.G.; Sugahara, K.N.; et al. Etchable plasmonic nanoparticle probes to image and quantify cellular internalization. Nat. Mater. 2014, 13, 904–911. [Google Scholar] [CrossRef]
- Huang, P.; Rong, P.; Lin, J.; Li, W.; Yan, X.; Zhang, M.G.; Nie, L.; Niu, G.; Lu, J.; Wang, W.; et al. Triphase interface synthesis of plasmonic gold bellflowers as near-infrared light mediated acoustic and thermal theranostics. J. Am. Chem. Soc. 2014, 136, 8307–8313. [Google Scholar] [CrossRef] [PubMed]
- Naik, G.V.; Shalaev, V.M.; Boltasseva, A. Alternative plasmonic materials: Beyond gold and silver. Adv. Mater. 2013, 25, 3264–3294. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hainfeld, J.F.; Lin, L.; Slatkin, D.N.; Dilmanian, F.A.; Vadas, T.M.; Smilowitz, H.M. Gold nanoparticle hyperthermia reduces radiotherapy dose. Nanomed. Nanotech. Biol. Med. 2014, 10, 1609–1617. [Google Scholar] [CrossRef]
- Day, E.S.; Thompson, P.A.; Zhang, L.; Lewinski, N.A.; Ahmed, N.; Drezek, R.A.; Blaney, S.M.; West, J.L. Nanoshell-mediated photothermal therapy improves survival in a murine glioma model. J. Neurooncol. 2011, 104, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Mackey, M.A.; Ali, M.R.K.; Austin, L.A.; Near, R.D.; El-Sayed, M.A. The most effective gold nanorod size for plasmonic photothermal therapy: Theory and in vitro experiments. J. Phys. Chem. B 2014, 118, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Menon, J.U.; Jadeja, P.; Tambe, P.; Vu, K.; Yuan, B.; Nguyen, K.T. Nanomaterials for photo-based diagnostic and therapeutic applications. Theranostics 2013, 3, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jasinski, J.B.; Howell, J.L.; Patel, D.; Stephens, D.P.; Gobin, A.M. Tunability and stability of gold nanoparticles obtained from chloroauric acid and sodium thiosulfate reaction. Nanoscale Res. Lett. 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Alkilany, A.M.; Murphy, C.J. Toxicity and cellular uptake of gold nanoparticles: What we have learned so far? J. Nanopart. Res. 2010, 12, 2313–2333. [Google Scholar] [CrossRef] [PubMed]
- Nazir, S.; Hussain, T.; Ayub, A.; Rashid, U.; MacRobert, A.J. Nanomaterials in combating cancer: Therapeutic applications and developments. Nanomed. Nanotechnol. Biol. Med. 2014, 10, 19–34. [Google Scholar] [CrossRef]
- Popp, M.K.; Oubou, I.; Shepherd, C.; Nager, Z.; Anderson, C.; Pagliaro, L. Photothermal therapy using gold nanorods and near-infrared light in a Murine Melanoma Model increases survival and decreases tumor volume. J. Nanomater. 2014, 2014, 450670. [Google Scholar] [CrossRef]
- Sotiriou, G.A.; Starsich, F.; Dasargyri, A.; Wurnig, M.C.; Krumeich, F.; Boss, A.; Leroux, J.C.; Pratsinis, S.E. Photothermal killing of cancer cells by the controlled plasmonic coupling of silica-coated Au/Fe2O3 Nanoaggregates. Adv. Funct. Mater. 2014, 24, 2818–2827. [Google Scholar] [CrossRef]
- Sharma, P.; Brown, S.C.; Singh, A.; Iwakuma, N.; Pyrgiotakis, G.; Krishna, V.; Knapik, J.A.; Barr, K.; Moudgil, B.M.; Grobmyer, S.R. Near-infrared absorbing and luminescent speckled for photothermal therapy. J. Mater. Chem. 2010, 20, 5182–5185. [Google Scholar] [CrossRef]
- Ayala-Orozco, C.; Urban, C.; Knight, M.W.; Urban, A.S.; Neumann, O.; Bishnoi, S.W.; Mukherjee, S.; Goodman, A.M.; Charron, H.; Mitchell, T.; et al. Au nanomatryoshkas as efficient near-infrared photothermal transducers for cancer treatment: Benchmarking against nanoshells. ACS Nano 2014, 8, 6372–6381. [Google Scholar] [CrossRef]
- Deng, H.; Zhong, Y.; Du, M.; Liu, Q.; Fan, Z.; Dai, F.; Zhang, X. Theranostic self-assembly structure of gold nanoparticles for NIR photothermal therapy and X-Ray computed tomography imaging. Theranostics 2014, 4, 904–918. [Google Scholar] [CrossRef] [PubMed]
- Marchesan, S.; Prato, M. Nanomaterials for (Nano)medicine. ACS Med. Chem. Lett. 2013, 4, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lugo, M.; Rinaldi, C. Thermal potentiation of chemotherapy by magnetic nanoparticles. Nanomedicine (Lond.) 2013, 8, 1689–1707. [Google Scholar] [CrossRef]
- Polakiewicza, A.; Dodiuka, H.; Keniga, S. Super-hydrophilic coatings based on silica nanoparticles. J. Adhes. Sci. Technol. 2014, 28, 466–478. [Google Scholar] [CrossRef]
- Wang, X.; Wu, G.; Zhou, B.; Shen, J. Thermal annealing effect on optical properties of binary TiO2-SiO2 sol-gel coatings. Materials 2013, 6, 76–84. [Google Scholar] [CrossRef]
- Khosroshahi, M.E.; Nourbakhsh, M.S.; Ghazanfari, L. Synthesis and biomedical application of SiO2/Au nanofluid based on laser-induced surface plasmon resonance thermal effect. J. Mod. Phys. 2011, 2, 944–953. [Google Scholar] [CrossRef]
- Amirthalingam, T.; Kalirajan, J.; Chockalingam, A. Use of silica-gold core shell structured nanoparticles for targeted drug delivery system. J. Nanomed. Nanotechnol. 2011, 2, 119–123. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, D.; Altantzis, T.; Heidari, H.; Balsc, S.; Liz-Marzán, L.M. A protecting group approach toward synthesis of Au–silica Janus nanostars. Chem. Commun. 2014, 50, 79–81. [Google Scholar] [CrossRef]
- Primo, A.; Cormaa, A.; Garcíaa, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, T.; Wang, C.; Zhang, J. Nonlinear-optical and fluorescent properties of Ag aqueous colloid prepared by silver nitrate reduction. J. Nanomater. 2010, 2010, 56. [Google Scholar]
- Prodan, A.M.; Iconaru, S.M.; Chifiriuc, C.M.; Bleotu, C.; Ciobanu, C.S.; Motelica-Heino, M.; Sizaret, S.; Predoi, D. Iron oxide magnetic nanoparticles: Characterization and toxicity evaluation by in vitro and in vivo assays. J. Nanomater. 2013, 2013, 5. [Google Scholar]
- Zhang, Z.; Wang, J.; Chen, C. Gold nanorods based platforms for light-mediated theranostics. Theranostics 2013, 3, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Cobley, C.M.; Au, L.; Chen, J.; Xia, Y. Targeting gold nanocages to cancer cells for photothermal destruction and drug delivery. Exp. Opin. Drug Deliv. 2010, 7, 577–587. [Google Scholar] [CrossRef]
- Rasch, M.R.; Sokolov, K.V.; Korgel, B.A. Limitations on the optical tunability of small diameter gold nanoshells. Langmuir 2009, 25, 11777–11785. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Hondow, N.S.; Krzemiński, L.; Brown, A.P.; Jeuken, L.J.C.; Routledge, M.N. Mechanism of cellular uptake of genotoxic silica nanoparticles. Part. Fibre Toxicol. 2012, 9, 29. [Google Scholar] [CrossRef] [PubMed]
- Hervault, A.; Thanh, N.T.K. Magnetic nanoparticle-based therapeutic agents for thermo-chemotherapy treatment of cancer. Nanoscale 2014, 6, 11553–11573. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.A.; Herranz, B.; Santos, H.A. Nanostructured porous Si-based nanoparticles for targeted drug delivery. Biomaterials 2012, 2, 296–312. [Google Scholar]
- Bardhan, R.; Lal, S.; Joshi, A.; Halas, N.M. Theranostic nanoshells: From probe design to imaging and treatment of cancer. Acc. Chem. Res. 2011, 44, 936–946. [Google Scholar] [CrossRef] [PubMed]
- Gong, T.; Goh, D.; Olivo, M.; Yong, K.T. In vitro toxicity and bioimaging studies of gold nanorods formulations coated with biofunctional thiol-PEG molecules and Pluronic block copolymers. Beilstein J. Nanotechnol. 2014, 5, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Li, W.; Cobley, C.M.; Chen, J.; Xia, X.; Zhang, Q.; Yang, M.; Cho, E.C.; Brown, P.K. Gold nanocages: From synthesis to theranostic applications. Acc. Chem. Res. 2011, 44, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, H.; Yang, J.; Qiao, S.Z.; Du, X.-W. Pure gold nanocages by galvanic replacement reaction of magnesium nanoparticles. RSC Adv. 2014, 4, 1185–1188. [Google Scholar] [CrossRef]
- Wu, C.; Yu, C.; Chu, M. A gold nanoshell with a silica inner shell synthesized using liposome templates for doxorubicin loading and near-infrared photothermal therapy. Int. J. Nanomed. 2011, 6, 807–813. [Google Scholar]
- Leung, J.P.; Wu, S.; Chou, K.C.; Signorell, R. Investigation of sub-100 nm gold nanoparticles for laser-induced thermotherapy of cancer. Nanomaterials 2013, 3, 86–106. [Google Scholar] [CrossRef]
- Bardhan, R.; Chen, W.; Bartels, M.; Perez-Torres, C.; Botero, M.F.; McAninch, R.W.; Contreras, A.; Schiff, R.; Pautler, R.G.; Halas, N.J.; et al. Tracking of multimodal therapeutic nanocomplexes targeting breast cancer in vivo. Nano Lett. 2010, 10, 4920–4928. [Google Scholar] [CrossRef] [PubMed]
- Kessentini, S.; Barchiesi, D. Quantitative comparison of optimized nanorods, nanoshells and hollow nanospheres for photothermal therapy. Biomed. Opt. Express 2012, 3, 590–604. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.; Zhang, L.; Niu, W.; Qi, W.; Zhao, J.; Liu, Z.; Zhang, W.; Xu, G. One-pot synthesis of gold nanorods using binary surfactant systems with improved monodispersity, dimensional tunability and plasmon resonance scattering properties. Nanotechnology 2014, 25, 125601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xia, K.; Lu, X.; Li, G.; Chen, J.; Deng, Y.; Li, S.; Zhou, F.; He, N. Efficient and facile synthesis of gold nanorods with finely tunable plasmonic peaks from visible to Near-IR range. Chem. Mater. 2014, 26, 1794–1798. [Google Scholar] [CrossRef]
- El-Sayed, M.A.; Shabaka, A.A.; El-Shabrawy, O.A.; Yassin, N.A.; Mahmoud, S.S.; El-Shenawy, S.M.; Al-Ashqar, E.; Eisa, W.H.; Farag, N.M.; El-Shaer, M.A.; et al. Tissue distribution and efficacy of gold nanorods coupled with laser induced photoplasmonic therapy in ehrlich carcinoma solid tumor model. PLoS One 2013, 8, e76207. [Google Scholar] [CrossRef] [PubMed]
- Alcantar, N.A.; Aydil, E.S.; Israelachvili, J.A. Polyethylene glycol-coated biocompatible surfaces. J. Biomed. Mater. Res. 2000, 51, 343–351. [Google Scholar] [CrossRef] [PubMed]
- Chan, R.T.H.; Marçal, H.; Russell, R.A.; Holden, P.J.; Foster, L.J.R. Application of polyethylene glycol to promote cellular biocompatibility of polyhydroxybutyrate films. Int. J. Polym. Sci. 2011, 2011, 473045. [Google Scholar]
- Zhang, Z.; Wang, J.; Nie, X.; Wen, T.; Ji, Y.; Wu, X.; Zhao, Y.; Chen, C. Near infrared laser-induced targeted cancer therapy using thermoresponsive polymer encapsulated gold nanorods. J. Am. Chem. Soc. 2014, 136, 7317–7326. [Google Scholar] [CrossRef] [PubMed]
- Ankareddi, I.; Bailey, M.M.; Brazel, C.S.; Rasco, J.F.; Hood, R.D. Developmental toxicity assessment of thermoresponsive poly(N-isopropylacrylamide-co-acrylamide) oligomers in CD-1 mice. Birth Defects Res. B Dev. Reprod. Toxicol. 2008, 83, 112–116. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jabeen, F.; Najam-ul-Haq, M.; Javeed, R.; Huck, C.W.; Bonn, G.K. Au-Nanomaterials as a Superior Choice for Near-Infrared Photothermal Therapy. Molecules 2014, 19, 20580-20593. https://doi.org/10.3390/molecules191220580
Jabeen F, Najam-ul-Haq M, Javeed R, Huck CW, Bonn GK. Au-Nanomaterials as a Superior Choice for Near-Infrared Photothermal Therapy. Molecules. 2014; 19(12):20580-20593. https://doi.org/10.3390/molecules191220580
Chicago/Turabian StyleJabeen, Fahmida, Muhammad Najam-ul-Haq, Rabia Javeed, Christian W. Huck, and Guenther K. Bonn. 2014. "Au-Nanomaterials as a Superior Choice for Near-Infrared Photothermal Therapy" Molecules 19, no. 12: 20580-20593. https://doi.org/10.3390/molecules191220580
APA StyleJabeen, F., Najam-ul-Haq, M., Javeed, R., Huck, C. W., & Bonn, G. K. (2014). Au-Nanomaterials as a Superior Choice for Near-Infrared Photothermal Therapy. Molecules, 19(12), 20580-20593. https://doi.org/10.3390/molecules191220580