Preparation and In Vitro Evaluation of Glycyrrhetinic Acid-Modified Curcumin-Loaded Nanostructured Lipid Carriers
Abstract
:1. Introduction

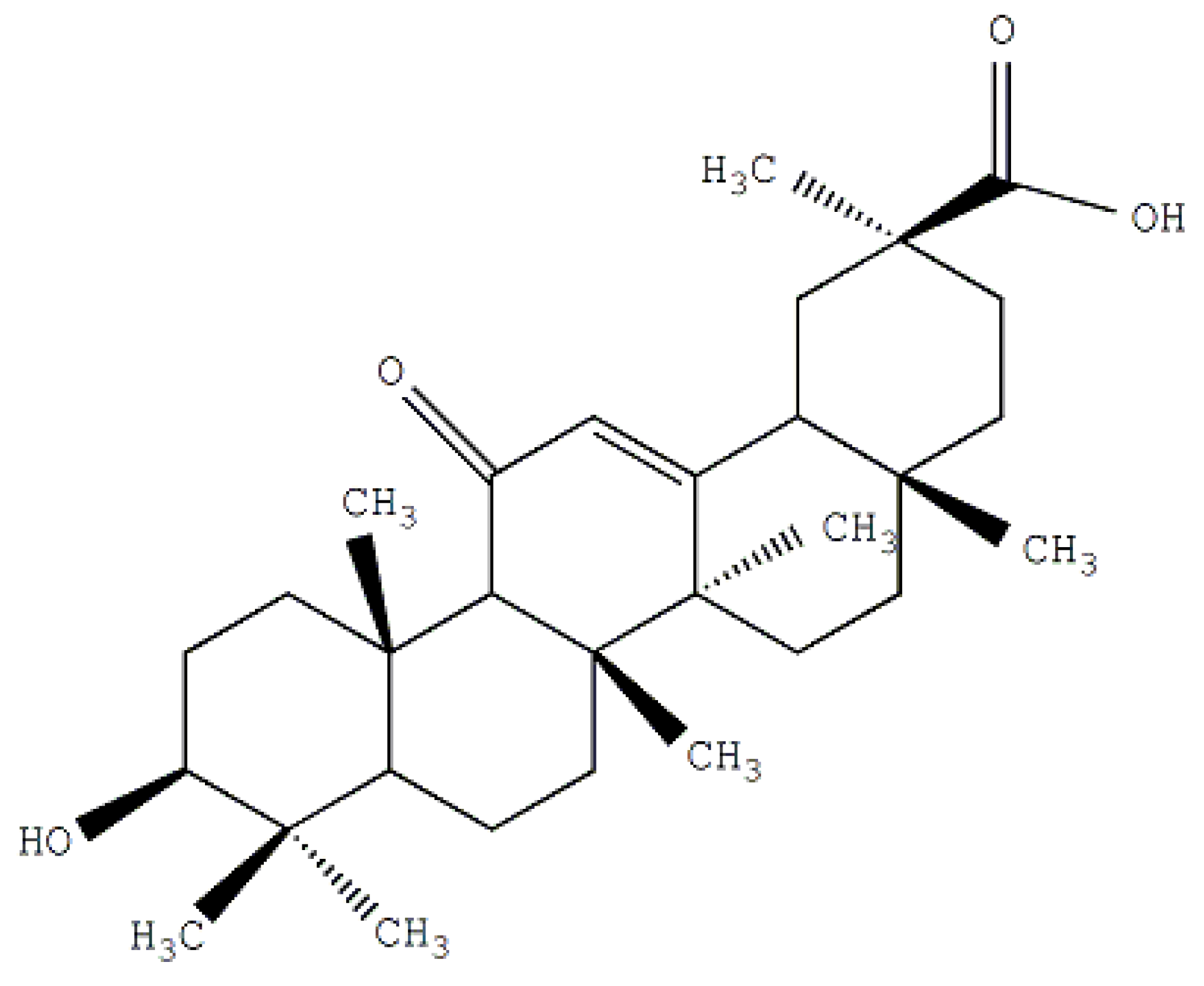
2. Results and Discussion
2.1. Synthesis and the Surface Characterization of GA-Phospholipid Derivative

2.1.1. FT-IR

2.1.2. 1H-NMR
2.2. Particle Characterization


| Type | Particle Characterization | |||
|---|---|---|---|---|
| Particle Sizes (nm) | Zeta Potentials (mV) | Encapsulation Efficiency (%) | Drug Loading Capacity (%) | |
| Cur-NLC | 58.3 ± 8.8 | −22.6 ± 0.88 | 93.48 ± 0.86 | 2.25 ± 0.32 |
| Cur-PEG-NLC | 102.4 ± 13.5 | −17.1 ± 0.53 | 97.12 ± 2.45 | 2.34 ± 0.28 |
| Cur-GA5%-PEG-NLC | 123.1 ± 15.6 | −16.2 ± 0.48 | 95.31 ± 2.18 | 2.30 ± 0.30 |
| Cur-GA10%-PEG-NLC | 128.4 ± 15.9 | −15.5 ± 0.37 | 93.11 ± 1.76 | 2.24 ± 0.22 |
| Cur-GA15%-PEG-NLC | 132.7 ± 16.7 | −14.8 ± 0.32 | 90.06 ± 1.12 | 2.17 ± 0.24 |
2.3. Cellular Selective Uptake of NLCs

2.4. Cytotoxicity to HepG2
| T (h) | IC50 (μg/mL) | |||
|---|---|---|---|---|
| Cur-Solution | Cur-NLC | Cur-PEG-NLC | Cur-GA10%-PEG-NLC | |
| 24 | 42.28 ± 9.40 ** | 8.00 ± 0.97 ** | 3.28 ± 0.16 | 2.91 ± 0.70 |
| 48 | 15.60 ± 1.28 ** | 3.87 ± 0.18 ** | 3.14 ± 1.27 | 2.22 ± 0.03 |
| 72 | 6.93 ± 0.61 ** | 1.70 ± 0.31 | 1.54 ± 0.04 | 1.34 ± 0.24 |

3. Experimental
3.1. General
3.2. Preparation of Cur-NLC
3.3. Synthesis of GA-Phospholipid Derivative (GA-PEG2000-DSPE)
3.4. Preparation of Cur-PEG-NLC and Cur-GA-PEG-NLC
3.5. FT-IR and 1H-NMR Analysis
3.6. Measurement of Particles Size and Zeta Potential
3.7. Transmission Electron Microscopy (TEM)
3.8. Drug Encapsulation
3.9. In Vitro Cellular Uptake and In Vitro Cytotoxicity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yallapu, M.M.; Jaqqi, M.; Chauhan, S.C. Curcumin nanoformulationd: A future nanomedicine for cancer. Drug Discov. Today 2012, 17, 71–80. [Google Scholar] [CrossRef]
- Huang, M.T.; Newmark, H.L.; Frenkel, K. Inhibitory effects of curcumin on tumorigenesis in mice. J. Cell. Biochem. Suppl. 1997, 27, 26–34. [Google Scholar]
- Siddiqui, A.M.; Cui, X.; Wu, R.; Dong, W.; Zhou, M.; Hu, M.; Simms, H.H.; Wang, P. The anti-inflammatory effect of curcumin in an experimental model of sepsis is mediated by up-regulation of peroxisome proliferatoractivated receptor-gamma. Crit. Care Med. 2006, 34, 1874–1882. [Google Scholar] [CrossRef]
- Kumar, K.; Rai, A.K. Proniosomal formulation of curcumin having anti-inflammatory and anti-arthritic activity in different experimental animal models. Pharmazie 2012, 67, 852–857. [Google Scholar]
- Menon, V.P.; Sudheer, A.R. Antioxidant and anti-inflammatory properties of curcumin. Adv. Exp. Med. Biol. 2007, 595, 105–125. [Google Scholar] [CrossRef]
- Paek, E.J.; Jeon, C.H.; Ko, G.; Kim, J.; Sohn, D.H. Protective effect of curcumin in rat liver injury induced by carbon tetrachloride. J. Pharm. Pharmacol. 2000, 52, 437–440. [Google Scholar] [CrossRef]
- Ireson, C.R.; Jones, D.J.; Orr, S.; Coughtrie, M.W.; Boocock, D.J.; Williams, M.L.; Farmer, P.B.; Steward, W.P.; Gescher, A.J. Metabolism of the cancer chemopreventive agent curcumin in human and rat intestine. Cancer Epidemiol. Biomarkers Prev. 2002, 11, 105–111. [Google Scholar]
- Maheshwari, R.K.; Singh, A.K.; Gaddipati, J.; Srimal, R.C. Multiple biological activities of curcumin: A short review. Life Sci. 2006, 78, 2081–2087. [Google Scholar] [CrossRef]
- Yang, C.L.; Liu, Y.Y.; Ma, Y.G.; Xue, Y.X.; Liu, D.G.; Ren, Y.; Liu, X.B.; Li, Y.; Li, Z. Curcumin blocks small cell lung cancer cells migration, invasion, angiogenesis, cell cycle and neoplasia through Janus kinase-STAT3 signalling pathway. PLoS One 2012, 7, e37960. [Google Scholar]
- Huang, M.T.; Smart, R.C.; Wong, C.Q.; Conney, A.H. Inhibitory effect of curcumin, chlorogenic acid, caffeic acid and ferulic acid on tumor promotion in mouse skin by 12-Otetradecanoylphorbol-13-acetate. Cancer Res. 1988, 48, 5941–5946. [Google Scholar]
- Pereira, M.A.; Grubbs, C.J.; Barnes, L.H.; Li, H.; Olson, G.R.; Eto, I.; Juliana, M.; Whitaker, L.M.; Kelloff, G.J.; Steele, V.E.; et al. Effects of the phytochemicals, curcumin and quercetin, upon azoxymethane-induced colon cancer and 7,12-dimethylbenz[a]anthracene-induced mammary cancer in rats. Carcinogenesis 1996, 17, 1305–1311. [Google Scholar] [CrossRef]
- Huang, M.T.; Loum, Y.R.; Mam, W.; Newmark, H.L.; Reuhl, K.R.; Conney, A.H. Ihibitory effects of dietary curcumin on forestomach, duodenal, and colon carcinogenesis in mice. Cancer Res. 1994, 54, 5841–5847. [Google Scholar]
- Rao, C.V.; Rivenson, A.; Simi, B.; Reddy, B.S. Chemoprevention of colon carcinogenesis by dietary curcumin, a naturally occurring plant phenolic compound. Cancer Res. 1995, 55, 259–266. [Google Scholar]
- Zhang, F.; Altorki, N.K.; Mestre, J.R.; Subbaramaiah, K.; Dannenberg, A.J. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 1999, 20, 445–451. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, B.; Chen, H.; Zhang, L. Anticancer activities of curcum in on human hepatocarcinoma cell line Sk-hep-1. Zhongguo Zhong Yao Za Zhi 2010, 35, 485–488. [Google Scholar]
- Churchill, M.; Chadburn, A.; Bilinski, R.T.; Bertagnolli, M.M. Inhibition of intestinal tumors by curcumin associated with changes in the intestinal immune cell profile. J. Surg. Res. 2000, 89, 169–175. [Google Scholar] [CrossRef]
- Muller, R.H.; Radtke, M.; Wissing, S.A. Nanostructured lipid matrices for improved microencapsulation of drugs. Int. J. Pharm. 2002, 242, 121–128. [Google Scholar] [CrossRef]
- Jenning, V.; Thunemann, A.F.; Gohla, S.H. Characterisation of a novel solid lipid nanoparticle carrier system based on binary mixtures of liquid and solid lipids. Int. J. Pharm. 2000, 199, 167–177. [Google Scholar] [CrossRef]
- Jenning, V.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) based on binary mixtures of liquid and solid lipids: A 1H-NMR study. Int. J. Pharm. 2000, 205, 15–21. [Google Scholar] [CrossRef]
- Mehnert, W.; Mader, K. Solid lipid nanoparticles: Production, characterization and applications. Adv. Drug. Deliv. Rev. 2001, 47, 165–196. [Google Scholar] [CrossRef]
- Muller, R.H.; Mader, K.; Gohla, S. Solid lipid nanoparticles (SLN) for controlled drug delivery-a review of the state of the art. Eur. J. Pharm. Biopharm. 2000, 50, 161–177. [Google Scholar] [CrossRef]
- Muller, R.H.; Gohla, S.; Dingler, A.; Schneppe, T. Large Scale Production of Solid Lipid Nanoparticles (SLNTM) and Nanosuspensions (DissoCubesTM). In Handbook of Pharmaceutical Controlled Release Technology; Wise, D.L., Ed.; Marcel Dekker Inc: New York, NY, USA, 2000; pp. 359–376. [Google Scholar]
- Uner, M. Preparation, characterization and physico-chemical properties of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC): Their benefits as colloidal drug carrier systems. Pharmazie 2006, 61, 375–386. [Google Scholar]
- Mao, S.J.; Hou, S.X.; He, R.; Zhang, L.K.; Wei, D.P.; Bi, Y.Q.; Jin, H. Uptake of albumin nanoparticle surface modified with glycyrrhizin by primary cultured rat hepatocytes. World J. Gastroenterol. 2005, 11, 3075–3079. [Google Scholar]
- Lin, A.; Liu, Y.; Huang, Y.; Sun, J.; Wu, Z.; Zhang, X.; Ping, Q. Glycyrrhizin surface-modified chitosan nanoparticles for hepatocyte-targeted delivery. Int. J. Pharm. 2008, 359, 247–253. [Google Scholar] [CrossRef]
- Mao, S.J.; Bi, Y.Q.; Jin, H.; Wei, D.P.; He, R.; Hou, S.X. Preparation, characterization and uptake by primary cultured rat hepatocytes of liposomes surface-modified withglycyrrhetinic acid. Pharmazie 2007, 62, 614–619. [Google Scholar]
- Kato, K.; Uchida, E.; Kang, E.T.; Uyama, Y.; Ikada, Y. Polymer surface with graft chains. Prog. Polym. Sci. 2003, 28, 209–259. [Google Scholar] [CrossRef]
- Nam, H.Y.; Kwon, S.M.; Chung, H.; Lee, S.Y.; Kwon, S.H.; Jeon, H.; Kim, Y.; Park, J.H.; Kim, J.; Her, S.; et al. Cellular uptake mechanism and intracellular fate of hydrophobically modified glycol chitosan nanoparticles. J. Control. Release 2009, 135, 259–267. [Google Scholar] [CrossRef]
- Zhou, L.L.; Wang, Y.; Liu, Q.F. Study on determination of entrapment efficiency of sinomenine liposomes. Zhongguo Zhong Yao Za Zhi 2006, 31, 731–734. [Google Scholar]
- Sample Availability: Samples of Cur-NLC, Cur-PEG-NLC and Cur-GA-PEG-NLC are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chu, Y.; Li, D.; Luo, Y.-F.; He, X.-J.; Jiang, M.-Y. Preparation and In Vitro Evaluation of Glycyrrhetinic Acid-Modified Curcumin-Loaded Nanostructured Lipid Carriers. Molecules 2014, 19, 2445-2457. https://doi.org/10.3390/molecules19022445
Chu Y, Li D, Luo Y-F, He X-J, Jiang M-Y. Preparation and In Vitro Evaluation of Glycyrrhetinic Acid-Modified Curcumin-Loaded Nanostructured Lipid Carriers. Molecules. 2014; 19(2):2445-2457. https://doi.org/10.3390/molecules19022445
Chicago/Turabian StyleChu, Yang, Dan Li, Yi-Fan Luo, Xiao-Jin He, and Ming-Yan Jiang. 2014. "Preparation and In Vitro Evaluation of Glycyrrhetinic Acid-Modified Curcumin-Loaded Nanostructured Lipid Carriers" Molecules 19, no. 2: 2445-2457. https://doi.org/10.3390/molecules19022445
APA StyleChu, Y., Li, D., Luo, Y.-F., He, X.-J., & Jiang, M.-Y. (2014). Preparation and In Vitro Evaluation of Glycyrrhetinic Acid-Modified Curcumin-Loaded Nanostructured Lipid Carriers. Molecules, 19(2), 2445-2457. https://doi.org/10.3390/molecules19022445




